SnS Thin Film Solar Cells: Perspectives and Limitations
Abstract
:1. Introduction
2. Materials and Deposition Methods
2.1. SnS Deposition
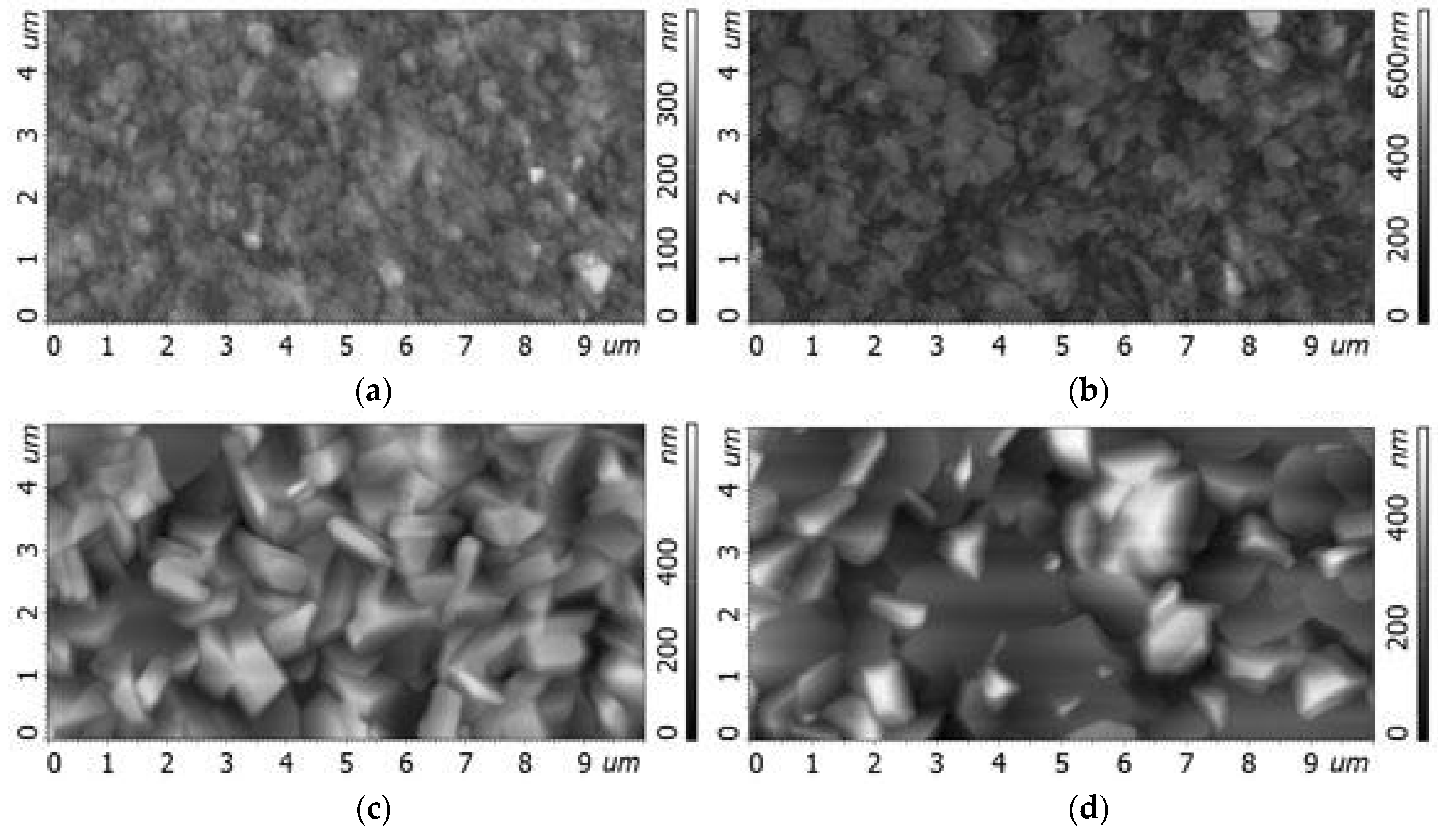
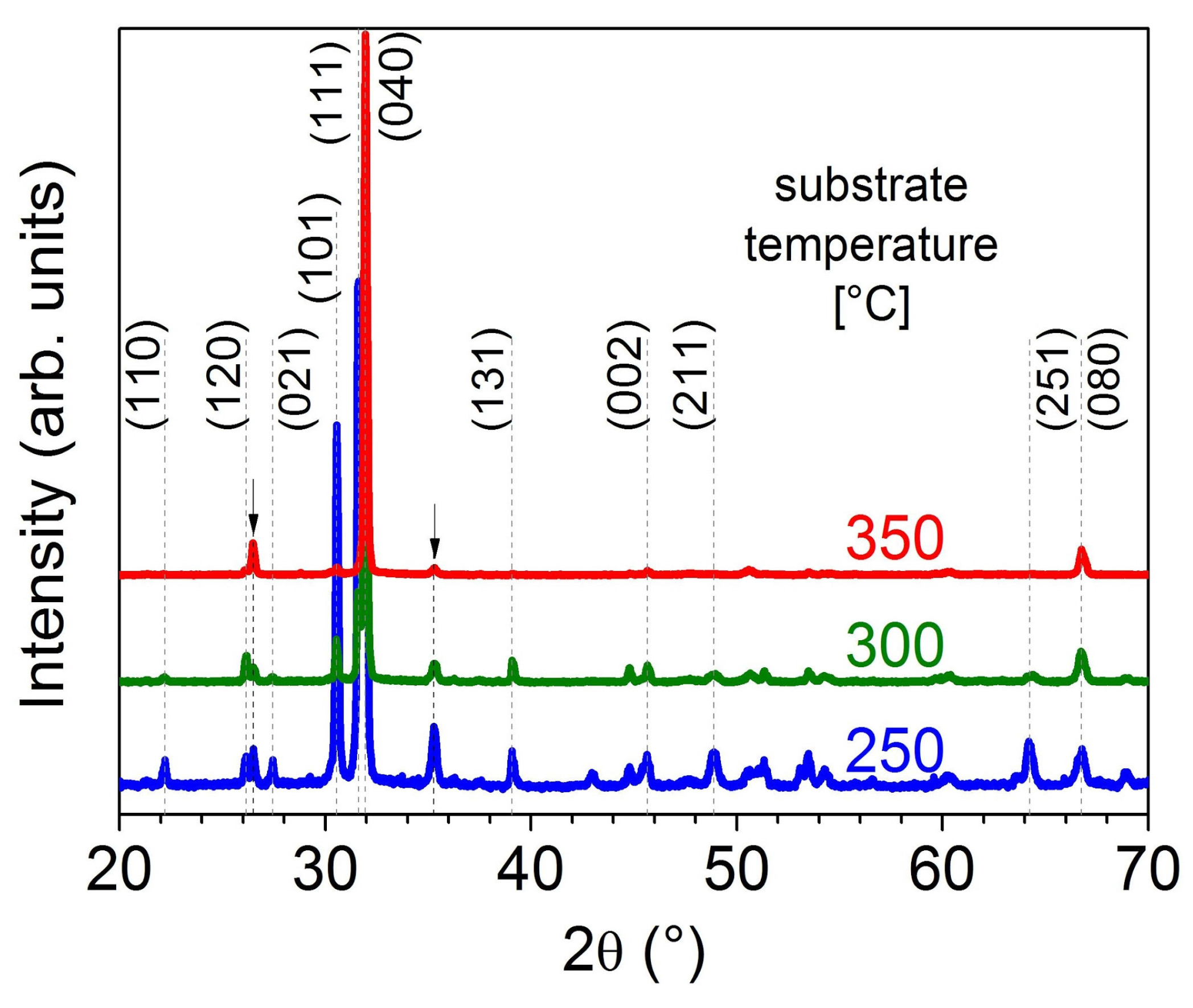
