Recent Developments in Accelerated Antibacterial Inactivation on 2D Cu-Titania Surfaces under Indoor Visible Light
Abstract
:1. Introduction
2. Bacterial Inactivation Performance on TiO2/Cu and Surface Properties
2.1. Short Description of the Main Issues of Concern Affecting the TiO2 Photocatalysis Application in Microbial Abatement
| Bacteria + [PES-TiO2] + light → [PES-TiO2*] Bacteria → Bacteria+ + PES-(TiO2)cbe- | (4) |
| PES-(TiO2) cbe- + O2 + H+ → HO2• E0 -0.05 NHE [43,56] | (5) |
| PES-(TiO2) cbe- + O2 ads → O2•−ads E0 -0.16 NHE [43,56] | (6) |
| PES-(TiO2) vbh+ + OH−ads → HO° E0 -1.90 NHE [49] | (7) |
| PES-(TiO2) vbh+ + H2Oads → HO•ads + H+ | (8) |
| O2•− + H+ ⇔ HO2• pKa 4.8 | (9) |
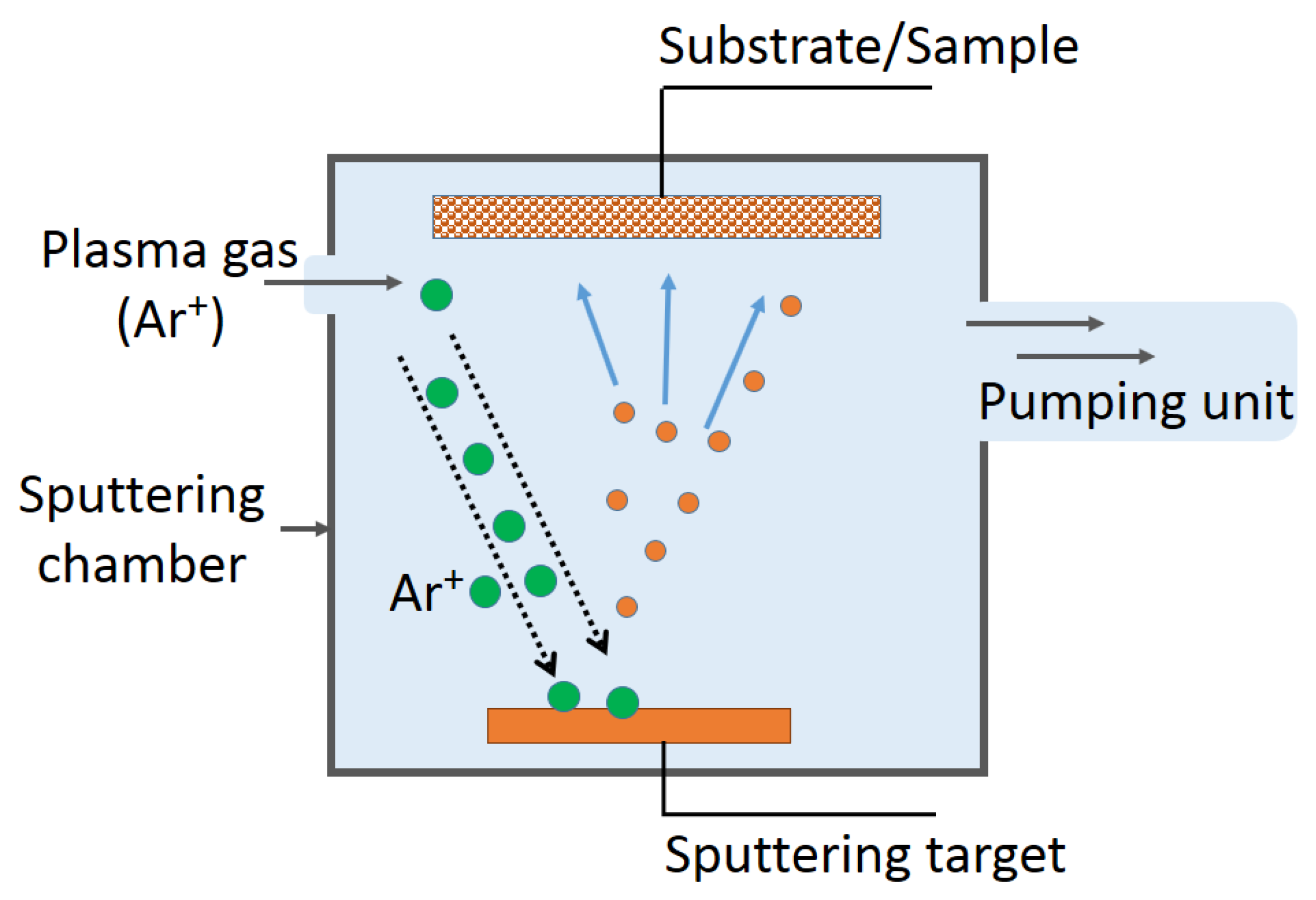
2.2. TiO2/Cu Synthesis Leading to Uniform, Adhesive, and Antibacterial Films
2.3. Cu-Loaded Sputtered Surfaces Active in the Dark and under Light, Leading to Microbial Abatement
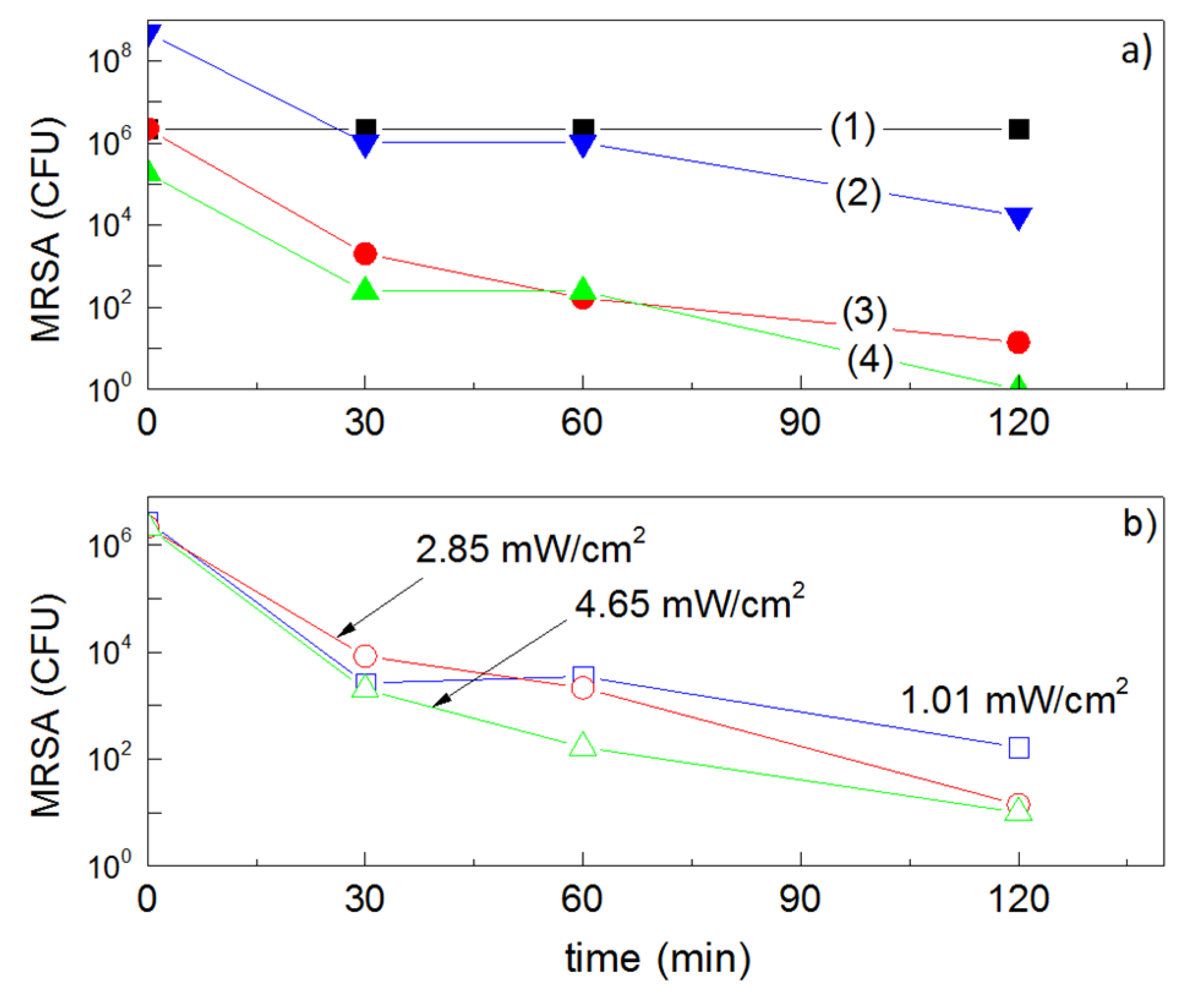
2.4. Behavior of Cu-Sputtered Surfaces in the Dark and under Hospital Settings (Indoor Actinic Light), Leading to MRSA-Isolate Inactivation
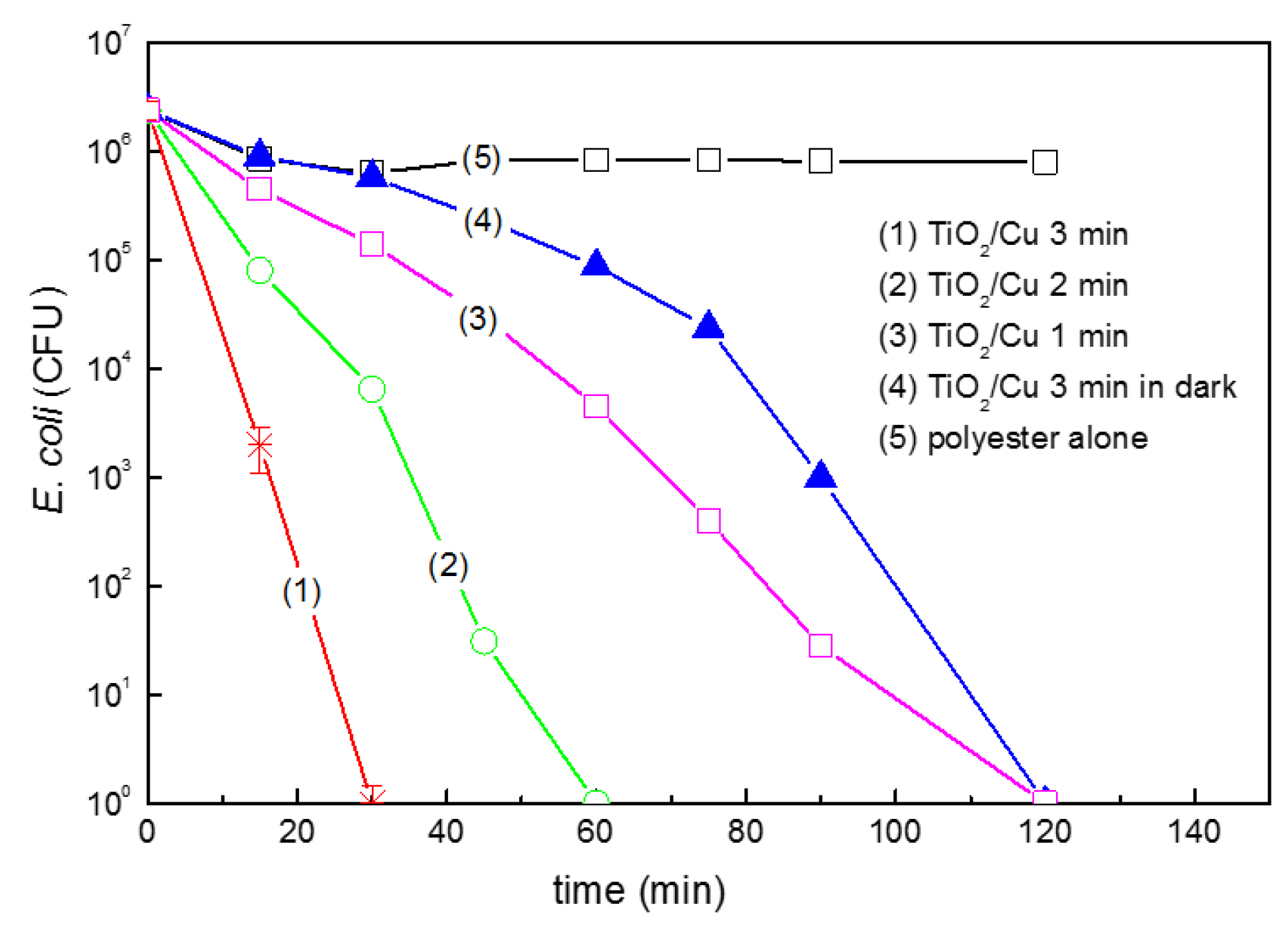
2.5. Photocatalytic/Catalytic Bactericidal Effects on E. coli and MRSA by TiO2/Cu Thin Films
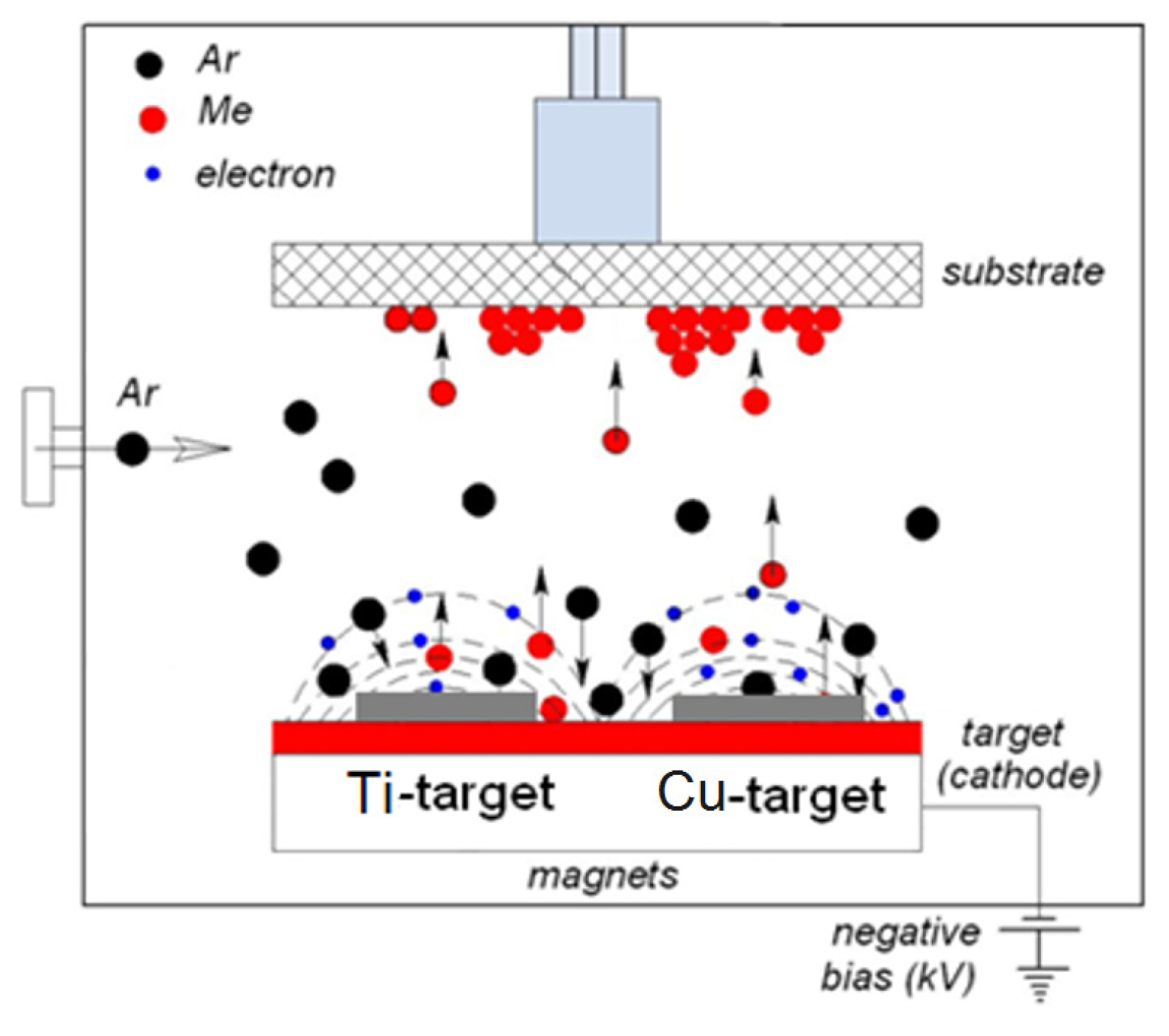
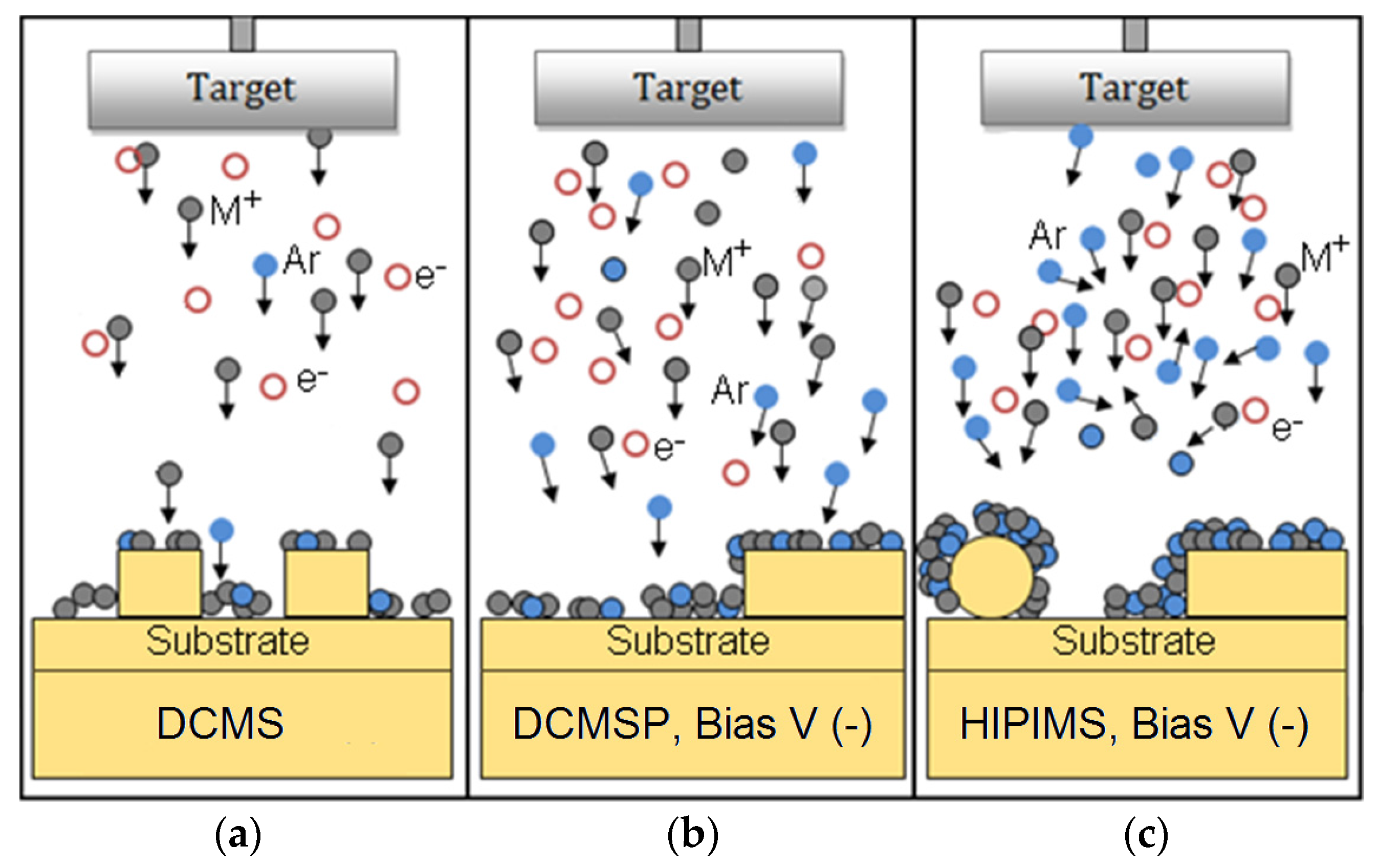
2.6. Current Work on TiO2/Cu Sputtered Surfaces by DCMS, DCMSP, and HIPIMS, Bactericidal Effects and Thin Film Properties
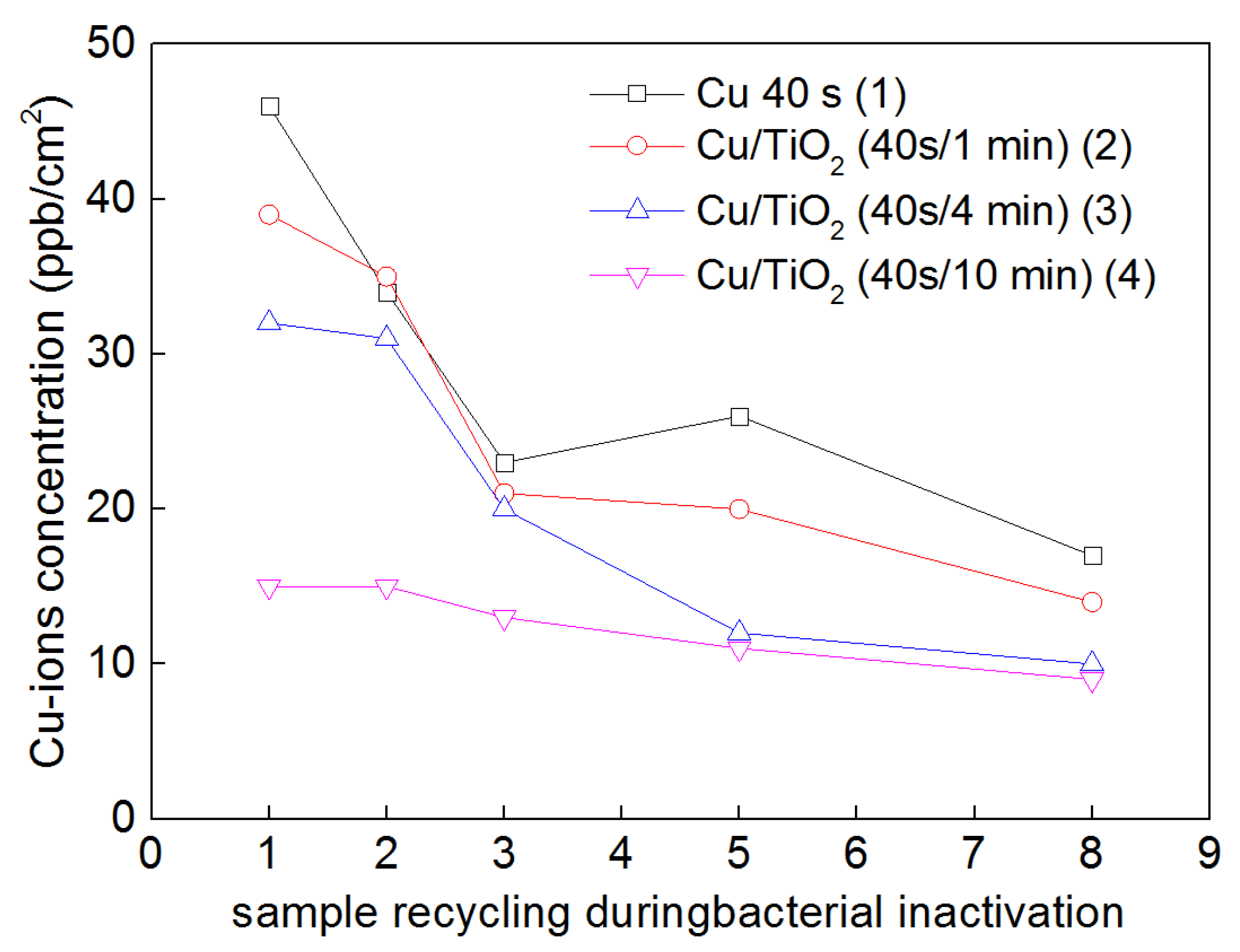
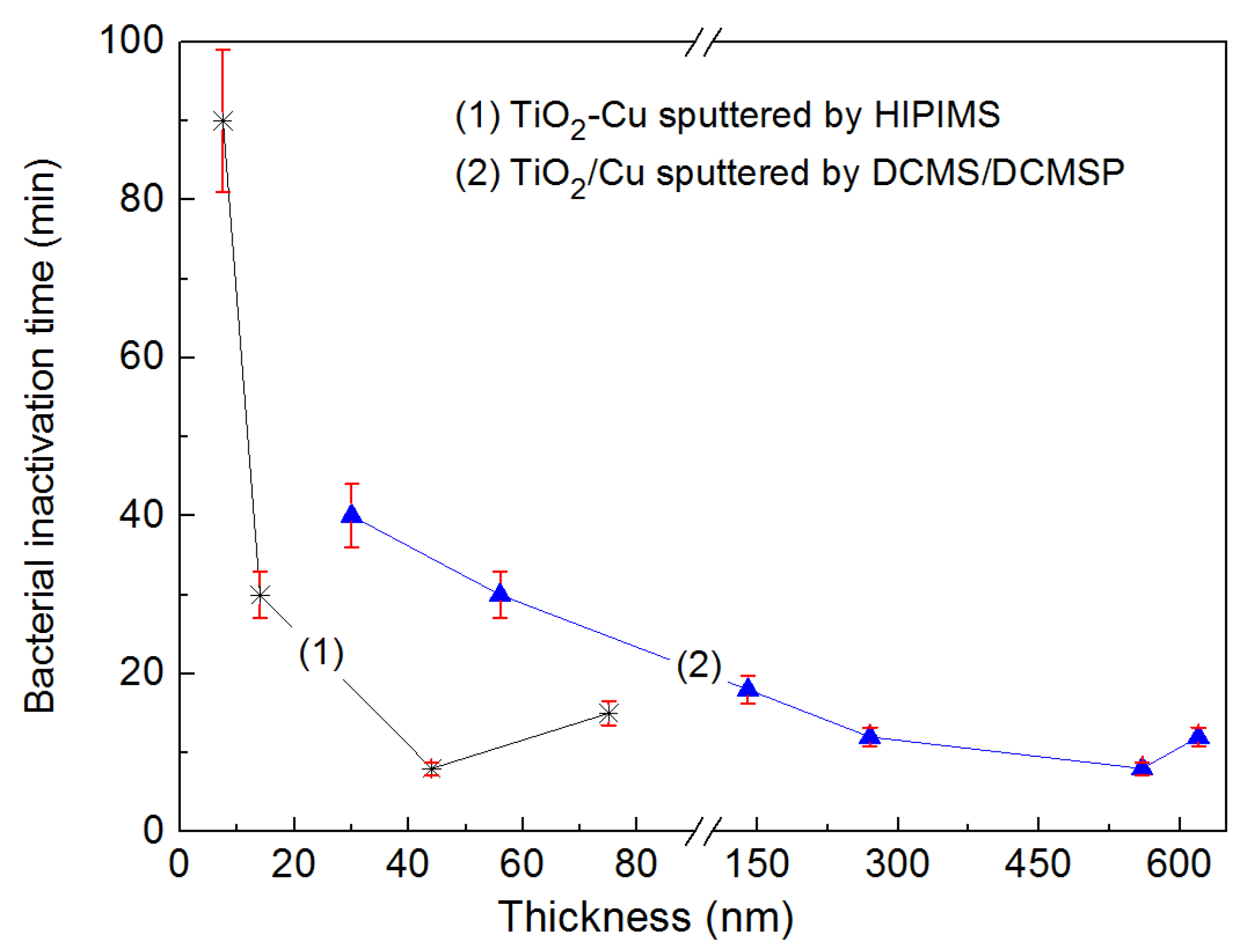
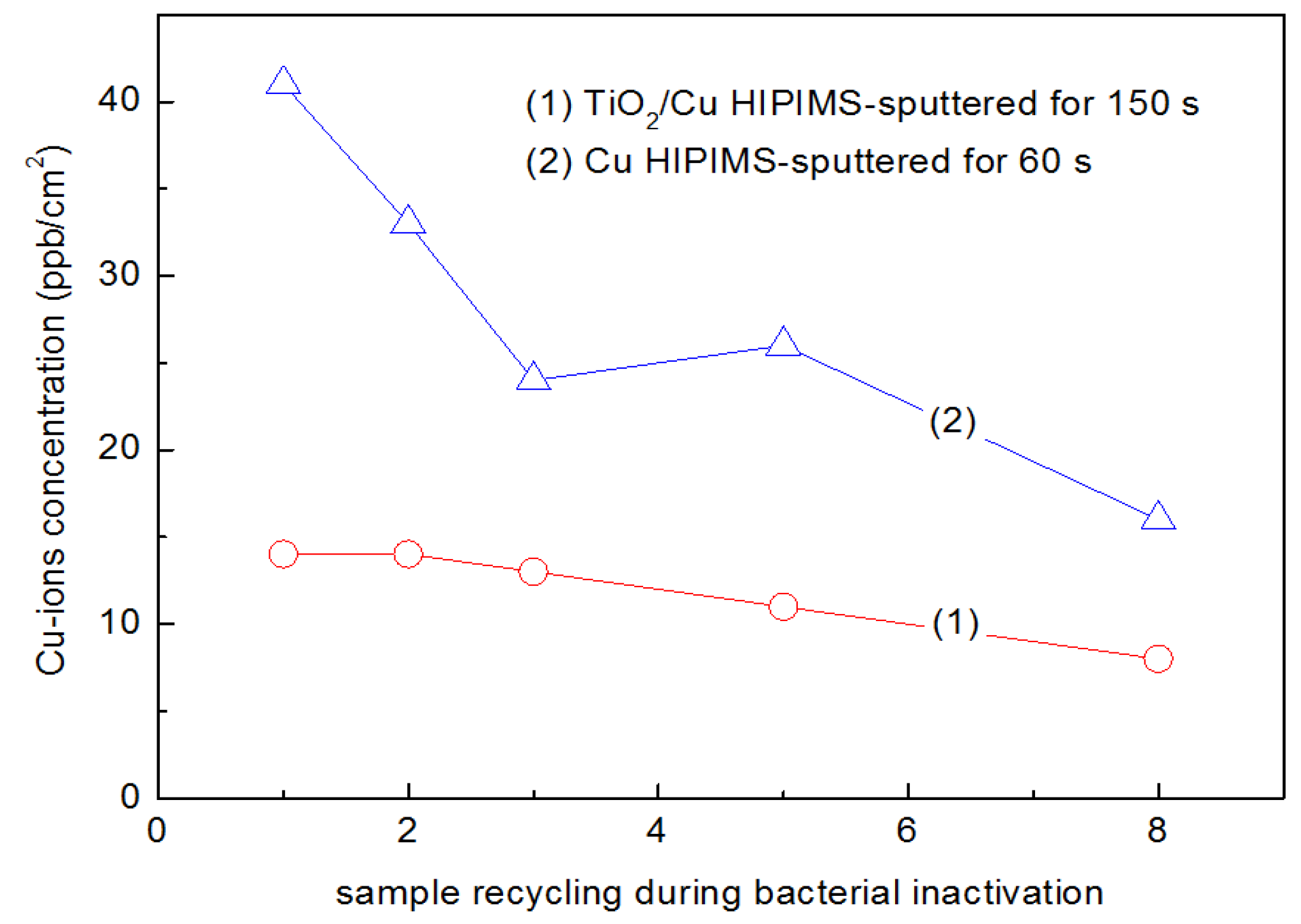
2.7. TiO2-Cu Films by HIPIMS: Comparison of the Bacterial Inactivation Performance with DCMS/DCMSP-Films
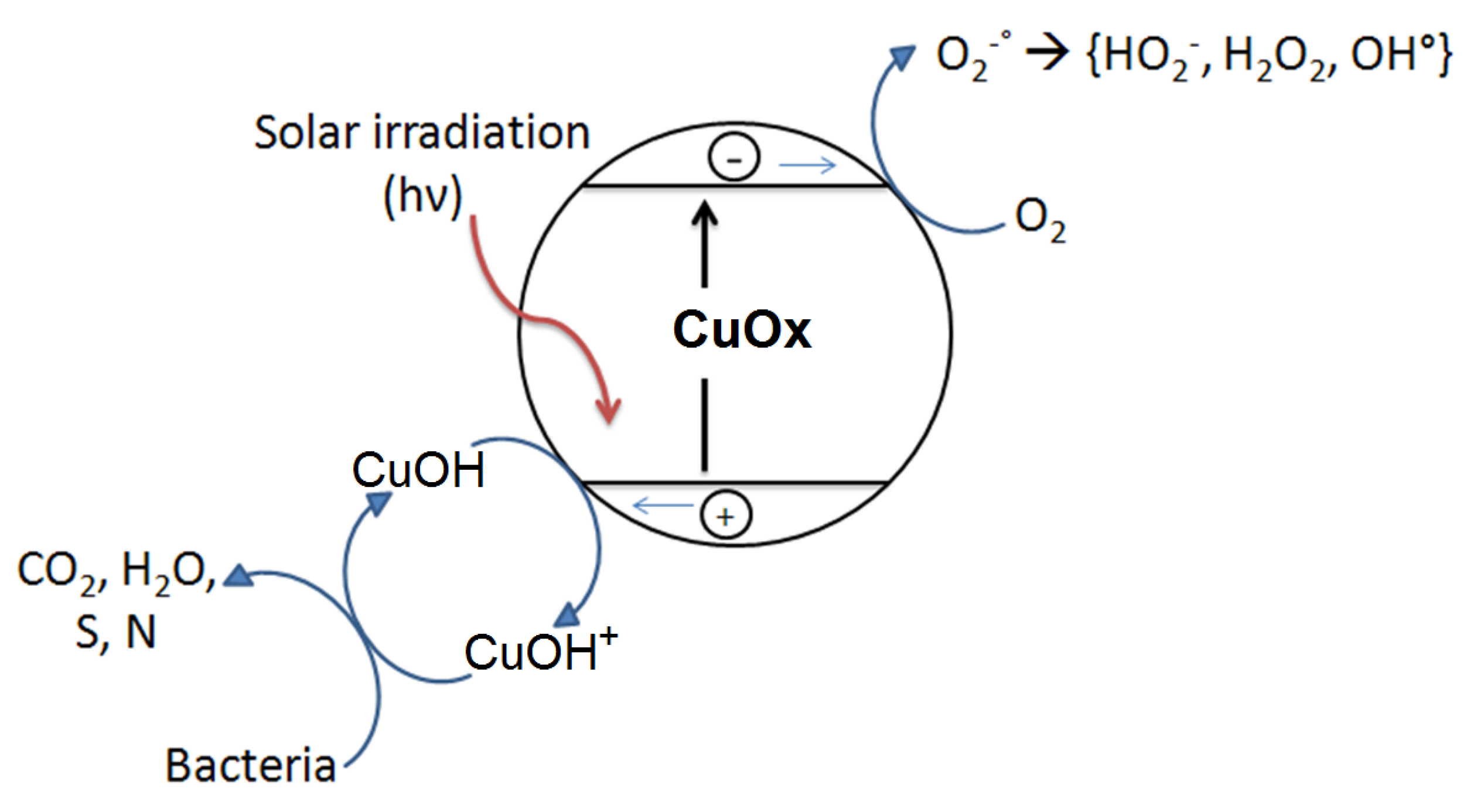
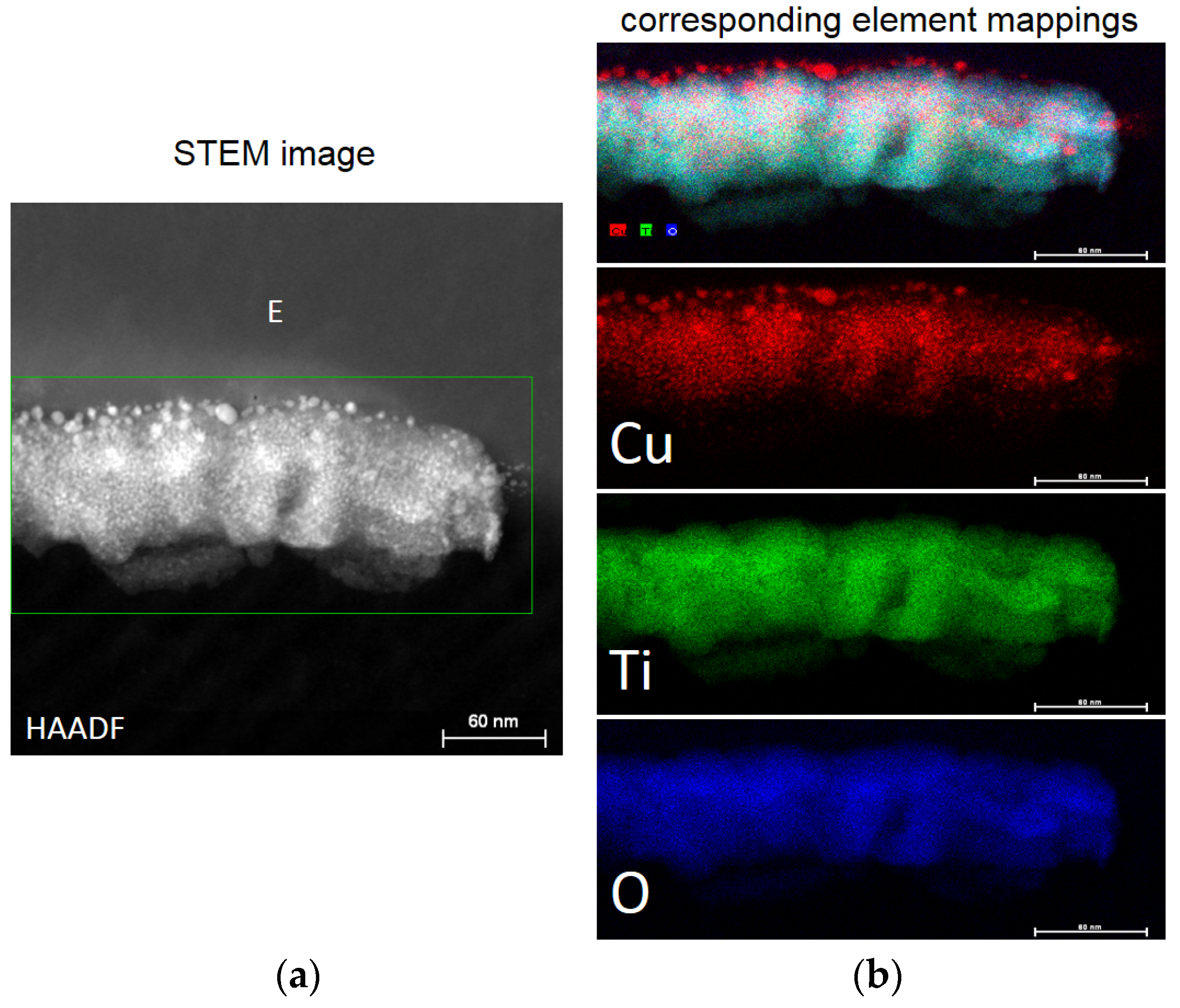
2.8. Interfacial Charge Transfer (IFCT) Suggested on TiO2-Cu Films Leading to Gram-Negative and Gram-Positive Bacterial Inactivation
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mikolay, A.; Hugget, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal activity of copper-deposited TiO2 films under weak UV-light illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Nozik, A. Photo-electrochemistry: Applications to Solar Energy Conversion. Annu. Rev. Phys. Chem. 1978, 189, 521–549. [Google Scholar]
- Yadava, H.; Otaria, S.; Kolia, V.; Malib, S.; Hong, C.; Pawara, S.; Delekara, S. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Bensimon, M.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Supported TiO2 films deposited at different energies: Implications of the surface compactness on the catalytic kinetics. Appl. Catal. B 2016, 191, 42–52. [Google Scholar] [CrossRef]
- Robin, S.; Soulimane, T.; Lavelle, S. Interactions of Biofilm-forming Bacteria with Abiotic Surfaces. In Biological Interactions with Surface Charges in Biomaterials; Tofail, S.A.M., Ed.; RSC: London, UK, 2012. [Google Scholar]
- Lejeune, P. Contamination of abiotic surfaces: What a colonizing bacterium sees and how blur it. Trends Microbiol. 2003, 11, 179–184. [Google Scholar] [CrossRef]
- Bernstein, R.; Freger, V.; Lee, J.; Kim, Y.; Lee, J.; Herzberg, M. Should I stay or should I go? Bacterial attachment vs. biofilm formation on surface-modified membranes. Biofouling 2014, 30, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Norris, P.; Piozzi, A.; Donello, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 2001, 48, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.-J.; Dunlop, M.S.P.; Hamilton, J.W.J.; Fernandez-Ibanes, P.; Polo-Lopez, I.; Sharma, K.P.; Vennard, M.S.A. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Canpoccia, D.; Montanaro, L.; Arciola, C. A review of the biomaterials technologies for infection resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Shahzad, B.; Li, M.; Wang, G.; Xu, D. Antimicrobial materials with medical applications. Mater. Technol. Adv. Biomater. 2015, 30, B30–B95. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.; Vaerenberg, S.; Hopkins, S.; Catry, B.; et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012, 17, 20316. [Google Scholar] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Clinical infectious diseases Infectious. Dis. Soc. Am. 2009, 48, 1–12. [Google Scholar]
- Bhalla, A.M.D.; Pultz, N.J.B.S.; Gries, D.M.M.D.; Ray, A.J.M.D.; Eckstein, E.C.R.N.; David, C.; Aron, M.D.; Donskey, C.J.M.D. Acquisition of Nosocomial Pathogens on Hands after Contact With Environmental Surfaces Near Hospitalized Patients. Infect. Control Hosp. Epidemiol. 2004, 25, 164–167. [Google Scholar] [CrossRef] [PubMed]
