Effects of Rare Earth Elements on Properties of Ni-Base Superalloy Powders and Coatings
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation and Property Test of Powders
2.2. Preparation and Property Test of Coatings
3. Results and Discussion
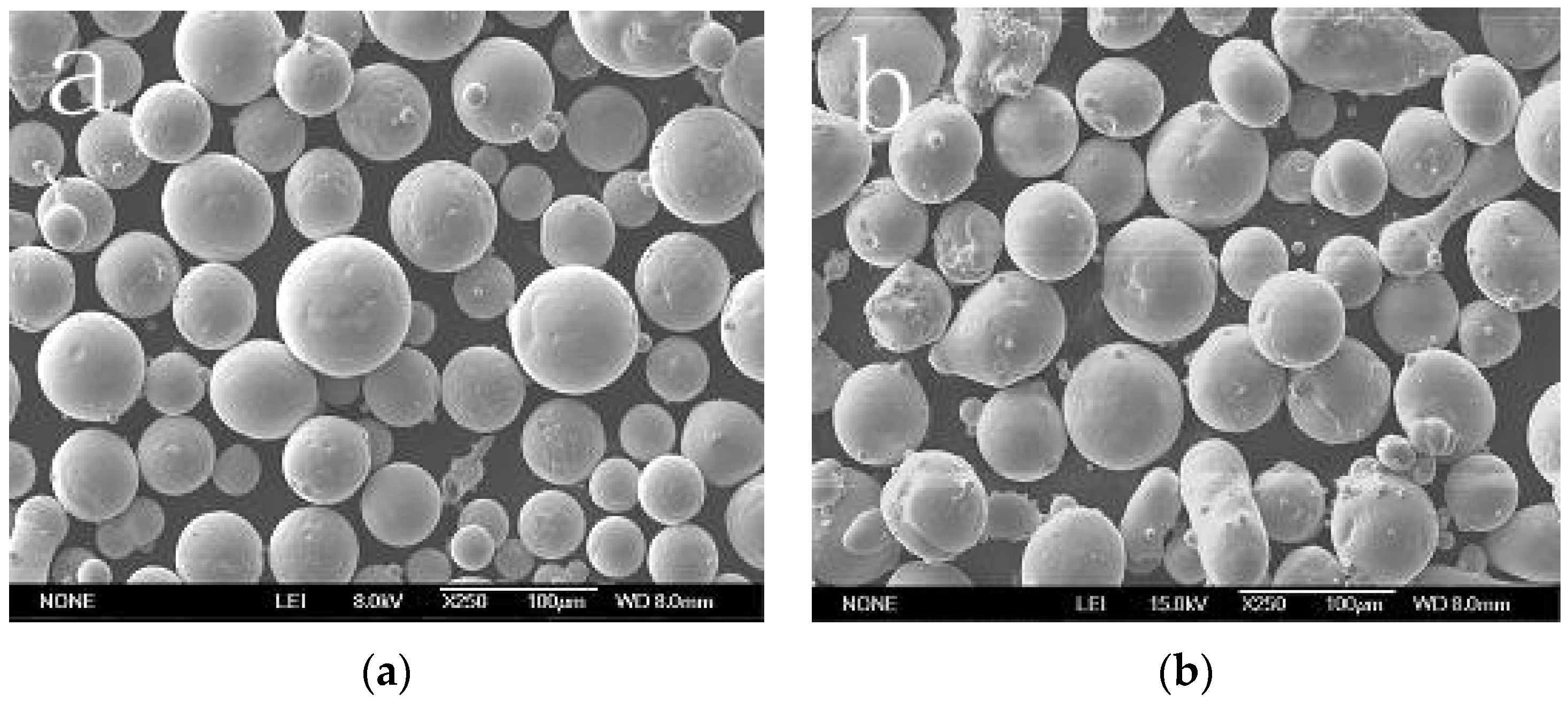
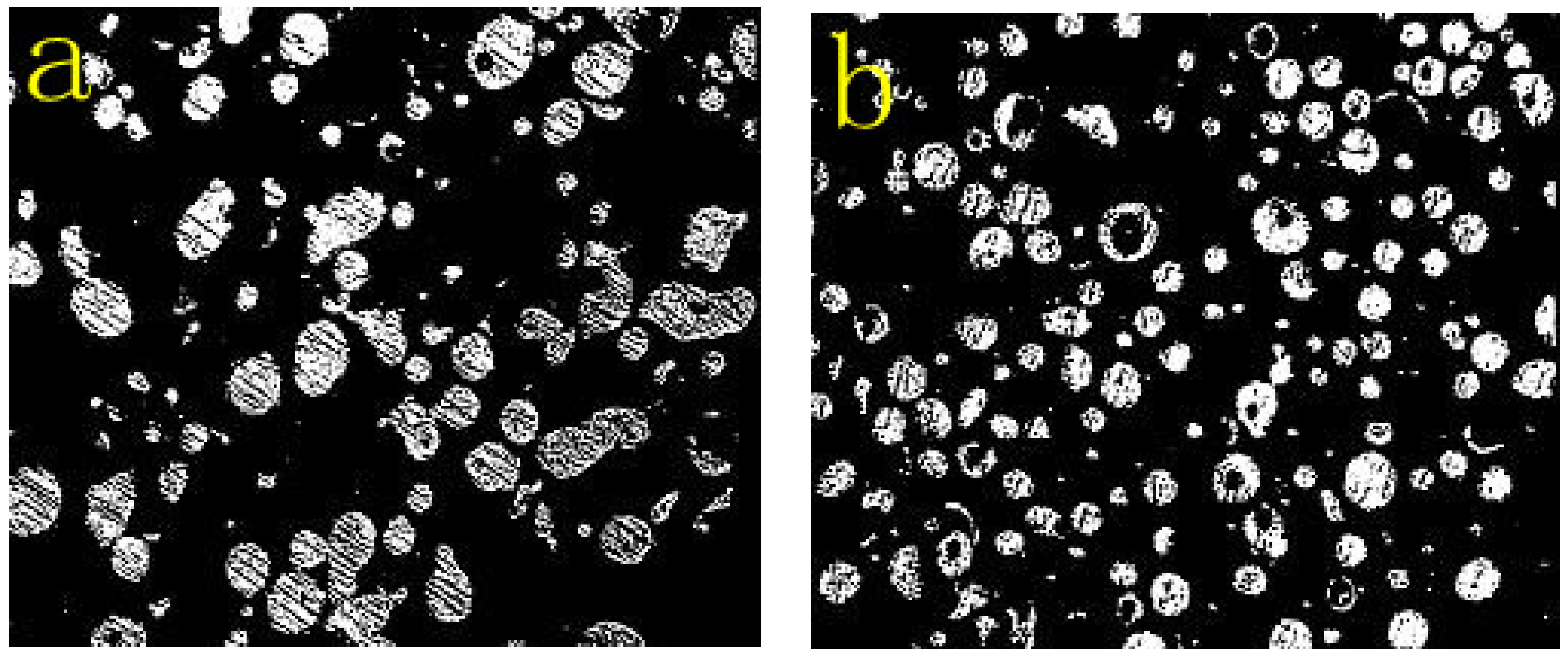
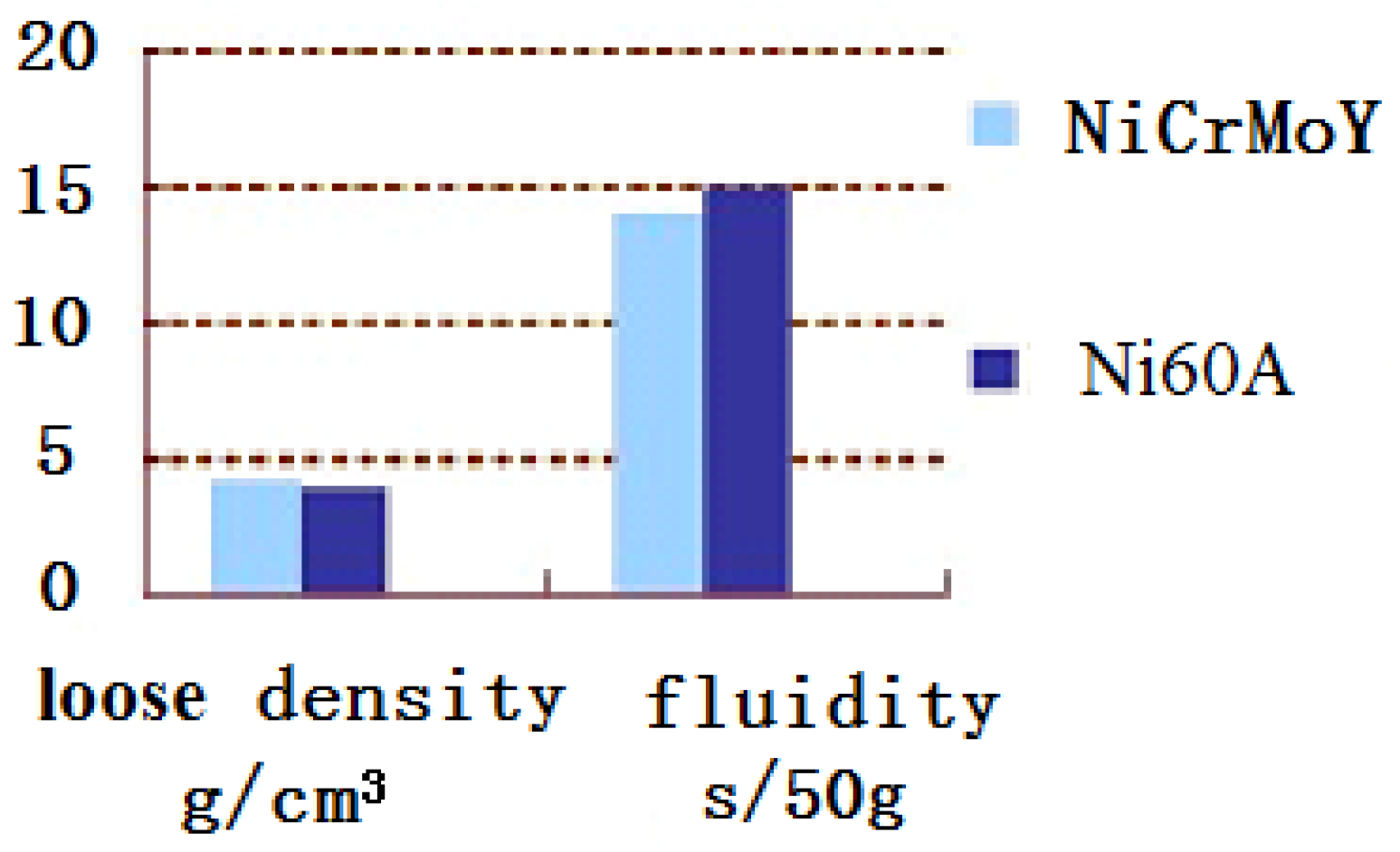
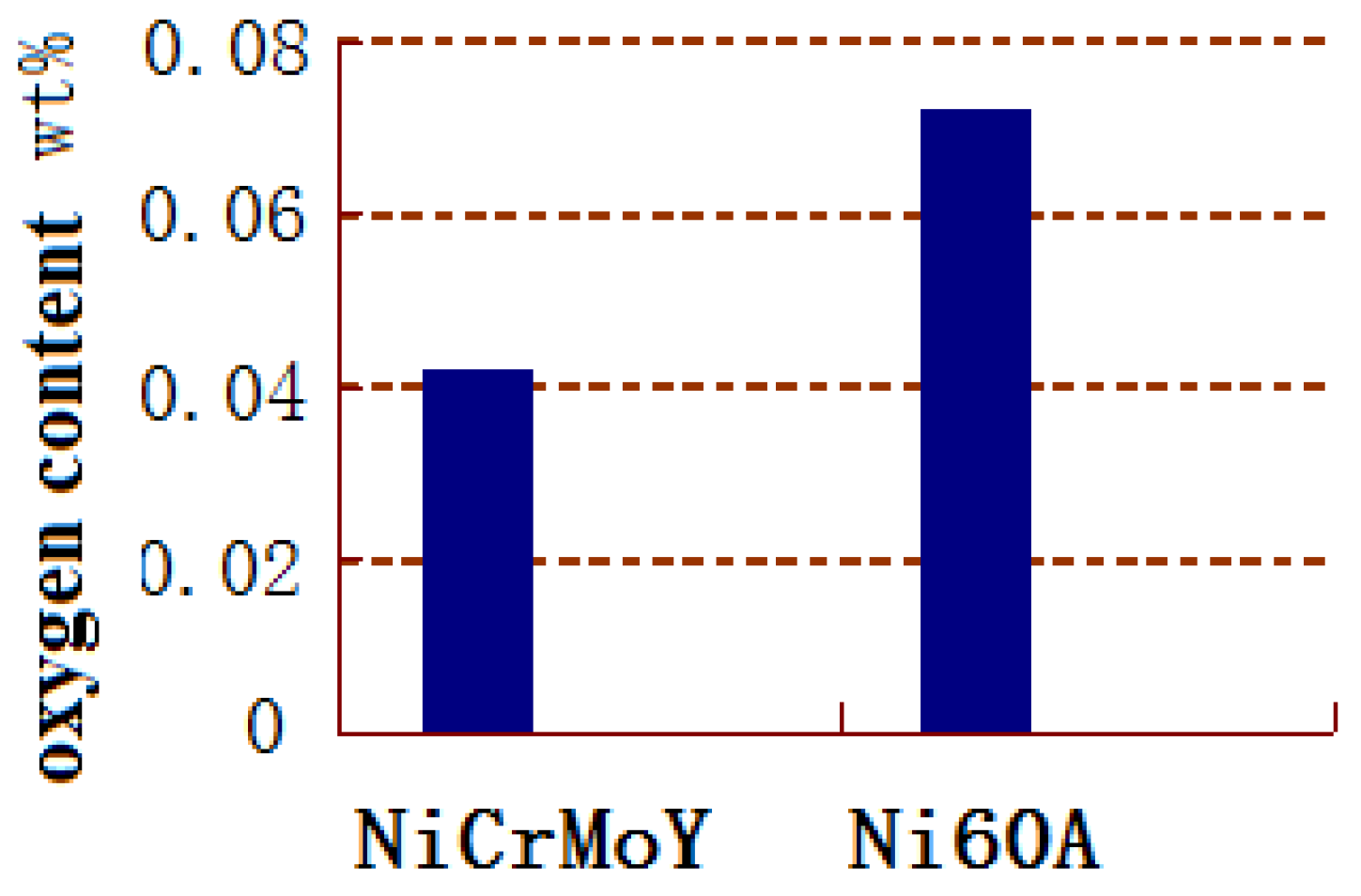