2.2. Substrate Temperature
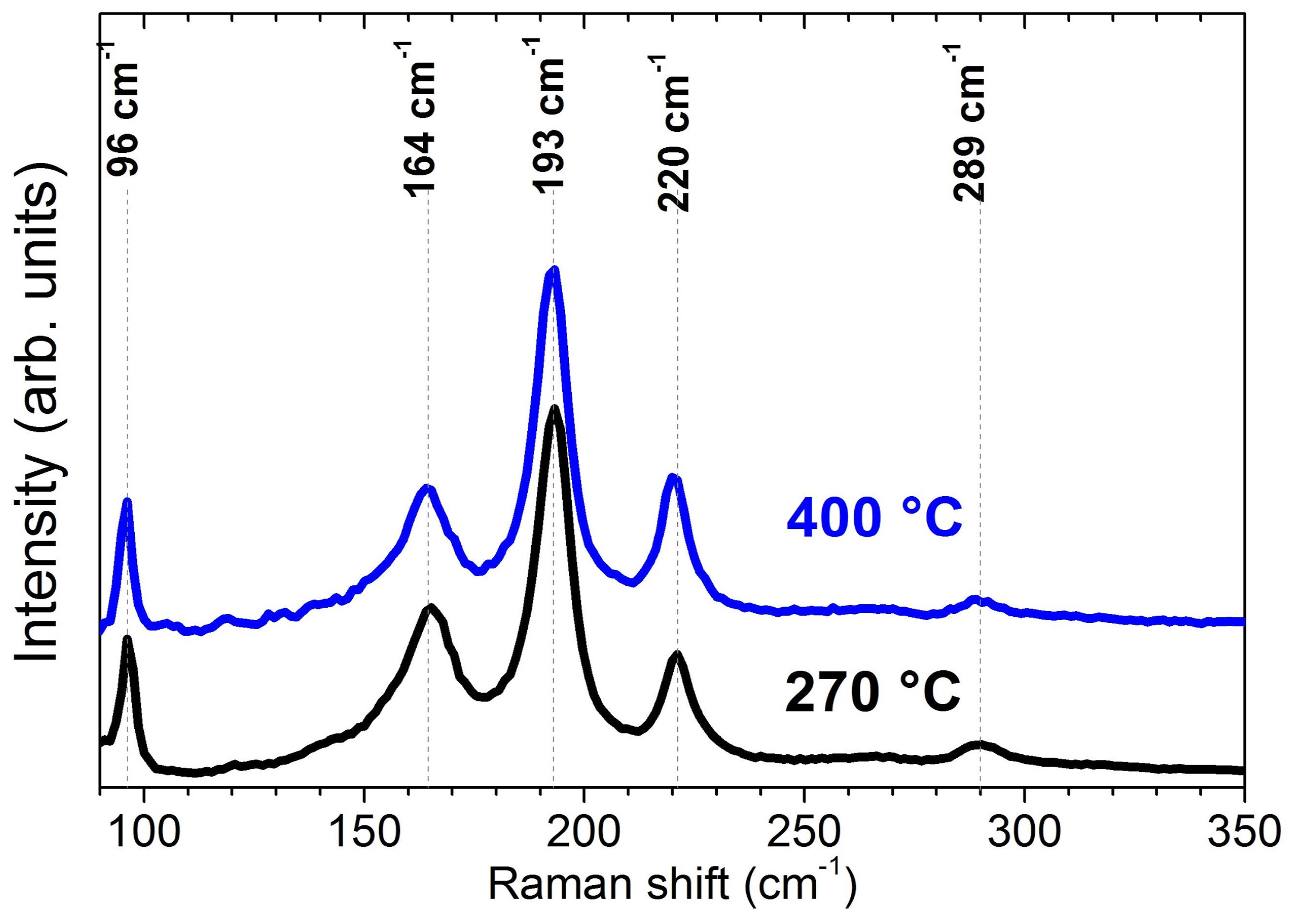
2.3. Evaporation of SnS: Reproducibility
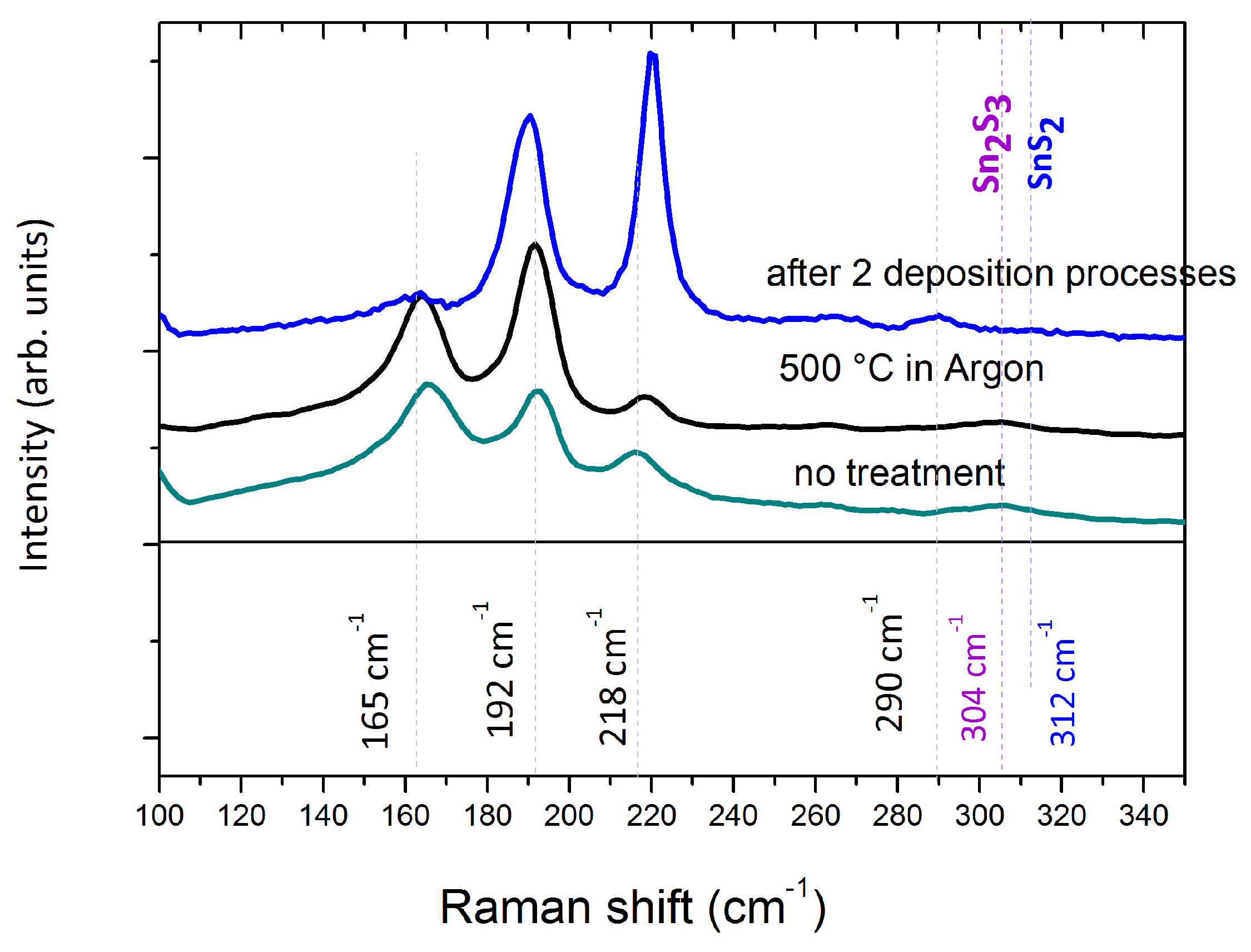
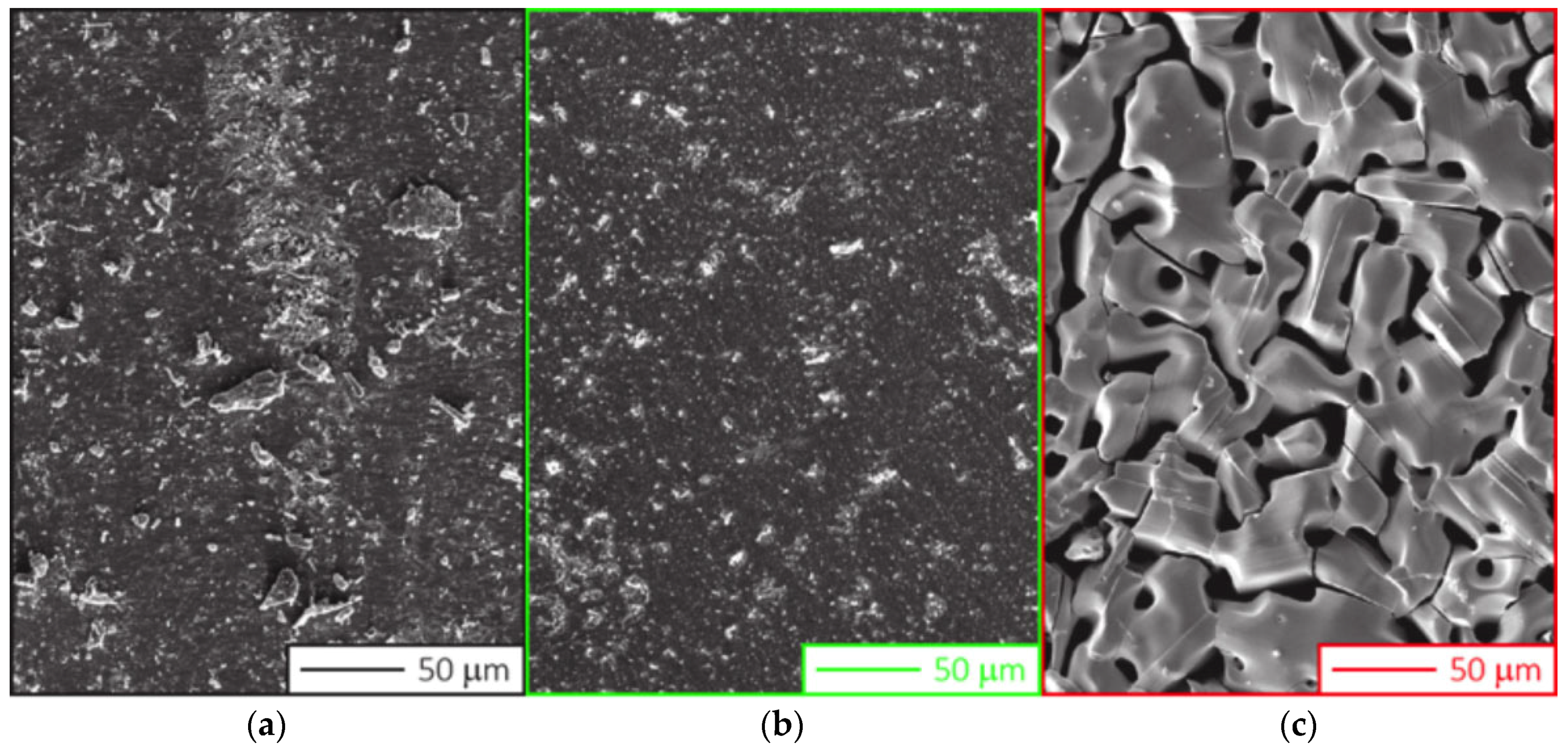
- SnS lumps annealing in vacuum in the Knudsen cell at 550 °C for 30 min; and
- SnS lumps annealing in a quartz tube furnace in controlled atmosphere–Ar at a pressure of 500 mbar at 500 °C for 60 min. A similar thermal treatment of the raw material has been proposed by Steinmann et al. [9] to clean the SnS powders from impurity phases.
- TRM SnS as deposited;
- TRM SnS after 60 min annealing in furnace at 500 °C;
- TRM SnS after two heating/cooling cycles, i.e., two deposition processes.