- French, G.L.; Otter, J.A.; Shannon, K.P.; Adams, N.M.; Watling, D.; Parks, M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004, 57, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhao, F.; Simoes, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Nakamura, N.; Komine, T. Continuous-Sterilization System That Uses Photosemiconductor Powders. Appl. Environ. Microbiol. 1988, 54, 1330–1333. [Google Scholar] [PubMed]
- Fujishima, A.; Hashimoto, K.; Watanabe, W. TiO2 Photocatalysis Fundamentals and Applications; Bkc Inc.: Tokyo, Japan, 1999. [Google Scholar]
- Taylor, K.; Roberts, J.; Roberts, J. The Challenge of Hospital Acquired Infections (HAI); National Audit Office: London, UK, 2002. [Google Scholar]
- Talon, D. The role of the hospital environment in the epidemiology of multi-resistant bacteria. J. Hosp. Infect. 1999, 43, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. The role of environmental cleaning in the control of hospital-acquired infection. J. Hosp. Infect. 2009, 73, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, S.; Kumar, R.; Chandrasekaran, N.; Mujerkee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low light exposure concentrations. Toxicol. Res. 2012, 1, 116–130. [Google Scholar]
- Zhang, L.; Luo, Z.; Song, L.; Shi, X.; Pan, Y.; Fan, Y.; Xu, Y. Effects and mechanisms of waterborne copper exposure influencing ovary development and related hormones secretion in yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 2016, 178, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.; Franklin, M.; Stewart, R. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. J. Antomicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemcal sterilization of microbial cells by semiconductor powders. FEMS Microb. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Foster, H.; Ditta, I.; Varghese, S.; Steele, A. Photocatlytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Pigeot-Rémy, S.; Simonet, F.; Errazuriz-Cerda, E.; Lazzaroni, J.; Atlan, D.; Gillard, C. Photocatalysis and disinfection of water: Identification of potential bacterial targets. Appl. Catal. B 2011, 104, 390–398. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Ulgig, K.; Morawski, A. The applicationot titanium dioxide for deactivation of bioparticulates: An overview. Catal. Today 2011, 169, 249–257. [Google Scholar] [CrossRef]
- Robertson, P.; Robertson, J.; Bahnemann, D. Removal of microorganisms and their chemical metabolites from water using semiconductors photocaatlysis. J. Hazard. Mater. 2012, 211–212, 162–171. [Google Scholar]
- Dalrymple, O.; Isaaks, W.; Stefanakos, E.; Trotz, M.; Goswami, D. Lipid vesicles a smodel membranes in photocatalytic disinfection studies. J. Photochem. Photobiol. A 2011, 221, 64–70. [Google Scholar] [CrossRef]
- Gamage, J.; Zhang, Z. Applications of Photocatalytic Disinfection: A review. Int. J. Photo-Energy 2010, 2010, 764870. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134–4143. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, O.; Stefanakos, E.; Trotz, M.; Goswami, Y. A review of the mechanism and modeling of photocatalytic disinfection. Appl. Catal. B 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Farr, S.; Kogoma, T. Oxidative stress responses in Escherrichia coli and Salmonella triphimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [PubMed]
- Kiwi, J.; Nadtochenko, V. New evidence for TiO2 photocatalysis during bilayer lipid-peroxidation. J. Phys. Chem. B 2004, 108, 17675–17684. [Google Scholar] [CrossRef]
- Linsebliger, A.; Lu, G.; Yates, J. Photocatalysis on TiO2 Surfaces. Principles, Mechanisms and Selected Results. Chem. Rev. 1996, 95, 735–758. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalyis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Daoud, W. Self-Cleaning Materials and Surfaces, a Nanotechnology Approach; Wiley: Chichester, UK, 2013. [Google Scholar]
- Griesser, H.J. Chapter 16: Uniform, adhesive and low cytotoxic films accelerating bacterial reduction in the dark and under visible light. In The Film Coatings for Biomaterials and Biomedical Applications; Rtimi, S., Pulgarin, C., Kiwi, J., Eds.; Elsevier Ltd.: London, UK, 2016. [Google Scholar]
- Kiwi, J.; Rtimi, S. Chapter 3: Environmentally mild self-cleaning processes on textile surfaces under daylight irradiation: Critical Issues. In Active Coatings for Smart Textiles; Hu, J., Ed.; Elsevier Ltd.: Cambridge, UK, 2016. [Google Scholar]