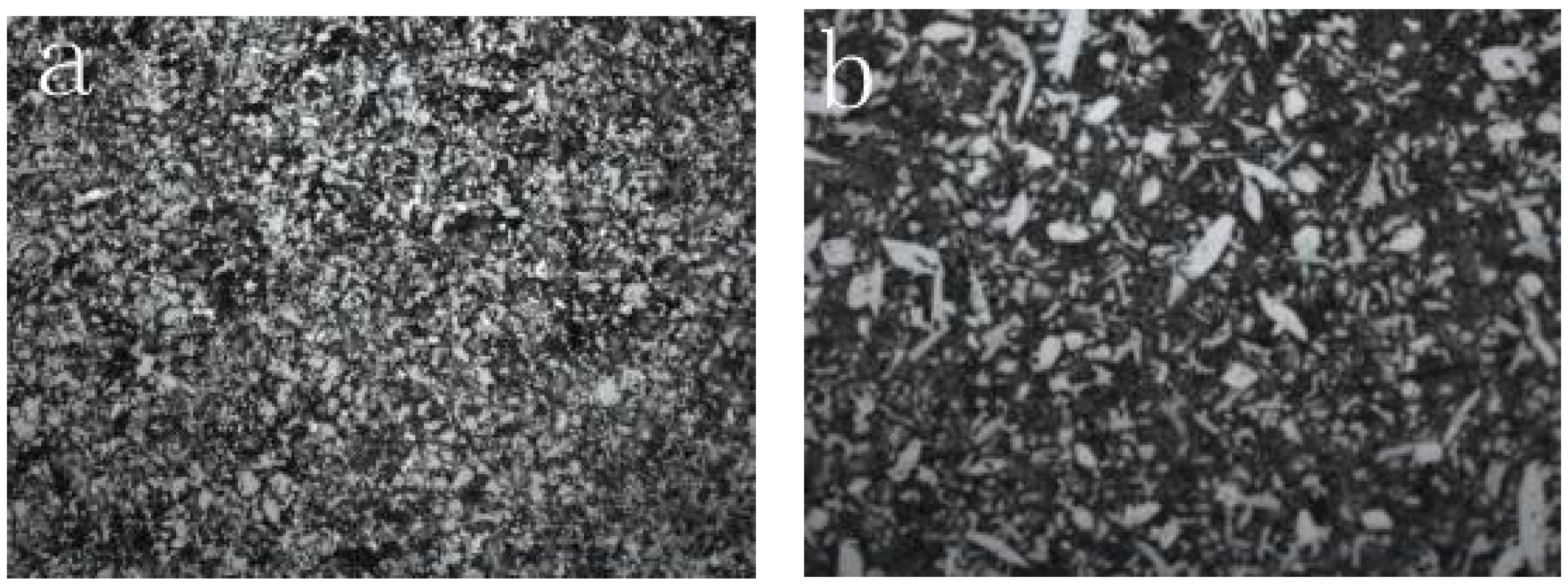
3.1. Properties of Powders
3.2. Properties of Coatings
4. Conclusions
- Due to the incorporation of the rare earth elements Mo, Nb, or Y, the majority of the NiCrMoY alloy particles exhibit better sphericity, smoother surface, fewer joint structures, larger apparent density, and lower flowability than those of particles without incorporation.
