Development of a Fabrication Process Using Suspension Plasma Spray for Titanium Oxide Photovoltaic Device
Abstract
:1. Introduction
2. Experimental Procedure
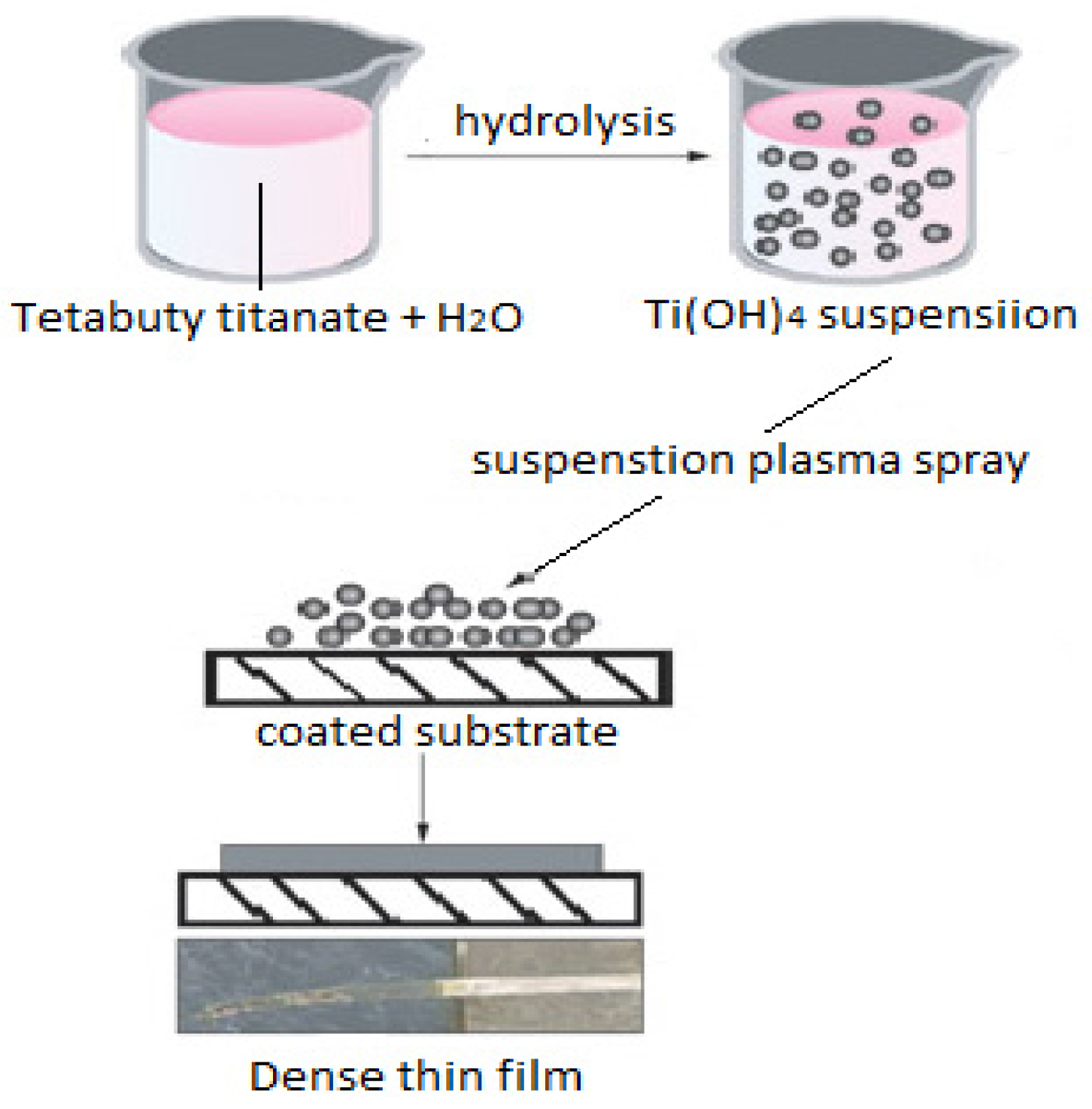
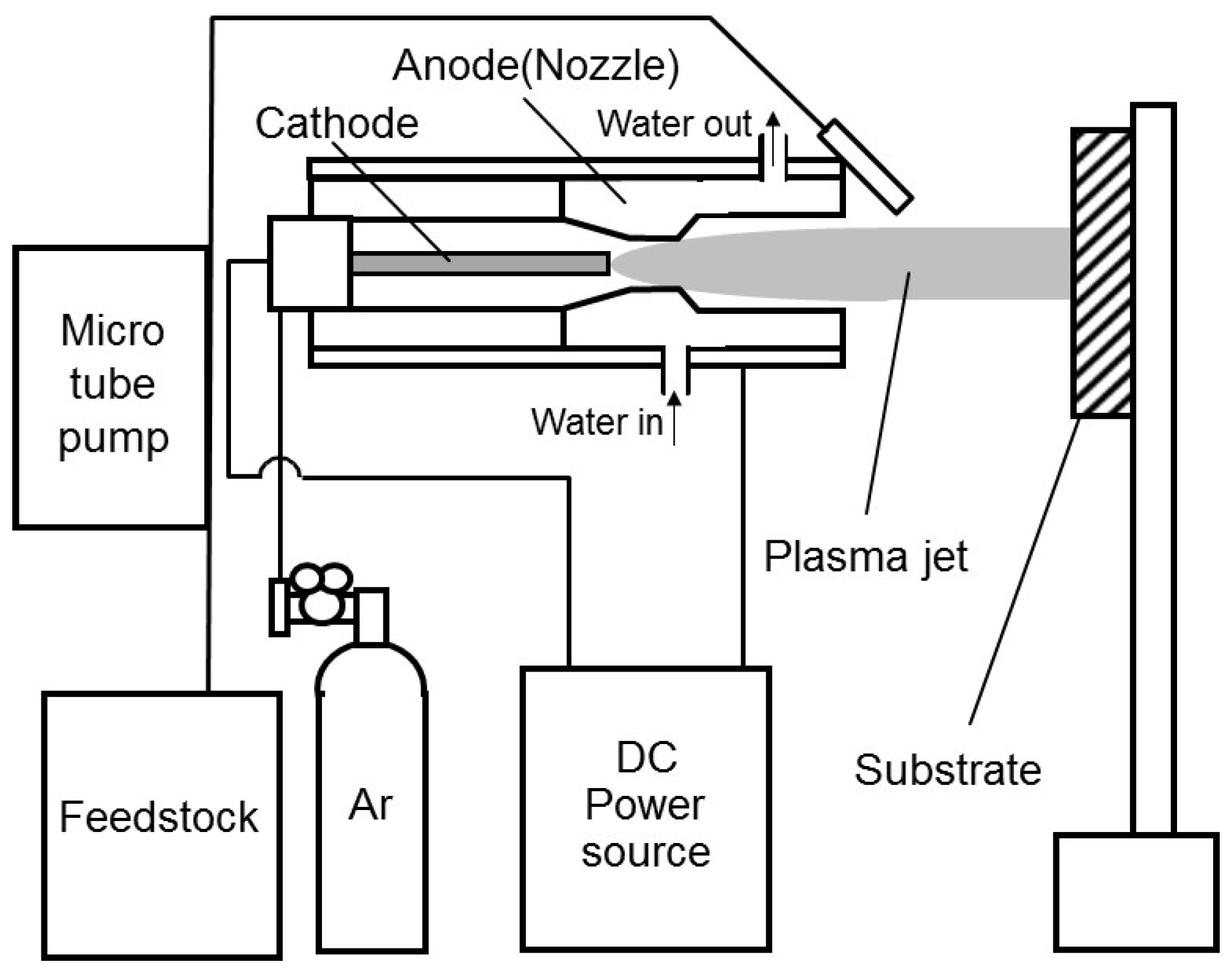
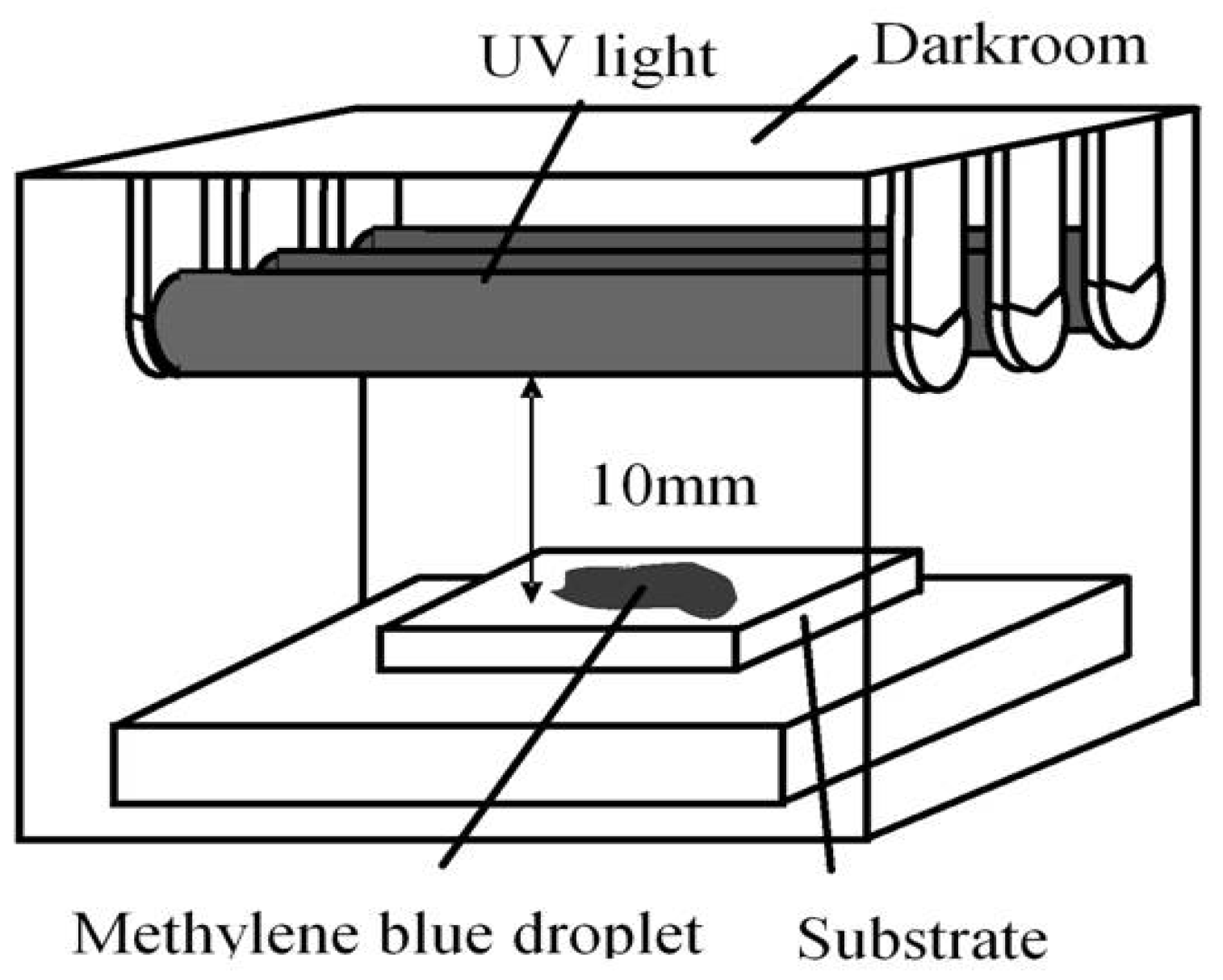
2.1. Method
2.2. Plasma Spray Parameters
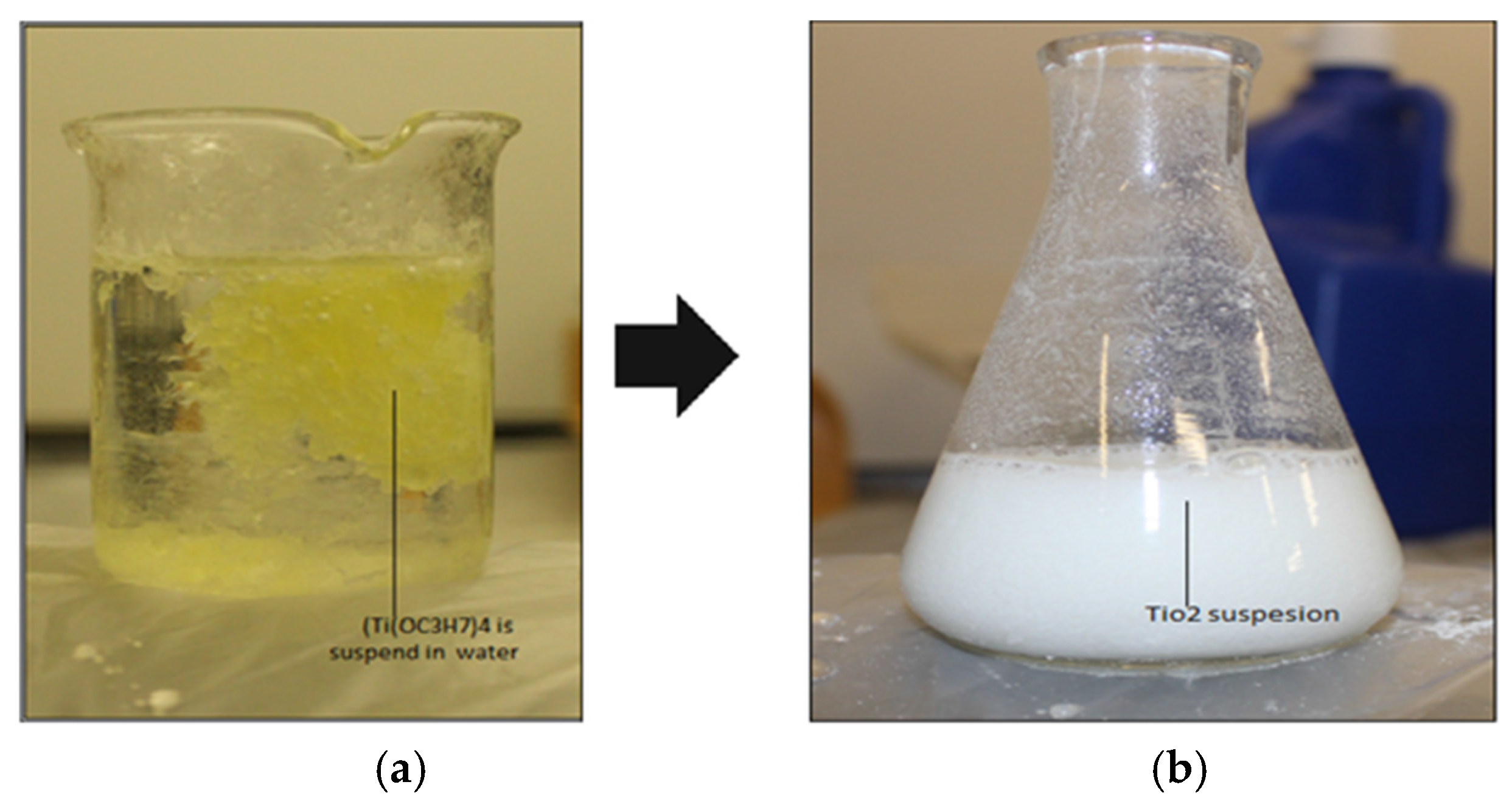
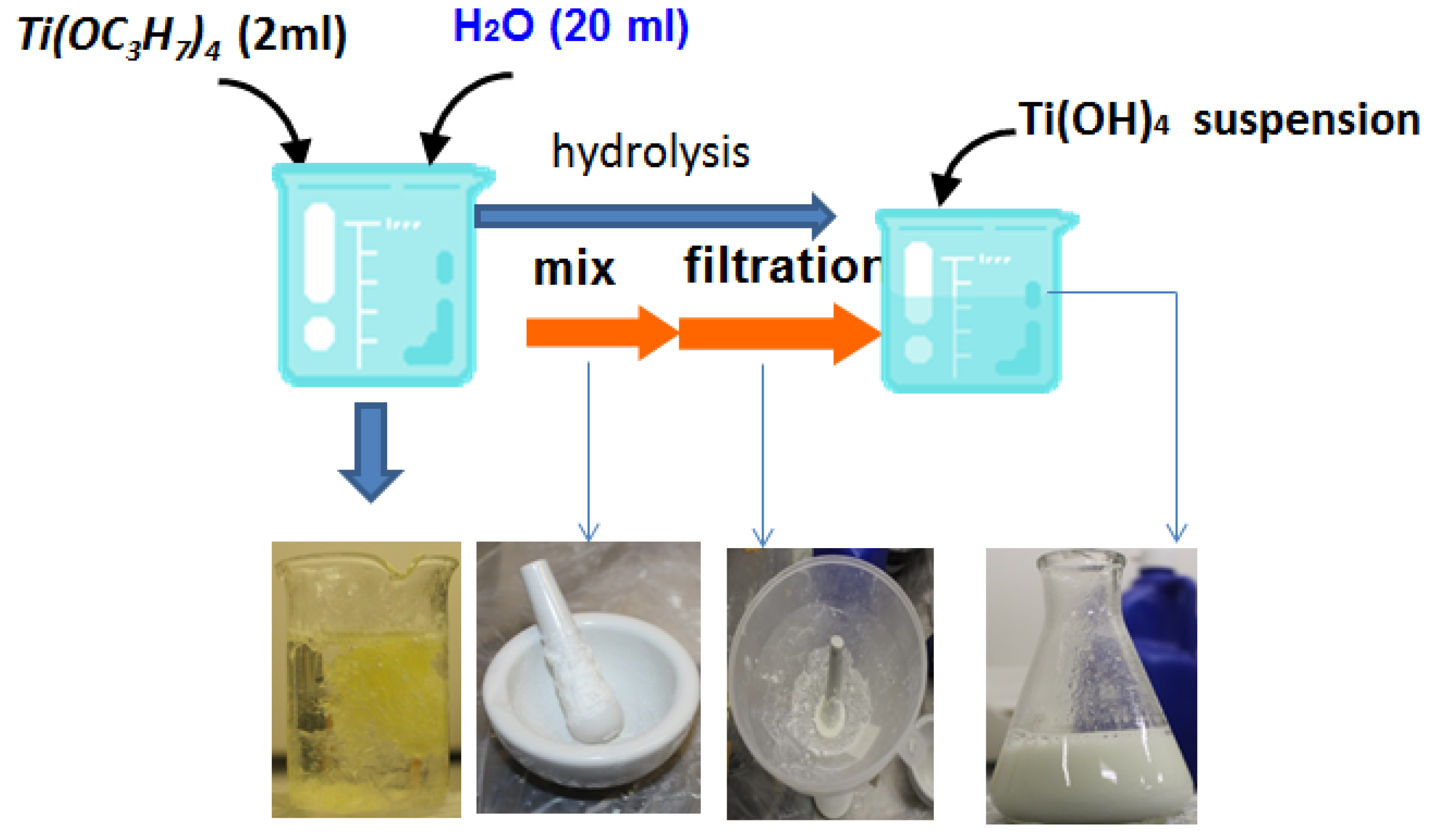
2.3. Suspension Preparation
Ti(OH)4 → TiO2 + 2H2O
2.4. Injection Method
3. Results and Discussion
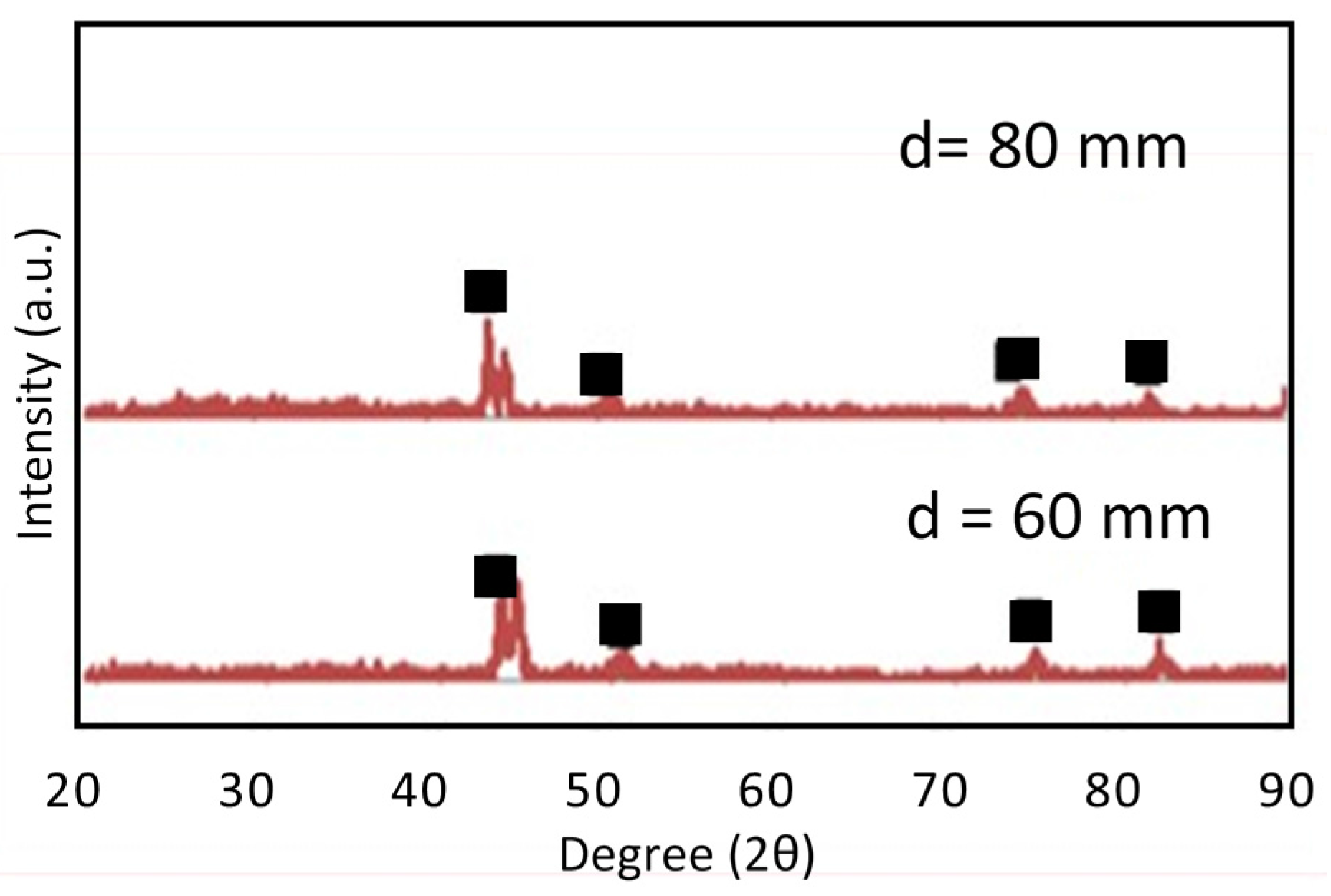