- Rtimi, S.; Sanjines, R.; Kiwi, J.; Pulgarin, C.; Bensimon, M.; Khmel, I.; Nadtochenko, V. Innovative photocatalysts (FeOx-TiO2): Transients by femtosecond laser pulse leading to bacterial inactivation under visible light. RSC Adv. 2015, 5, 101751–101759. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Nadtochenko, V.; Gostev, F.; Shelaev, I.; Kiwi, J. FeOx-TiO2 Film with Different Microstructiures Leading to Femtosecond Transients with Different Properties: Biological Implications under Visible Light. Nat. Sci. Rep. 2016, 6, 30113. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, T.; Pillai, C.S.; Seery, K.; Falaras, P.; Kontos, A.; Dunlop, M.; Dunlop, H.J.; Byrne, A.-J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, C.S.; Falaras, P.; O’Shea, K.; Byrne, A.-J.; Dionysiou, D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etacheri, V.; Di Valentin, C.; Schneider, D.; Bahnemann, D.; Pillai, C.S. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.; Pillai, C.S. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Fagan, R.; Cormack, M.; Dionysiou, D.; Pillai, S. A review on solar and visible light active TiO2 photocatalysts for treating bacteria, cynotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Devi, G.; Kavitha, R. A review on non-metal ion doped-titania for photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charges dynamic in enhancing the activity. Appl. Catal. B 2013, 140–168, 559–587. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.; Kaloudis, T.; O’Shea, K.; Dionysiou, D.; Hiskia, A. Assessment of the roles of reactive oxygen species in the UV and visible light photocatalytic degradation of cyanotoxins and water taste and odor compounds using C-TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, J.; Zhang, L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hashimoto, K.; Fujishima, K.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Enhancement of Photoinduced Hydrophilic Conversion Rate of TiO2 Film Electrode Surface of Anodic Polarization. J. Phys. Chem. B 2001, 105, 3023–3026. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and Photoinduced Hydrophilicity of Various Metal Oxide Films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Innovative semi-transparent nanocomposite films presenting photo-switchable behavior and leading to a reduction of the risk of infection under sunlight. RSC Adv. 2013, 3, 16345–16349. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kulik, A.; Kiwi, J. Innovative transparent non-scattering TiO2 bactericide films inducing increased E. coli cell fluidity. Surf. Coat. Technol. 2014, 254, 333–343. [Google Scholar] [CrossRef]
- Pulgarin, C.; Kiwi, J.; Nadtochenko, V. Mechanism of the photocatalytic destruction of bacteria by TiO2 films leading to cell-wall damages and bacterial lysis. Appl. Catal. B 2012, 128, 179–183. [Google Scholar] [CrossRef]
- Jayachandran, Y.; Narayandass, S. The effect of thickness of Ti-nitride coatings on bacterial adhesion. Trends Biomater. Artif. Organs 2010, 24, 90–93. [Google Scholar]
- Rio, L.; Kusiak, E.; Kiwi, J.; Pulgarin, C.; Trampuz, C.; Bizzini, A. Comparative methods to evaluate the bactericidal activity of copper-sputtered surfaces against methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 8176–8182. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kumar, I.; SalmonI, I.; Chance, B. The 650 and chromophore in Escherichia coli is oxy oxygenated compound, not the oxidized form of cytochrome oxidase: An hypothesis. J. Gen. Microbiol. 1983, 129, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Van Loosdrecht, L.; Lyklema, M.; Norde, W.; Schraa, G.; Zender, A. The role of bacterial cell-wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987, 53, 1893–1990. [Google Scholar] [PubMed]
- Xu, L.; Wellia, D.; Amal, R.; Liao, W.; Loo, J.; Tan, T. Fabrication of Highly Ordered TiO2 Nanorod/Nanotube Adjacent Array for Photo-electrochemical Applications. Langmuir 2010, 2, 1122–1128. [Google Scholar]
- Hogt, A.; Dankert, J.; Feijen, J. Adhesion of Staphyloccocus epidermis and Staphyloccocus Saprophyticus to a hydrophobic biomaterial. J. Gen. Microbiol. 1985, 131, 2485–2491. [Google Scholar] [PubMed]
- Fletcher, R.; Loeb, M. Influence of the substratum characteristics on the attachment of a marine pseudomonas to solid surfaces. Appl. Environ. Microbiol. 1979, 37, 67–72. [Google Scholar] [PubMed]
- Loveland, P.; Ryan, J.; Amy, G.; Harvey, R. The reversibility of virus attachment to mineral surfaces. Colloid Surf. A Physicochem. Eng. Aspects 1996, 107, 205–221. [Google Scholar] [CrossRef]