- The particle-size distribution of NiCrMoY alloy powders shows a single peak and fits a normal distribution with a median particle diameter of 68.3 µm, an apparent density of 4.300 g/cm3, and a flowability of 14.07 s.
- The microstructure of the NiCrMoY alloy coatings exhibits a finer structure and better hardness by the incorporation of appropriate amounts of Mo and Nb, and small amount of Y elements.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsai, Y.L.; Wang, S.F.; Bor, H.Y.; Hsu, Y.F. Effects of Zr addition on the microstructureand mechanical behavior of a fine-grained nickel-based superalloy at elevated temperatures. Mater. Sci. Eng. A 2014, 607, 294–301. [Google Scholar] [CrossRef]
- Scrivani, A.; Bardi, U.; Carrafiello, L.; Lavacchi, A.; Niccolai, F.; Rizzi, G. A comparative study of high velocity oxygen fuel, vacuum plasma spray, and axial plasma spray for the deposition of CoNiCrAlY bond coat alloy. J. Therm. Spray Technol. 2003, 12, 504–507. [Google Scholar] [CrossRef]
- Safai, S.; Herman, H. Microstructural investigation of plasma-sprayed aluminum coatings. Thin Solid Films 1977, 45, 295–307. [Google Scholar] [CrossRef]
- Christensen, R.J.; Tolpygo, V.K.; Clarke, D.R. The influence of the reactive element yttriumon the stress in alumina scales formed by oxidation. Acta Mater. 1997, 45, 1761–1766. [Google Scholar] [CrossRef]
- Paul, A.; Elmrabet, S.; Odriozola, J.A. Low cost rare earth elements deposition method for enhancing the oxidation resistance at high temperature of Cr2O3 and Al2O3 forming alloys. J. Alloys Compd. 2001, 323, 70–73. [Google Scholar] [CrossRef]
- Stringer, J.; Wallwork, G.R.; Wilcox, B.A.; Hed, A.Z. Effect of a thoria dispersion on high-temperature oxidation of chromium. Corros. Sci. 1972, 12, 625–636. [Google Scholar] [CrossRef]
- Antill, J.; Peakall, K. Influence of an alloy addition of yttrium on the oxidation behavior of an austenitic and a ferritic SS in carbon dioxide. J. Iron Steel Inst. 1967, 205, 1136–1142. [Google Scholar]
- Tien, J.K.; Pettit, F.S. Mechanism of oxide adherence on Fe–25Cr–4Al(Y or Sc) alloys. Metall. Trans. 1972, 3, 1587–1599. [Google Scholar] [CrossRef]
- Thanneeru, R.; Patil, S.; Deshpande, S. Effect of trivalent rare earth dopants in nanocrystalline ceria coatings for high-temperature oxidation resistance. Acta Mater. 2007, 55, 3457–3466. [Google Scholar] [CrossRef]
- Xiu, S.; Lei, W.; Yang, L. Effects of temperature and rare earth content on oxidation resistance of Ni-based superalloy. Prog. Nat. Sci. Mater. Int. 2011, 21, 227–235. [Google Scholar]
- He, H.; Liu, Z.; Wang, W.; Zhou, C. Microstructure and hot corrosion behavior of Co–Si modified aluminide coating on nickel based superalloys. Corros. Sci. 2015, 100, 466–473. [Google Scholar] [CrossRef]
- Elsaß, M.; Frommherz, M.; Scholz, A.; Oechsner, M. Interdiffusion in MCrAlY coated nickel-base superalloys. Surf. Coat. Technol. 2016, 307, 565–573. [Google Scholar] [CrossRef]
- Tan, J.; Looney, L.; Hashmi, M. Component repair using HVOF thermal spraying. J. Mater. Process. Technol. 1999, 92, 203–208. [Google Scholar] [CrossRef]
- Hu, C.; Hou, S. Failure analysis of plungers sprayed by Ni-based alloy on hydraulic feedback subsurface pump. J. Chin. Soc. Corros. Prot. 2012, 32, 80–84. [Google Scholar]