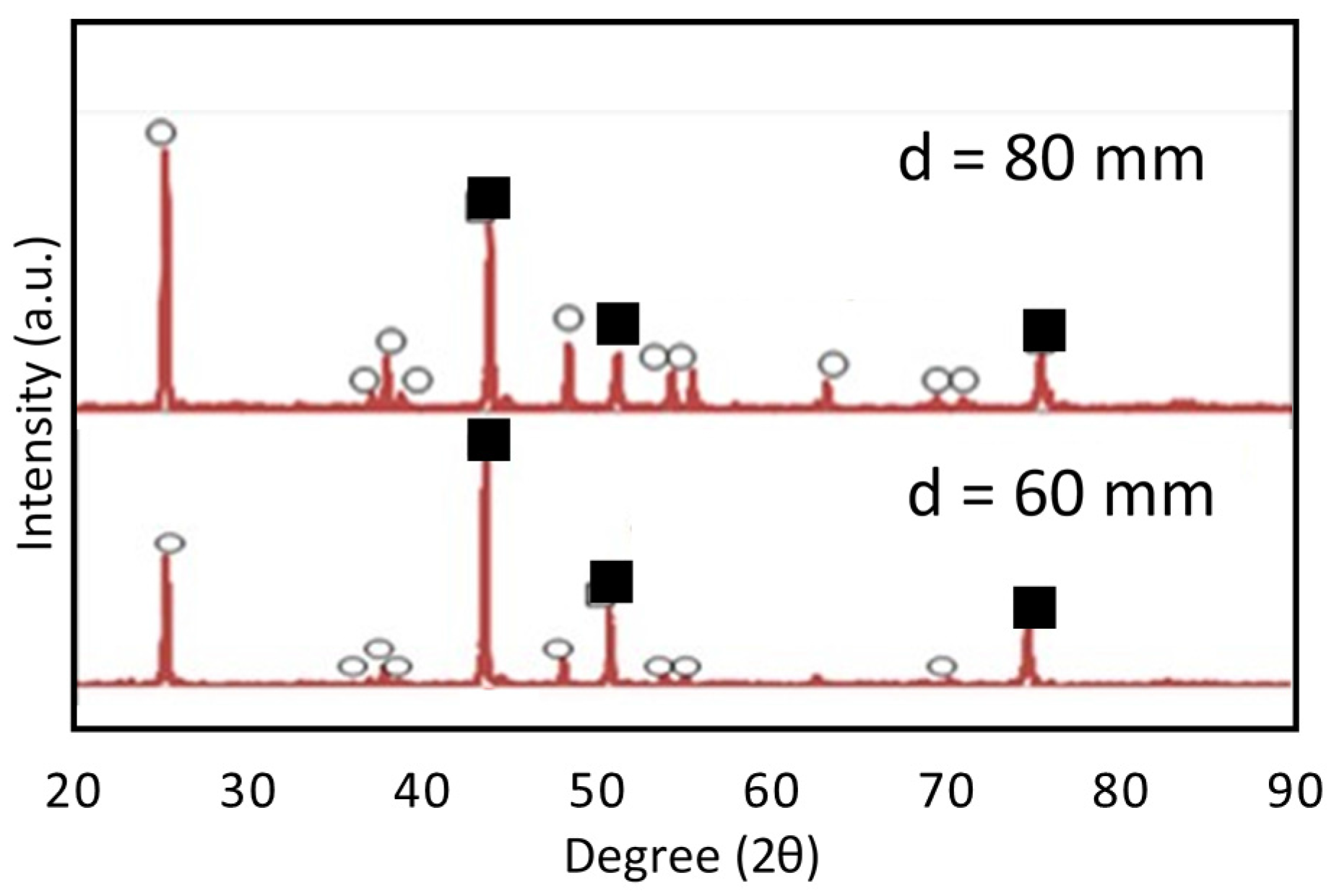
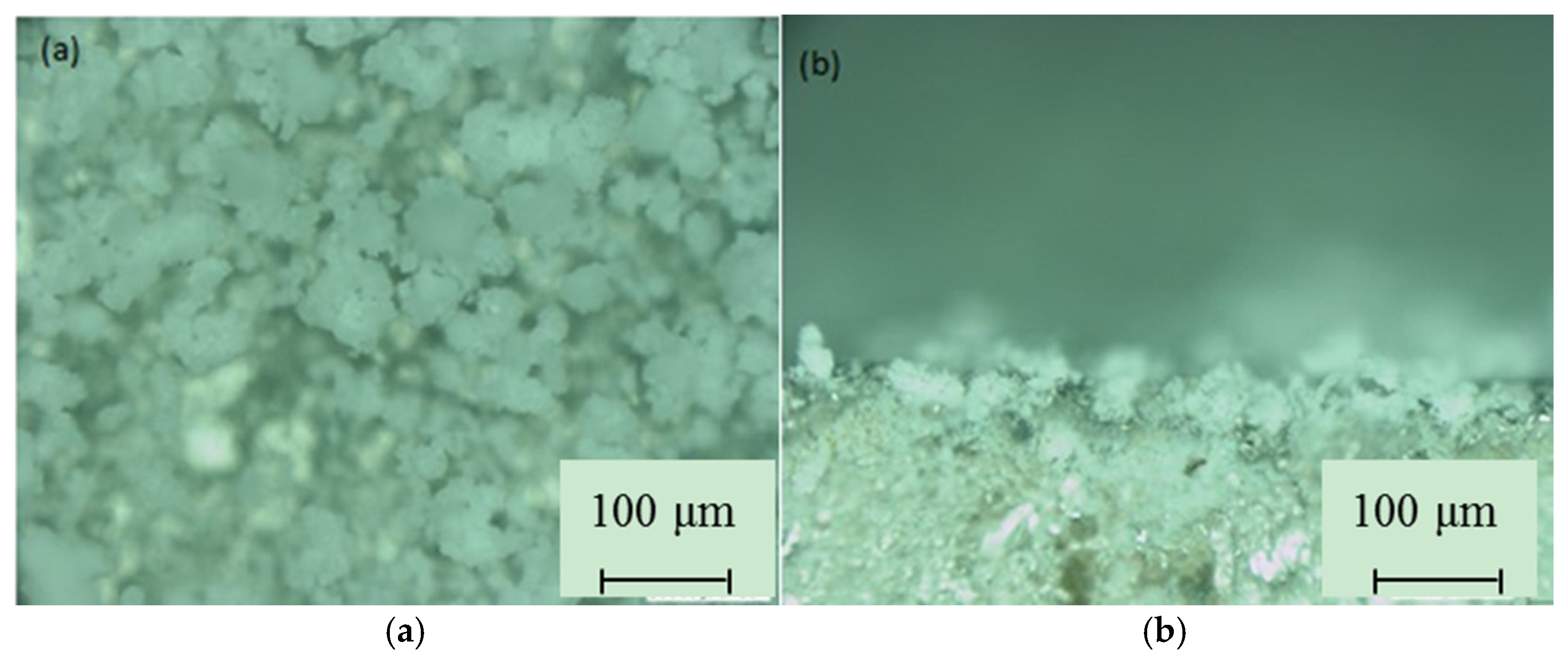
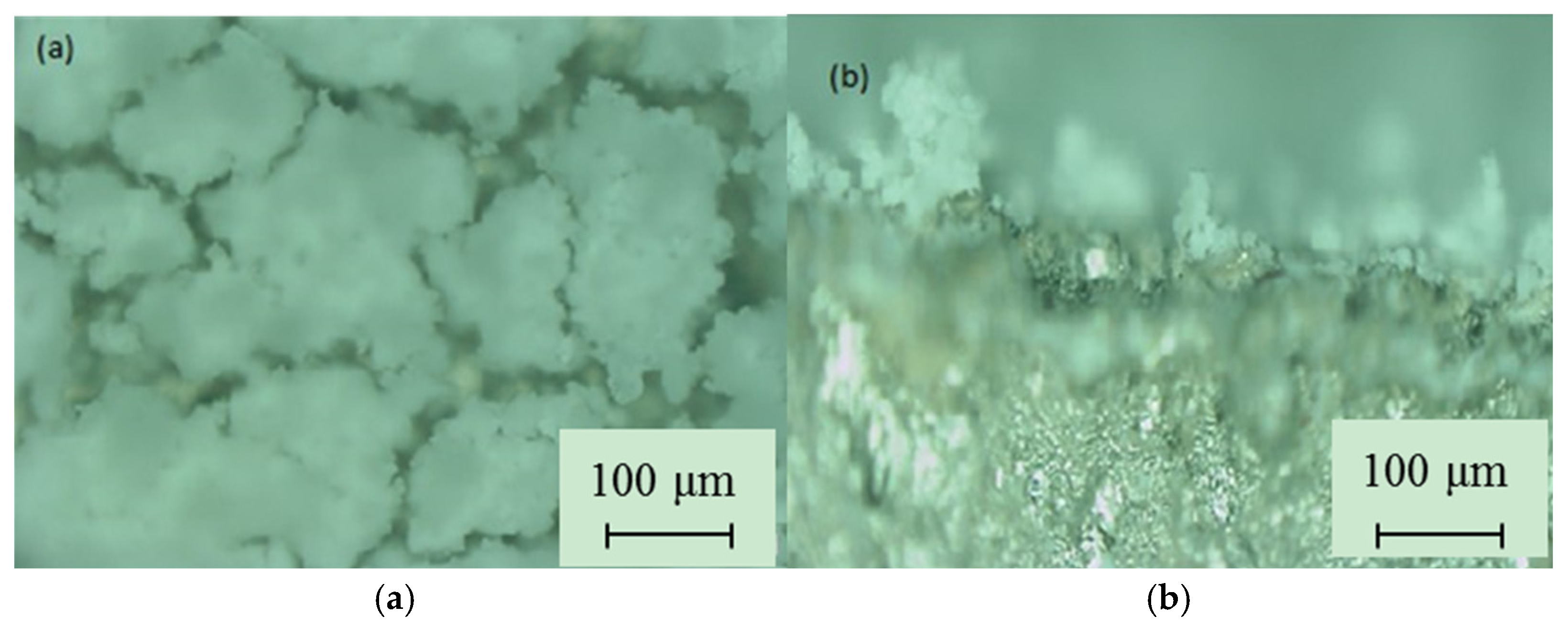
3.1. SPS Titanium Oxide Film
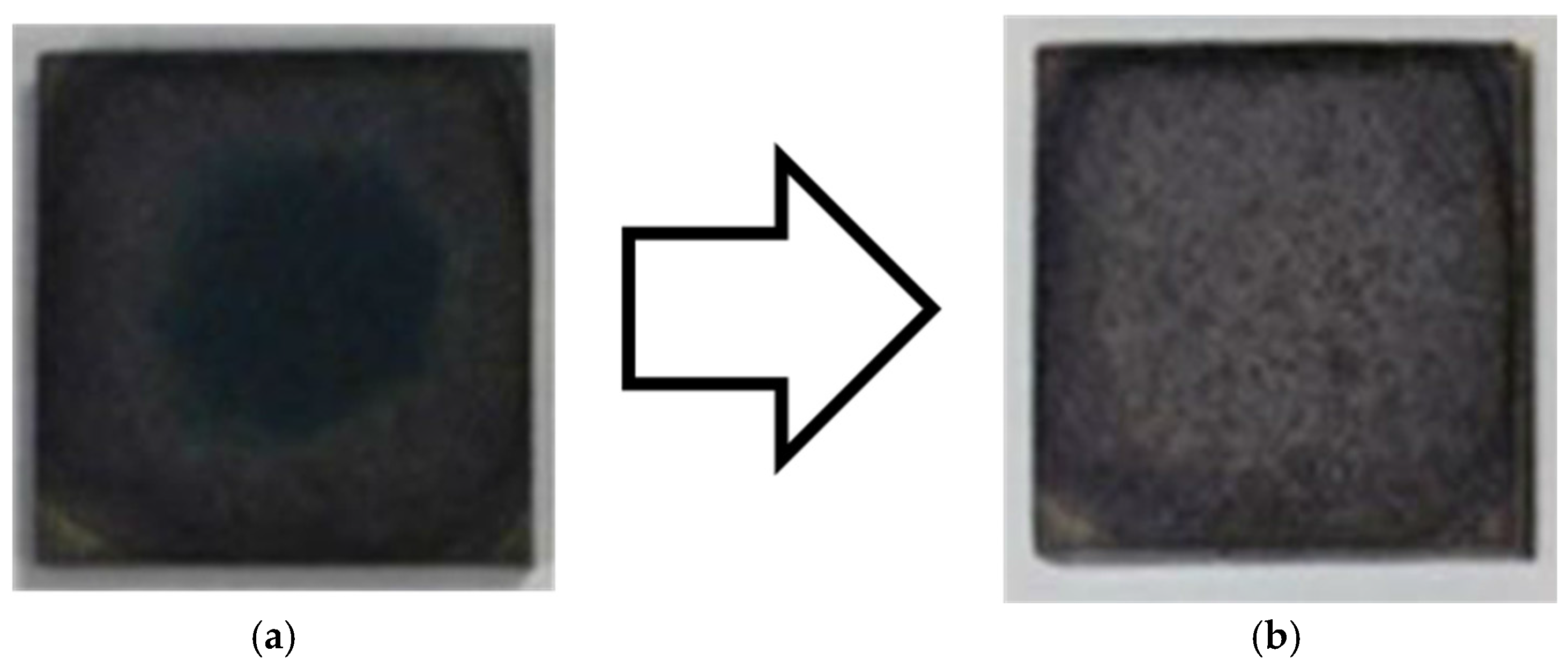
3.2. Photocatalytic Activity of TiO2 Coating
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sima, C.; Waldhauser, W.; Lacknar, J.; Kahn, J.; Kahn, M.; Nicolae, I.; Viespe, C.; Grigoriu, C.; Manea, A. Properties of TiO2 thin films deposited by RF magnetron sputtering. J. Optoelectron. Adv. Mater. 2007, 9, 1446–1449. [Google Scholar]