- Zhukova, L.; Kiwi, J.; Nikandrov, V. Effect of the nanoparticles on Escherichia coli cell division capacity at pH 4–4.5 in the absence of UV-irradiation. J. Colloids Surf. Bio-Interfaces 2012, 97, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Nesic, J.; Rtimi, R.; Hebert, C.; Pulgarin, C.; Roglic, G.; Kiwi, J. New evidence for TiO2 uniform surfaces leading to complete bacterial reduction in the dark: Critical issues. Colloids Surf. B Biointerfaces 2014, 123, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Ulrich, B.; Sinnet, R.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simler, S.; Brunner, H.; Vonmont, H.; Burkhart, M.; et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 2008, 156, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (ENM) for different regions and at different resolutions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Shereef, A.; Wu, J.; Binh, C.; Kelly, J.; Gaillard, J.; Gray, K. Effects of the material morphology on the phototoxicity of nano-TiO2 to bacteria. Environ. Sci. Technol. 2013, 49, 8113–8123. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Rincon, A.G.; Pulgarin, C. Photocatlytical inactivation of E. coli: Effect of the continuous-intermittent light intensity and of suspended fixed TiO2 concentration. Appl. Catal. B 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Rincon, A.G.; Pulgarin, C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: Post-irradiation events in the dark and assessment of the effective disinfection time. Appl. Catal. B 2004, 112, 99–112. [Google Scholar] [CrossRef]
- Miyauchi, M.; Irie, H.; Liu, M.; Qiu, X.; Yu, H.; Sunada, K.; Hashimoto, K. Visible-Light-Sensitive Photocatalysis: Nanocluster-Grafted Titanium Dioxide for Indoor Environmental remediation. J. Phys. Chem. Lett. 2016, 7, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yoriya, S.; Chumphu, A.; Pookmanee, P.; Laithong, W.; Thepa, S.; Songprakorp, R. Multi-layered TiO2 films towards enhancement of Escherichia coli inactivation. Materials 2016, 9, 808. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Kinetics and mechanism for transparent polyethylene-TiO2 films mediated self-cleaning leading to MB dye discoloration under sunlight irradiation. App. Cat. B Environ. 2015, 162, 236–244. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; McMurray, T.A.; Hamilton, J.W.; Byrne, J.A. Inactivation of clinically relevant pathogens by photocatalytic coatings. Photochem. Photobiol. A 2010, 196, 113–119. [Google Scholar] [CrossRef]
- McCullagh, C.; Robertson, C.; Bahnemann, D.; Robertson, K. The application of TiO2 photocatalysisfor disinfection of water contaminated with pathogenic micro-orgnisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Amenazaga-Madrid, P.; Silveyra-Morales, R.; Cordoba-Fierro, L.; Orianda-Borunda, E.; Soli, J. TEM evidence of ultrastructural alteration on Pseudomonas aeruginosa by photocatlytic TiO2 thin films. J. Photochem. Photobiol. B 2003, 70, 45–50. [Google Scholar] [CrossRef]
- Mendez-Hermida, F.; Ares-Mazas, E.; McGuigan, G.; Boyle, M.; Sichel, C.; Fernandez-Ibanez, P. Disinfection of drinking water contaminated with Crystosporidium parvum oocysts under natural sunlight and using the photocatlyst TiO2. J. Photochem. Photobiol. B 2007, 66, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Kong, S.; Orr, P.; Miller, C.; Henry, E. Photocatalytic inactivation of coliform bacteria and viruses in secondary waste water effluents. Water Res. 1995, 29, 95–100. [Google Scholar] [CrossRef]
- Lincous, A.; Carter, J.; Locuson, B.; Oulette, J.; Slattery, K.; Smith, A. Photocatalytic inhibition of algae growth using TiO2, WO3 and cocatalysts modifications. Environ. Sci. Technol. 2000, 34, 4752–4758. [Google Scholar] [CrossRef]
- Sichel, C.; de Cara, M.; Tello, J.; Blanco, J.; Fernandez-Ibanez, P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B 2007, 74, 152–160. [Google Scholar] [CrossRef]
- Kuhn, P.; Chaberny, F.; Massholder, K.; Stickler, M.; Benz, W.; Sonntag Gerdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium oxide und UV-light. Chemosphere 2003, 53, 51–57. [Google Scholar] [CrossRef]
- Zhang, L.; Dillert, R.; Bahnemann, D.; Vormoor, M. Photo-induced hydrophilicity and self-cleaning: Models and reality. Energy Environ. Sci. 2012, 5, 7491–7507. [Google Scholar] [CrossRef]
- Nakano, T.; Baba, S. Gas pressure effects on thickness uniformity and circumvented deposition during sputter deposition. Vacuum 2006, 80, 647–840. [Google Scholar] [CrossRef]
- Dunill, C.W.; Aiken, Z.A.; Pratten, M.; Wilson, M.; Parkin, P.I. Sulfur- and Nitrogen-Doped Titania Biomaterials via APCVD. Chem. Vap. Depos. 2010, 16, 50–54. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D. Photoactive and Antibacterial Thin Films on Stainless Steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Foster, H.A.; Sheel, D.W.; Shel, P.; Evans, P.; Varghese, S.; Rutschke, N.; Yates, M.H. Antimicrobial activity of titania/silver and titania/copper films prepared by CVD. J. Photochem. Photobiol. A 2010, 216, 283–289. [Google Scholar] [CrossRef]