Efficiency
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schneikart, A.; Schimper, H.-J.; Klein, A.; Jaegermann, W. Efficiency limitations of thermally evaporated thin-film SnS solar cells. J. Phys. D Appl. Phys. 2013, 46, 305109. [Google Scholar] [CrossRef]
- Katagiri, H.; Jimbo, K.; Maw, W.S.; Oishi, K.; Yamazaki, M.; Araki, H. Development of CZTS-based thin film solar cells. Thin Solid Films 2009, 517, 2455–2460. [Google Scholar] [CrossRef]
- Rampino, S.; Armani, N.; Bissoli, F.; Bronzoni, M.; Calestani, D.; Calicchio, M. 15% efficient Cu(In,Ga)Se2 solar cells obtained by low-temperature pulsed electron deposition. Appl. Phys. Lett. 2012, 101, 132107. [Google Scholar] [CrossRef]
- Todorov, T.K.; Reuter, K.B.; Mitzi, D.B. High-efficiency solar cell with earth-abundant liquid-processed absorber. Adv. Mater. 2010, 22, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Barkhouse, D.A.R.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Mitzi, D.B. Device characteristics of a 10.1% hydrazine-processed Cu2ZnSn(Se,S)4 solar cell. Prog. Photovolt. Res. Appl. 2012, 20, 6–11. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Marchionna, S.; Garattini, P.; Le Donne, A.; Acciarri, M.; Tombolato, S.; Binetti, S. Cu2ZnSnS4 solar cells grown by sulphurisation of sputtered metal precursors. Thin Solid Films 2013, 542, 114–118. [Google Scholar] [CrossRef]
- Kawano, Y.; Chantana, J.; Minemoto, T. Impact of growth temperature on the properties of SnS film prepared by thermal evaporation and its photovoltaic performance. Curr. Appl. Phys. 2015, 15, 897–901. [Google Scholar] [CrossRef]
- Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Brandt, R.E.; Poindexter, J.R. 3.88% Efficient tin sulfide solar cells using congruent thermal evaporation. Adv. Mater. 2014, 26, 7488–7492. [Google Scholar] [CrossRef] [PubMed]
- Sinsermsuksakul, P.; Sun, L.; Lee, S.W.; Park, H.H.; Kim, S.B.; Yang, C. Overcoming efficiency limitations of SnS-based solar cells. Adv. Energy Mater. 2014, 4, 1400496. [Google Scholar] [CrossRef]
- Herzenberg, R. Kolbeckina, un nuevo mineral de estaño—Buenos Aires. Rev. Miner. 1932, 4, 33–35. [Google Scholar]
- Ehm, L.; Knorr, K.; Dera, P.; Krimmel, A.; Bouvier, P.; Mezouar, M. Pressure-induced structural phase transition in the IV–VI semiconductor SnS. J. Phys. Condens. Matter. 2004, 16, 3545–3554. [Google Scholar] [CrossRef]
- Albers, W.; Haas, C.; van der Maesen, F. The preparation and the electrical and optical properties of SnS crystals. J. Phys. Chem. Solids 1960, 15, 306–310. [Google Scholar] [CrossRef]
- Vidal, J.; Lany, S.; D’Avezac, M.; Zunger, A.; Zakutayev, A.; Francis, J. Band-structure, optical properties, and defect physics of the photovoltaic semiconductor SnS. Appl. Phys. Lett. 2012, 100, 032104. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Conibeer, G. Physical properties of very thin SnS films deposited by thermal evaporation. Thin Solid Films 2011, 520, 837–841. [Google Scholar] [CrossRef]
- Devika, M.; Reddy, N.K.; Ramesh, K.; Ganesam, V.; Gopal, E.S.R.; Ramakrishna, K.T.R. Influence of substrate temperature on surface structure and electrical resistivity of the evaporated tin sulphide films. Appl. Surf. Sci. 2006, 253, 1673–1676. [Google Scholar] [CrossRef]
- Devika, M.; Reddy, N.K.; Ramesh, K.; Gopal, E.S.R. Influence of annealing on physical properties of evaporated SnS films. Semicond. Sci. Technol. 2006, 21, 1125. [Google Scholar] [CrossRef]
- Devika, M.; Reddy, N.K.; Prashantha, M.; Ramesh, K.; Reddy, S.V.; Hahn, Y.B. The physical properties of SnS films grown on lattice-matched and amorphous substrates. Phys. Status Solidi 2010, 207, 1864–1869. [Google Scholar] [CrossRef]
- Hasan, B.A. Dieletectric Properties of Vacuum Evaporated SnS Thin Films. Int. J. Nanotechnol. Adv. Mater. 2013, 1, 87–93. [Google Scholar]
- Cheng, S.; Zhong, N.; Huang, C.; Chen, G. SnS films prepared by sulfuration of Sn precursor layers. Adv. Mater. Devices Sens. Imaging II 2005, 5633, 303–307. [Google Scholar]