- Cimpean, A.; Popescu, S.; Ciofrangeanu, C.M.; Gleizes, A.N. Effects of LPMOCVD prepared TiO2 thin films on the in vitro behavior of gingival fibroblasts. Mater. Chem. Phys. 2011, 125, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Oja, I.; Mere, A.; Krunks, M.; Solterbeck, C.-H.; Es-Souni, M. Properties of TiO2 Films Prepared by the Spray Pyrolysis Method. Solid State Phenom. 2004, 99, 259–264. [Google Scholar] [CrossRef]
- Kajitvichyanukula, P.; Ananpattarachaia, J.; Pongpomb, S. Sol–gel preparation and properties study of TiO2 thin film for photocatalytic reduction of chromium(VI) in photocatalysis process. Sci. Technol. Adv. Mater. 2005, 6, 352–358. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Ding, C.; Chu, P.K. In vitro bioactivity of plasma-sprayed TiO2 coating after sodium hydroxide treatment. Surf. Coat. Technol. 2006, 200, 5487–5492. [Google Scholar] [CrossRef]
- Li, C.-J.; Yang, G.-J.; Wang, Y.-Y.; Li, C.-X.; Ye, F.-X.; Ohmori, A. Phase Formation during Deposition of TiO2 Coatings through High Velocity Oxy-Fuel Spraying. Mater. Trans. 2006, 47, 1690–1696. [Google Scholar] [CrossRef]