- Caputo, G.; Nobile, C.; Buonsanti, R.; Lipp, T.; Manna, L.; Cingolani, R.; Cozzoli, P.; Atanssiou, A. Determination of surface properties of various substrates using TiO2 nano-rod coatings with tunable characteristics. J. Mater. Sci. 2008, 43, 3474–3480. [Google Scholar] [CrossRef]
- Irie, H.; Washizuka, S.; Hashimoto, K. Hydrophilicity on carbon-doped TiO2 thin films under visible light. Thin Solid Films 2006, 510, 21–25. [Google Scholar] [CrossRef]
- Scanlon, D.; Dunnill, W.D.; Buckeridge, J.; Shevlin, S.; Logsdail, S.; Scott, S.; Woodley, M.; Richard, C.; Catlow, A.; Powell, M.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Teixeira, V.; Portinha, A.; Magalhães, A.; Newton, R.; Coutinho, P. Iron-doped photocatalytic TiO2 sputtered coatings on plastics for self-cleaning applications. Mater. Sci. Eng. B 2007, 138, 144–150. [Google Scholar] [CrossRef]
- Miron, C.; Roca, A.; Cozorici, P.; Sirghi, L. Photo-induced bactericidal activity activity of TiO2 tin films obtained by radiofrequency magnetron sputtering deposition. J. Optoelectr. Adv. Mater. 2004, 7, 915–919. [Google Scholar]
- Plowman, R.; Graves, R.; Griffin, N.; Taylor, L. The rate of added cost of hospital acquired infections. J. Hosp. Infect. 2001, 47, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Rossnagel, S.; Hopwood, J. Magnetron sputter deposition with high levels of metal ionization. Appl. Phys. Lett. 1993, 63, 3285–3287. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, D.R. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Kousznetsov, V.; Macak, K.; Schneider, J.; Helmersson, U.; Petrov, I. A novel pulsed magnetron sputter technique utilizing very high target power densities. Surf. Coat. Technol. 1999, 122, 290–293. [Google Scholar] [CrossRef]
- Castro, C.; Pulgarin, C.; Osorio, P.; Giraldo, S.; Kiwi, J. Structure-reactivity relations of the Cu-cotton sputtered layers during E. coli inactivation in the dark and under light. J. Photochem. Photobiol. A 2010, 216, 295–302. [Google Scholar] [CrossRef]
- Osorio, P.; Sanjines Ruales, C.; Castro, C.; Pulgarin, C.; Rengifo, J.-A.; Lavanchy, J.-A.; Kiwi, J. Antimicrobial Cu-functionalized surfaces prepared by bipolar asymmetric DC-pulsed magnetron sputtering (PMS). J. Photochem. Photobiol. A 2011, 220, 70–76. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Pulgarin, C.; Lavanchy, J.-C.; Kiwi, J. Growth of TiO2/Cu films by HIPIMS for accelerated bacterial loss of viability. Surf. Coat. Technol. 2013, 232, 804–813. [Google Scholar] [CrossRef]
- Li, C.; Tian, X.; Gong, C.; Xu, J. The improvement of high power impulse magnetron sputtering performance by an external unbalanced magnetic field. Vacuum 2016, 133, 98–104. [Google Scholar] [CrossRef]
- Nikaido, H.J. Prevention of Drug Access to Bacterial Targets. Permeability Barriers and Active Flux. Biol. Chem. 1994, 269, 3905–3909. [Google Scholar]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper impregnated products with potential biocidal activities. J. FASEB 2008, 18, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Biocidal Textiles can help fight nosocomial infections. Med. Hypothesis 2008, 70, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.; Lavie, Y.; Grinfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.; Marikovsky, M. Molecular Mechanisms of enhanced wound healing by copper oxide-impregated dressing. Wound Rep. Reg. 2010, 18, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper an Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Applerot, G.; Abu-Mukh, R.; Irzh, R.; Charmet, J.; Keppner, J.; Laux, L.; Guilbert, G.; Gedanken, A. Decorative parylene coated glass with ZnO nano-particles for antibacterial applications. ACS Appl. Mater. Interfaces 2010, 2, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Applerot, N.; Perkas, N.; Wehrshuetz-Sigl, E.; Hasman, E.G.; Guebitz, G.; Gedanken, A. CuO-cotton nanocomposite: Formation, Morphology and bacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Torres, A.; Ruales, C.; Pulgarin, C.; Aimable, A.; Bowen, P.; Kiwi, J. Enhanced Inactivation of E. coli by RF-plasma Pre-treated Cotton/CuO (65 m2/g) under Visible Light. Appl. Mater. Interfaces 2010, 1, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Kusiak-Nejman, E.; Morawski, A.; Ehiasarian, A.; Baghriche, O.; Pulgarin, C.; Mielczarski, E.; Mielczarski, J.; Kulik, A.; Kiwi, J. E. coli Inactivation by High Power Impulse Magnetron Sputtered (HIPIMS) Cu-Surfaces. J. Phys. Chem. C 2011, 115, 21113–21119. [Google Scholar] [CrossRef]