- Hartman, K.; Johnson, J.L.; Bertoni, M.I.; Recht, D.; Aziz, M.J.; Scarpulla, M.A. SnS thin-films by RF sputtering at room temperature. Thin Solid Films 2011, 519, 7421–7424. [Google Scholar] [CrossRef]
- Banai, R.E.; Lee, H.; Motyka, M.A.; Chandrasekharan, R.; Podraza, N.J.; Brownson, J.R.S. Optical properties of sputtered SnS thin films for photovoltaic absorbers. IEEE J. Photovolt. 2013, 3, 1084–1089. [Google Scholar] [CrossRef]
- Jiang, F.; Shen, H.; Gao, C.; Liu, B.; Lin, L.; Shen, Z. Preparation and properties of SnS film grown by two-stage process. Appl. Surf. Sci. 2011, 257, 4901–4905. [Google Scholar] [CrossRef]
- Sousa, M.G.; Da Cunha, A.F.; Fernandes, P.A. Annealing of RF-magnetron sputtered SnS2 precursors as a new route for single phase SnS thin films. J. Alloys Compd. 2014, 592, 80–85. [Google Scholar] [CrossRef]
- Reddy, K.T.R.; Reddy, P.P.; Datta, P.K.; Miles, R.W. Formation of polycrystalline SnS layers by a two-step process. Thin Solid Films 2002, 403–404, 116–119. [Google Scholar] [CrossRef]
- Tanuševski, A.; Poelman, D. Optical and photoconductive properties of SnS thin films prepared by electron beam evaporation. Sol. Energy Mater. Sol. Cells 2003, 80, 297–303. [Google Scholar] [CrossRef]
- Nozaki, H.; Onoda, M.; Sekita, M.; Kosuda, K.; Wada, T. Variation of lattice dimensions in epitaxial SnS films on MgO (001). J. Solid State Chem. 2005, 178, 245–252. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H.; Sun, L. Preparation and properties of zinc blende and orthorhombic SnS films by chemical bath deposition. Appl. Surf. Sci. 2011, 257, 6750–6755. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H. Influence of the deposition parameters on the properties of orthorhombic SnS films by chemical bath deposition. Thin Solid Films 2012, 520, 3523–3527. [Google Scholar] [CrossRef]
- Gode, F.; Guneri, E.; Baglayan, O. Effect of tri-sodium citrate concentration on structural, optical and electrical properties of chemically deposited tin sulfide films. Appl. Surf. Sci. 2014, 318, 227–233. [Google Scholar] [CrossRef]
- Jayasree, Y.; Chalapathi, U.; Bhaskar, U.P.; Raja, V.S. Effect of precursor concentration and bath temperature on the growth of chemical bath deposited tin sulphide thin films. Appl. Surf. Sci. 2012, 258, 2732–2740. [Google Scholar] [CrossRef]
- Yue, G.H.; Peng, D.L.; Yan, P.X.; Wang, L.S.; Wang, W.; Luo, X.H. Structure and optical properties of SnS thin film prepared by pulse electrodeposition. J. Alloys Compd. 2009, 468, 254–257. [Google Scholar] [CrossRef]
- Sato, N.; Ichimura, M.; Arai, E.; Yamazaki, Y. Characterization of electrical properties and photosensitivity of SnS thin films prepared by the electrochemical deposition method. Sol. Energy Mater. Sol. Cells 2005, 85, 153–165. [Google Scholar] [CrossRef]
- Ghosh, B.; Das, M.; Banerjee, P.; Das, S. Fabrication of vacuum-evaporated SnS/CdS heterojunction for PV applications. Sol. Energy Mater. Sol. Cells 2008, 92, 1099–1104. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H.; Sun, L.; Huang, H.; Lu, L.; Cai, H. Preparation of SnS films with zinc blende structure by successive ionic layer adsorption and reaction method. Mater. Lett. 2010, 64, 2177–2179. [Google Scholar] [CrossRef]
- Reddy, K.T.R.; Reddy, P.P.; Miles, R.W.; Datta, P.K. Investigations on SnS films deposited by spray pyrolysis. Opt. Mater. 2001, 17, 295–298. [Google Scholar] [CrossRef]
- Sajeesh, T.H.; Warrier, A.R.; Kartha, C.S.; Vijayakumar, K.P. Optimization of parameters of chemical spray pyrolysis technique to get n and p-type layers of SnS. Thin Solid Films 2010, 518, 4370–4374. [Google Scholar] [CrossRef]
- Messaoudi, M.; Aida, M.S.; Attaf, N.; Bezzi, T.; Bougdira, J.; Medjahdi, G. Deposition of tin(II) sulfide thin films by ultrasonic spray pyrolysis: Evidence of sulfur exo-diffusion. Mater. Sci. Semicond. Process. 2014, 17, 38–42. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Heo, J.; Noh, W.; Hock, A.S.; Gordon, R.G. Atomic Layer Deposition of Tin Monosulfide Thin Films. Adv. Energy Mater. 2011, 1, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; George, S.M. Tin Monosulfide Thin Films Grown by Atomic Layer Deposition Using Tin 2, 4-Pentanedionate and Hydrogen Sulfide. J. Phys. Chem. 2010, 114, 17597–17603. [Google Scholar] [CrossRef]