- Yang, G.-J.; Li, C.-J.; Han, F.; Li, W.-Y.; Ohmori, A. Low Temperature Deposition and Characterization of TiO2 Photocatalytic Film through Cold Spray. Appl. Surf. Sci. 2008, 254, 3979–3982. [Google Scholar] [CrossRef]
- Yamada, M.; Isago, H.; Nakano, H.; Fukumoto, M. Cold Spraying of TiO2 Photocatalyst Coating with Nitrogen Process Gas. J. Therm. Spray Technol. 2010, 19, 1218–1223. [Google Scholar] [CrossRef]
- Ando, Y.; Tobe, S.; Tahara, H. Photo-catalytic TiO2 film deposition by atmospheric TPCVD. Vacuum 2006, 80, 1278–1283. [Google Scholar] [CrossRef]
- Popescu, S.; Jerby, E.; Meir, Y.; Barkay, Z.; Ashkenazi, D.; Mitchell, J.B.A.; Le Garrec, J.L.; Narayanan, T. Plasma column and nano-powder generation from solid titanium by localized microwaves in air. J. Appl. Phys. 2015, 118, 023302. [Google Scholar] [CrossRef]
- Ando, Y. Titanium Oxide Film Deposition on Acrylic Resin by Atmospheric TPCVD. IEEE Trans. Plasma Sci. 2009, 37, 2202–2206. [Google Scholar] [CrossRef]


| Process Parameter | Value |
|---|---|
| Sandblasting realized | Always |
| Working gas composition | Ar |
| Working gas flow rate, L/min | 5–15 |
| Spray distance, mm | 100–150 |
| Discharge current, A | 60–50 |
| Constrictor diameter of plasma torch nozzle, mm | 6 |
| Feedstock | Ti(OH)4 suspension * |
| Feedstock feed rate, mL/min | 11 |
| Feedstock injection port, diameter, mm | 0.4 |
| Deposition distance, mm | 60–80 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi A, H.S.; Ando, Y. Development of a Fabrication Process Using Suspension Plasma Spray for Titanium Oxide Photovoltaic Device. Coatings 2017, 7, 40. https://doi.org/10.3390/coatings7030040
Hadi A HS, Ando Y. Development of a Fabrication Process Using Suspension Plasma Spray for Titanium Oxide Photovoltaic Device. Coatings. 2017; 7(3):40. https://doi.org/10.3390/coatings7030040
Chicago/Turabian StyleHadi A, Hsian Sagr, and Yasutaka Ando. 2017. "Development of a Fabrication Process Using Suspension Plasma Spray for Titanium Oxide Photovoltaic Device" Coatings 7, no. 3: 40. https://doi.org/10.3390/coatings7030040