- Haenle, M.; Fritsche, M.; Zietz, C.; Bader, M.; Heidenau, F. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: In vitro effectiveness against MRSA and mechanical properties. J. Mater. Sci. Mater. Med. 2011, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, C.; Bensimon, M.; Kiwi, J. New Evidence for Cu-decorated binary-oxides mediating the bacterial inactivation/mineralization in aerobic media. Colloids Surf. B Biointerfaces 2016, 144, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I. Epitaxial Growth, Part B; IBM Academic Press: New York, NY, USA, 1975; pp. 382–436. [Google Scholar]
- Ehasarian, P.A. High-power impulse magnetron sputtering and its applications. Pure Appl. Chem. 2010, 82, 1247–1258. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Baghriche, O.; Kiwi, J. Accelerated inactivation obtained by HIPIMS sputtering on low cost surfaces with concomitant reduction in the metal-semiconductor content. RSC Adv. 2013, 3, 13127–13130. [Google Scholar] [CrossRef]
- Mishra, A.; Kelly, J.-P.; Bradley, W.-J. The 2D-plasma potential distribution in a HIPIMS discharge. J. Appl. Phys D Appl. Phys. 2011, 44, 425201. [Google Scholar] [CrossRef]
- Von Keudell, A.; Hecimovic, A.; Maszl, C. Control of High Power Pulsed Magnetron Discharge by Monitoring the Current Voltage Characteristics. Contrib. Plasma Phys. 2016, 56, 918–926. [Google Scholar] [CrossRef]
- Petrov, I.; Myers, J.E.; Green, J.R.; Abelson, J. Mass and energy resolved detection of ions and neutral sputtered species incident at the substrate during reactive magnetron sputtering of Ti in mixed Ar+N2 mixture. J. Vac. Sci. Technol. A 1994, 12, 2846–2851. [Google Scholar] [CrossRef]
- Kousznetsov, V.; Mazak, K.; Schneider, J.; Helmersson, U.; Petrov, I. Ionized sputter-deposition using an extremely high plasma density pulsed magnetron discharge. Surf. Coat. Technol. 1999, 12, 20–295. [Google Scholar]
- Song, Y.; Kwon, Y.; Choi, G.; Lee, W. Photocatalyric activity of Cu/TiO2 with oxidation state of surface loaded copper. Bull. Korean Chem. Soc. 1999, 20, 957–960. [Google Scholar]
- Yu, J.; Ran, J. Facile preparation and enhanced photocatlytic activity H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364–1371. [Google Scholar] [CrossRef]
- Lopez-Ayala, S.; Rincon, E.M. Catalytic and photocatalytic performance of mesoporous CuxO-TiO2. J. Photochem. Photobiol. A 2011, 222, 249–257. [Google Scholar] [CrossRef]
- Rodriguez-Torres, C.; Golmar, F.; Cabrera, A.; Errico, L.; Mudara-Navarro, M.A.; Renteria, M.; Sanchez, F.; Duhalde, S. Magnetic and structural study of Cu-doped TiO2 thin films. Appl. Surf. Sci. 2007, 254, 365–367. [Google Scholar] [CrossRef]
- Audronis, M.; Hinder, S.; Mack, P.; Bellido-Gonzales, V.; Bussey, D.; Mathews, A.; Baker, M. A comparison of reactive plasma pretreatments on PET substrates by Cu and Ti pulsed-DC and HIPIMS discharges. Thin Solid Films 2011, 520, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Ballo, M.; Rtimi, S.; Mancini, S.; Kiwi, J. Bactericidal activity and mechanism of action of copper sputtered flexible surfaces against multidrug-resistant pathogens. Appl. Microbiol. Cell Physiol. 2016, 100, 5945–5953. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Keevil, C.W. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Sands, K.; Vineyard, G.; Platt, R. Surgical site infections occurring after hospital discharge. J. Infect. Dis. 1996, 173, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Gharsa, H.; Dziri, R.; Klibi, N.; Chairat, S.; Lozano, C.; Torres, C.; Bellaaj, R.; Ben Slama, K. Environmental Staphylococcus aureus contamination in a Tunisian hospital. J. Chemother. 2016, 28, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, D.; Krans, T.; Espirito Santo, C.; Elowsky, C.; Domaille, D.; Chang, C.; Grass, G. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Espirito Santo, C.; Lam, E.; Elowsky, C.; Quaranta, D.; Domaille, D.; Chang, C.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shilla, R.; Christian, P.; Elliot, T.S. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Heidenau, F.; Mittelmeir, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G. A novel antibacterial titania coating: Metal ion toxicity in vitro surface colonization. J. Mater. Sci. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, J. Intravascular catheter infections. J. Hosp. Infect. 2010, 73, 323–330. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. What is the predominant source of intravascular catheter infections? Clin. Infect. Dis. 2011, 2, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Ballo, M.; Rtimi, S.; Pulgarin, C.; Hopf, N.; Berthet, A.; Kiwi, J.; Moreillon, P.; Entenza, J.; Bizzini, A. In vitro and in vivo effectiveness of an innovative silver-copper nanoparticle coating of catheters to prevent methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2016, 60, 5349–5356. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Noyce, J.; Michels, H.; Keeevil, C. Potential action of Cu surfaces on meticillin-resistant Staphylococcus aureus. Appl. Microbiol. 2010, 109, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Zietz, C.; Fritsche, A.; Finke, B.; Stranak, V.; Haenle, M.; Hippler, R.; Mittelmeier, W.; Bader, R. Analysis of the release characteristics of Cu-treated antimicrobial implants surface using atomic absorption spectrometry. Bioorg. Chem. Appl. 2012, 2012, 850390. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Teoh, W.; Matrquis, C.; Amal, R. Cytotoxyc Origin of Copper (II)Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate and Medical Salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxycity of Ag, CuO, and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Park, H.; Kim, J.; Kim, H.; Lee, H.; Yoon, J.; Lee, C. Microbial inactivation by Cupric Ion in Combination with H2O2: Role of Reactive Oxidants. Environ. Sci. Technol. 2013, 47, 13661–13667. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Pang, H.; Xu, S.; Lu, Q. Copper based nanostructures: Promising antibacterial agents and photocatalysis. Chem. Commun. 2009, 3571–3573. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A.; Perkas, N.; Perelshtein, I.; Applerot, G.; Lipovsky, A.; Nitzan, Y.; Lubart, R. Chapter 9. Innovative Inorganic Nanoparticles with Antimicrobial Properties Attached to Textiles by Sonochemistry. In Cavitation: A Novel Energy-Efficient Technique for the Generation of Nanomaterials; Manickam, S., Ashokkumar, M., Eds.; Pan Stanford Publishing: Singapore, 2014; pp. 263–300. [Google Scholar]
- Sedighi, A.; Montazer, M.; Samadi, N. Synthesis of nano-Cu2O on cotton: Morphological, physical, biological and optical sensing characteristics. Carbohydr. Polym. 2014, 110, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. The relationship of copper to DNA damage and damage prevention in humans. Mutant. Res. 2012, 733, 83–91. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryck, D.; Yo koyama, T.; Hashimoto, K. Visible light sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10671–10766. [Google Scholar] [CrossRef]
- Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) onto TiO2 and WO3 photocatalysts. Chem. Phys. Lett. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Ishiguro, H.; Yao, Y.; Nakano, R.; Hara, M.; Sunada, K.; Hashimoto, K.; Kajioka, J.; Fujishima, A.; Kubota, Y. Photocatalytic activity of Cu2+/TiO2-coated cordierite foam inactivates bacteriophages and Legionella pneumophila. Appl Catal. B 2013, 129, 56–61. [Google Scholar] [CrossRef]
- Yates, M.H.; Brook, L.A.; Ditta, B.I.; Evans, P.; Foster, H.A.; Sheel, W.D.; Steele, A. Photo-induced self-cleaning and biocidal bahavior of titania and copper oxide multilayers. J. Photochem. Photoboiol. A 2008, 197, 197–205. [Google Scholar] [CrossRef]
- Kelly, J.P.; Li, H.; Benson, S.P.; Whitehead, K.A.; Verran, J.; Arnell, D.R.; Iordanova, I. Comparison of the tribological and antimicrobial propertiesof CrN/Ag, ZrN/Ag, TiN/Ag and TiN/Cu nancomposite coatings. Surf. Coat Technol. 2010, 205, 1606–1610. [Google Scholar] [CrossRef]
- Caballero, L.; Whitehead, A.K.; Allen, S.N.; Verran, J. Inactivation of Esherichia coli on immobilized TiO2 using fluorescent light. J. Photochem. Photobiol. A 2009, 202, 92–98. [Google Scholar] [CrossRef]
- Kelly, P.J.; Barker, M.P.; Ostovarpour, S.; Ratova, M.; West, T.G.; Iordanova, I.; Bradley, W.J. Deposition of photocatalytic titania coatings on polymer substrates by HIPIMS. Vacuum 2012, 86, 1880–1882. [Google Scholar] [CrossRef]
- Leyland, N.; Podporska, A.; Caroll, J.; Browne, J.; Hinder, S.; Quilty, B.; Pillai, C.S. Highly efficient F, Cu-doped TiO2 antibacterial visible light active photocatalytic coatings to combat hospital acquired infections. Nat. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Pillai, S.C.; Carrol, J. Dublin Institute of Technology. A Surface Coating. U.S. Patent 9,210,934, 15 December 2015. [Google Scholar]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Innovative TiO2/Cu nanosurfaces inactivating bacteria in the minute range under low intensity actinic light. ACS Appl. Mater. Interfaces 2012, 4, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.H. Swanson, Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health Part A Toxic. Hazard. Subst. Environ. Eng. 2006, 4, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Effect of the spectral properties of TiO2, Cu, TiO2/Cu sputtered films on the bacterial inactivation under low intensity actinic light. J. Photochem. Photobiol. A 2013, 251, 50–56. [Google Scholar] [CrossRef]