- Jiang, T.; Ozin, G.A. New directions in tin sulfide materials chemistry. J. Mater. Chem. 1998, 8, 1099–1108. [Google Scholar] [CrossRef]
- Ristov, M.; Grozdanov, I.; Mitreski, M. Chemical deposition of TIN (II) sulphide films. Thin Solid Films 1989, 173, 53–58. [Google Scholar] [CrossRef]
- Sánchez-Juárez, A.; Tiburcio-Silver, A.; Ortiz, A. Fabrication of SnS2/SnS heterojunction thin film diodes by plasma-enhanced chemical vapor deposition. Thin Solid Films 2005, 480–481, 452–456. [Google Scholar] [CrossRef]
- Reddy, K.T.R.; Reddy, N.K.; Miles, R.W. Photovoltaic properties of SnS based solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 3041–3046. [Google Scholar] [CrossRef]
- Ghosh, B.; Das, M.; Banerjee, P.; Das, S. Fabrication and optical properties of SnS thin films by SILAR method. Appl. Surf. Sci. 2008, 254, 6436–6440. [Google Scholar] [CrossRef]
- Malaquias, J.; Fernandes, P.A.; Salomé, P.M.P.; Da Cunha, A.F. Assessment of the potential of tin sulphide thin films prepared by sulphurization of metallic precursors as cell absorbers. Thin Solid Films 2011, 519, 7416–7420. [Google Scholar] [CrossRef]
- Bashkirov, S.A.; Gremenok, V.F.; Ivanov, V.A.; Lazenka, V.V.; Bente, K. Tin sulfide thin films and Mo/p-SnS/n-CdS/ZnO heterojunctions for photovoltaic applications. Thin Solid Films 2012, 520, 5807–5810. [Google Scholar] [CrossRef]
- Steichen, M.; Djemour, R.; Gu, L.; Siebentritt, S.; Dale, P.J. Direct Synthesis of Single-Phase p-Type SnS by Electrodeposition from a Dicyanamide Ionic Liquid at High Temperature for Thin Film Solar Cells. J. Phys. Chem. C 2013, 117, 4383–4393. [Google Scholar] [CrossRef]
- Ikuno, T.; Suzuki, R.; Kitazumi, K.; Takahashi, N.; Kato, N.; Higuchi, K. SnS thin film solar cells with Zn1−xMgxO buffer layers. Appl. Phys. Lett. 2013, 102, 193901. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Hartman, K.; Kim, S.B.; Heo, J.; Sun, L.; Park, H.H. Enhancing the efficiency of SnS solar cells via band-offset engineering with a zinc oxysulfide buffer layer. Appl. Phys. Lett. 2013, 102, 53901. [Google Scholar] [CrossRef] [Green Version]
- Park, H.H.; Heasley, R.; Sun, L.; Steinmann, V.; Jaramillo, R.; Hartman, K. Co-optimization of SnS absorber and Zn(O,S) buffer materials for improved solar cells. Prog. Photovolt. Res. Appl. 2015, 23, 901–908. [Google Scholar] [CrossRef]
- Jaramillo, R.; Steinmann, V.; Yang, C.; Hartman, K.; Chakraborty, R.; Poindexter, J.R. Making Record-efficiency SnS Solar Cells by Thermal Evaporation and Atomic Layer Deposition. J. Vis. Exp. 2015, 99, 52705. [Google Scholar] [CrossRef] [PubMed]
- Schneikart, A. Herstellung und Charakterisierung von SnS-Dünnschichtsolarzellen. Ph.D. Thesis, Technische Universitat Darmstadt, Darmstadt, Germany, 2014. [Google Scholar]
- Noguchi, H.; Setiyadi, A.; Tanamura, H.; Nagatomo, T.; Omoto, O. Characterization of vacuum-evaporated tin sulfide film for solar cell materials. Sol. Energy Mater. Sol. Cells 1994, 35, 325–331. [Google Scholar] [CrossRef]
- Chandrasekhar, H.R.; Humphreys, R.G.; Zwick, U.; Cardona, M. Infrared and Raman spectra of the IV-VI compounds SnS and SnSe. Phys. Rev. B 1977, 15, 2177–2183. [Google Scholar] [CrossRef]
- Reddy, V.R.M.; Gedi, S.; Park, C.; Miles, R.W.; Reddy, K.T.R. Development of sulphurized SnS thin film solar cells. Curr. Appl. Phys. 2015, 15, 588–598. [Google Scholar] [CrossRef]
- Mare, S.D.; Salavei, A.; Piccinelli, F.; Romeo, A. Analysis of SnS Growth and Post Deposition Treatment by Congruent Physical Vapor Deposition. In Proceedings of 31st European Photovoltaic Solar Energy Conference and Exhibition—EU PVSEC 2015, Hamburg, Germany, 14–18 September 2015; pp. 1349–1352.