- Dong, G.; Yan, B.; Deng, Q.; Yu, T. Effect of niobium on the microstructure and wear resistance of nickel-based alloy coating by laser cladding. Rare Metal Mater. Eng. 2011, 40, 973–977. [Google Scholar]
- Tang, Y.; Yang, J. Influence of rare earth on wear ability of spray welding layer. Hot Work. Technol. 2001, 1, 32–33. [Google Scholar]
- Lu, Z.; Tian, Y.; Zhu, C. Study on Ni60AA alloy powder coating by high frequency induction heating thermal. Spray Technol. 2012, 4, 44–46. [Google Scholar]
- ASTM E1019-11 Standard Test Methods for Determination of Carbon, Sulfur, Nitrogen, and Oxygen in Steel, Iron, Nickel, and Cobalt Alloys by Various Combustion and Fusion Techniques; ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM E2594-09 Standard Test Method for Analysis of Nickel Alloys by Inductively Coupled Plasma Atomic Emission Spectrometry (Performance-Based Method); ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM E354-14 Standard Test Methods for Chemical Analysis of High-Temperature, Electrical, Magnetic, and Other Similar Iron, Nickel, and Cobalt Alloys; ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 4938 Steel and Iron-Determination of Nickel Content-Gravimetric or Titrimetric Method; International Organization for Standardization: Geneva, Switzerland, 1988.
- Wang, Y.; Shen, D.; Liao, B. Effect of rare earth on Ni-based spontaneous melting alloy by laser cladding. Appl. Laser 2003, 3, 139–141. [Google Scholar]
- Zhang, C. Research on RE Micro-Alloy Effect in Self-Fusion Alloy. Master’s Thesis, Shenyang University of Technology, Shenyang, Liaoning, China, 2005. [Google Scholar]
- Liang, B.; Zhang, Z. Development and application in Ni-based powders containing rare earth elements. J. Lanzhou Polytech. Coll. 2007, 1, 28–30. [Google Scholar]
- Ma, Y.; Huang, B.; Fan, J. Effect of rare earth Y on preparation of nanometer W-Ni-Fe composite powder. Rare Metal Mater. Eng. 2005, 34, 1135–1138. [Google Scholar]
- Yuan, H.; Li, Z.; Xu, W. The study of Argon atomized superalloy powders. Powder Metall. Ind. 2010, 4, 1–5. [Google Scholar]

| Alloy | Chemical Compositions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | B | Si | Cr | Fe | Mo | Cu | Nb | Y | Ni | |
| Ni60A | 0.98 | 2.91 | 3.96 | 16.4 | 3.2 | – | – | – | – | Bal. |
| NiCrMoY | 1.0 | 2.85 | 4.0 | 16.5 | 3.0 | 2.5 | 1.5 | 0.5 | 0.15 | Bal. |
| Parameters | Gas | |
|---|---|---|
| O2 | C2H2 | |
| Pressure (MPa) | 1.2 | 0.1 |
| Flow rate (m3/h) | 1.6–1.8 | 1.2–1.5 |
| Spray rate (kg/h) | 7.0 | |
| Spray distance (mm) | 150–200 | |
| Thickness (mm) | 250 | |
| Remelting voltage (V) | 550 | |
| Remelting current (A) | 280 | |
| Coatings | HRC | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Average | |
| Ni60A | 60.5 | 60.5 | 61.0 | 60.0 | 60.5 | 60.5 |
| NiCrMoY | 62.0 | 62.5 | 63.0 | 62.5 | 63.0 | 62.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Hou, S. Effects of Rare Earth Elements on Properties of Ni-Base Superalloy Powders and Coatings. Coatings 2017, 7, 30. https://doi.org/10.3390/coatings7020030
Hu C, Hou S. Effects of Rare Earth Elements on Properties of Ni-Base Superalloy Powders and Coatings. Coatings. 2017; 7(2):30. https://doi.org/10.3390/coatings7020030
Chicago/Turabian StyleHu, Chunlian, and Shanglin Hou. 2017. "Effects of Rare Earth Elements on Properties of Ni-Base Superalloy Powders and Coatings" Coatings 7, no. 2: 30. https://doi.org/10.3390/coatings7020030