- Kiwi, J.; Morrison, C. Heterogeneous photocatalysis. Dynamics of charge transfer in lithium-doped anatase-based catalyst powders with enhanced water photocleavage under ultraviolet irradiation. J. Phys. Chem. 1984, 88, 6146–6152. [Google Scholar] [CrossRef]
- De Romanha, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of copper in light of new insights of copper homeostasis. J. Trace Elements Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Ballo, M.; Laub, D.; Pulgarin, C.; Entenza, J.; Bizzini, A.; Kiwi, J. Duality in the Eschericia coli and methicillin resistant Staphylococcus aureus reduction mechanism under actinic light on innovative co-sputtered surfaces. Appl. Catal. A 2015, 498, 185–191. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Ding, M.; Shomodara, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid Cux/TiO2 nanocomposites as Risk-Reduction Materials in Indoor Environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Grey, B.; Steck, R. Concentrations of Copper Thought to be Toxic to Esherichia coli Can Induce the Viable but Nonculturable Condition. Appl. Environ. Microbiol. 2001, 67, 5325–5327. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Nadtochenko, V.; Lavanchy, J.-C.; Kiwi, J. Preparation and Mechanism of Cu-decorated TiO2-ZrO2 Films Showing Accelerated Bacterial Inactivation. ACS Appl. Mater. Interfaces 2015, 7, 12832–12839. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Accelerated self-cleaning by Cu-promoted binary-oxides under sunlight. Appl. Catal. B 2016, 180, 648–655. [Google Scholar] [CrossRef]
- Neubert, S.; Mitoraj, D.; Shevlin, S.; Pulisova, P.; Heimann, M.; Du, Y.; Gregory, C.; Goh, K.; Pacia, M.; Kruczał, K.; et al. Highly efficient rutile TiO2 photocatalyst with single Cu(II) and Fe(III) surface catalytic sites. J. Mater. Chem. A 2016, 4, 3127–3133. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikanda, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocaatlytic disinfection of bacteria under visible light. J. Colloid Interfaces Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.; Green, S.; Michels, H.; Keevil, C. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 2010, 76, 5390–5401. [Google Scholar] [CrossRef] [PubMed]
- Eshed, M.; Lellouche, J.; Matalon, S.; Gedanken, A.; Banin, M. Sonochemical coatings of ZnO and CuO nanoparticles inhibit streptococcus mutans biofilm formation on teeth model. Langmuir 2012, 28, 12288–12295. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Liu, W. The roles of the surface-doped metal-ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B 2014, 156–157, 466–475. [Google Scholar] [CrossRef]
- Lemire, A.; Harrison, J.; Turner, R. Antimicrobial activity of metals: Mechanism, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Lee, B. Disinfection of Sthphylococcus aureus in indoor aerosols using Cu-TiO2 deposited on glassfibers under visible light irradiation. J. Photochem. Photobiol. A 2015, 307–308, 16–22. [Google Scholar] [CrossRef]
- Liu, L.; Giu, X.; Sun, C.; Li, H.; Deng, Y.; Gao, F.; Dong, L. In situ loading of ultrasmall Cu2O particles on TiO2 nanosheets to enhance the visible light photoactivity. Nanoscale 2012, 4, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Musil, J. Flexible hard nanocomposite coatings. RSC Adv. 2015, 5, 60482–60495. [Google Scholar] [CrossRef]
- Musil, J.; Blazek, J.; Fajfrlik, K.; Cerstvy, R. Flexible antibacterial Al-Cu-N films. Surf. Coat. Technol. 2015, 264, 114–120. [Google Scholar] [CrossRef]
- Musil, J.; Karel, M.; Fajfrlik, K.; Cerstvy, R.J. Flexible antibacterial Zr-Cu-N thin films resistant to cracking. Vac. Sci. Technol. A 2015, 34. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rtimi, S.; Pulgarin, C.; Kiwi, J. Recent Developments in Accelerated Antibacterial Inactivation on 2D Cu-Titania Surfaces under Indoor Visible Light. Coatings 2017, 7, 20. https://doi.org/10.3390/coatings7020020
Rtimi S, Pulgarin C, Kiwi J. Recent Developments in Accelerated Antibacterial Inactivation on 2D Cu-Titania Surfaces under Indoor Visible Light. Coatings. 2017; 7(2):20. https://doi.org/10.3390/coatings7020020
Chicago/Turabian StyleRtimi, Sami, Cesar Pulgarin, and John Kiwi. 2017. "Recent Developments in Accelerated Antibacterial Inactivation on 2D Cu-Titania Surfaces under Indoor Visible Light" Coatings 7, no. 2: 20. https://doi.org/10.3390/coatings7020020