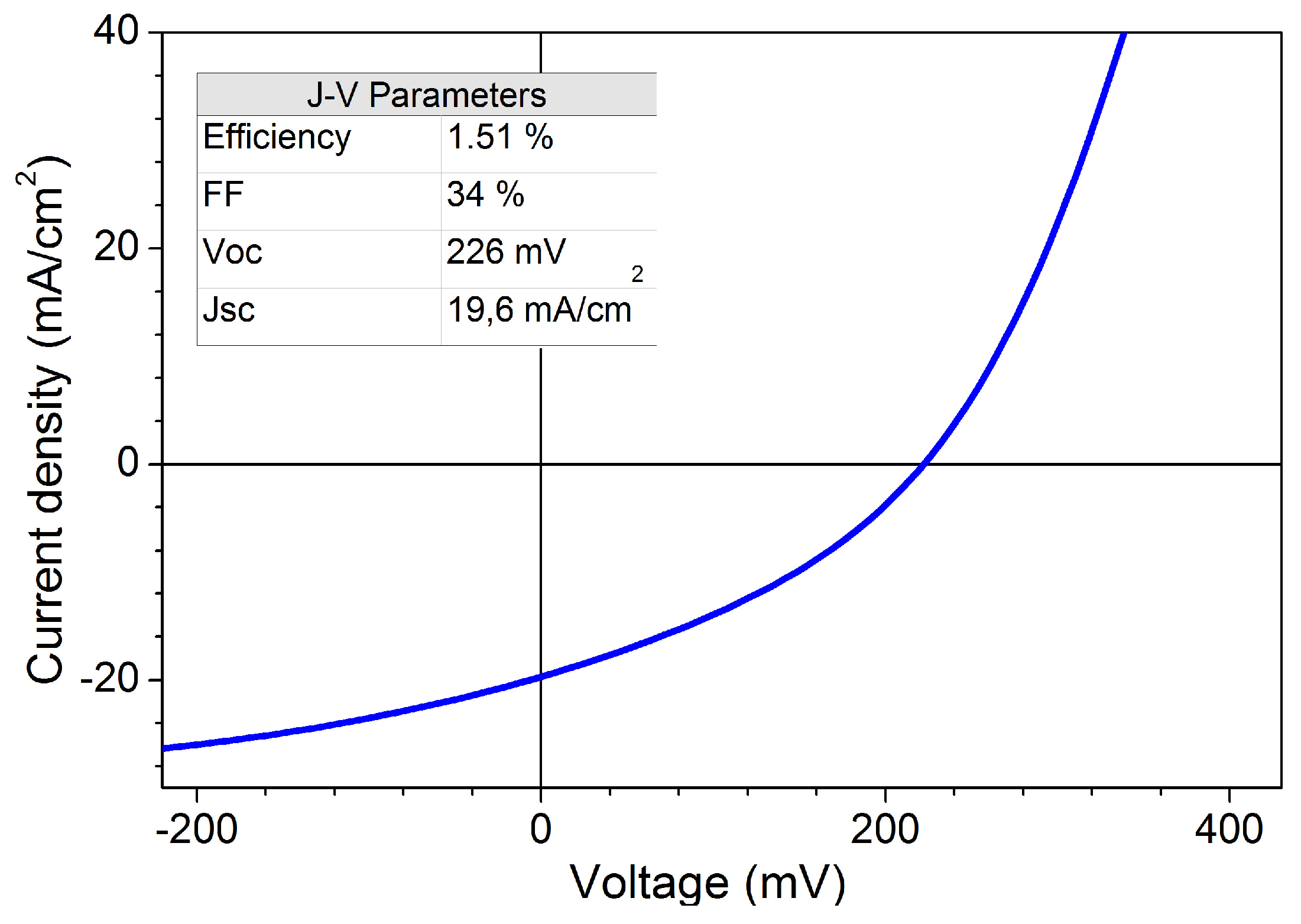

| SnS Deposition | Buffer Layer | Efficiency | Reference |
|---|---|---|---|
| Spray pyrolysis | CdS:In | 1.3% | [44] |
| Thermal Evaporation | CdS | 1.6% | [1] |
| Thermal Evaporation | CdS | 2.5% | [8] |
| RF-sputtering | Zn1−xMgxO | 2.1% | [49] |
| Pulsed-CVD | Zn(O,S) | 2.9% | [51] |
| VE | Zn(O,S) | 3.88% | [9] |
| ALD | Zn(O,S) | 4.36% | [10] |
| Material Treatment | S/Sn Ratio |
|---|---|
| Before treament from TRM | 1.09 |
| 60 m @ 500 °C, 500 mbar | 1.08 |
| After 2nd deposition | 1.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mare, S.; Menossi, D.; Salavei, A.; Artegiani, E.; Piccinelli, F.; Kumar, A.; Mariotto, G.; Romeo, A. SnS Thin Film Solar Cells: Perspectives and Limitations. Coatings 2017, 7, 34. https://doi.org/10.3390/coatings7020034
Di Mare S, Menossi D, Salavei A, Artegiani E, Piccinelli F, Kumar A, Mariotto G, Romeo A. SnS Thin Film Solar Cells: Perspectives and Limitations. Coatings. 2017; 7(2):34. https://doi.org/10.3390/coatings7020034
Chicago/Turabian StyleDi Mare, Simone, Daniele Menossi, Andrei Salavei, Elisa Artegiani, Fabio Piccinelli, Arun Kumar, Gino Mariotto, and Alessandro Romeo. 2017. "SnS Thin Film Solar Cells: Perspectives and Limitations" Coatings 7, no. 2: 34. https://doi.org/10.3390/coatings7020034







