Ozone Resistance, Water Permeability, and Concrete Adhesion of Metallic Films Sprayed on a Concrete Structure for Advanced Water Purification
Abstract
:1. Introduction
2. Experimental Plan
2.1. Outline
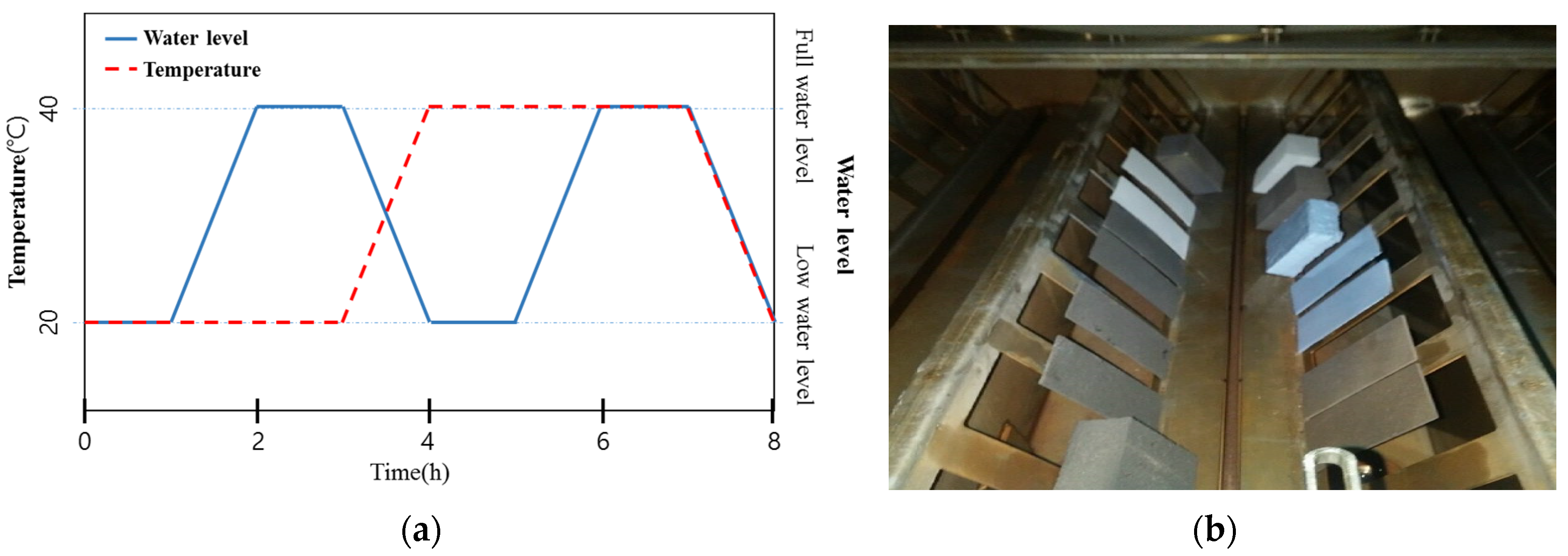
2.2. Ozone Treatment
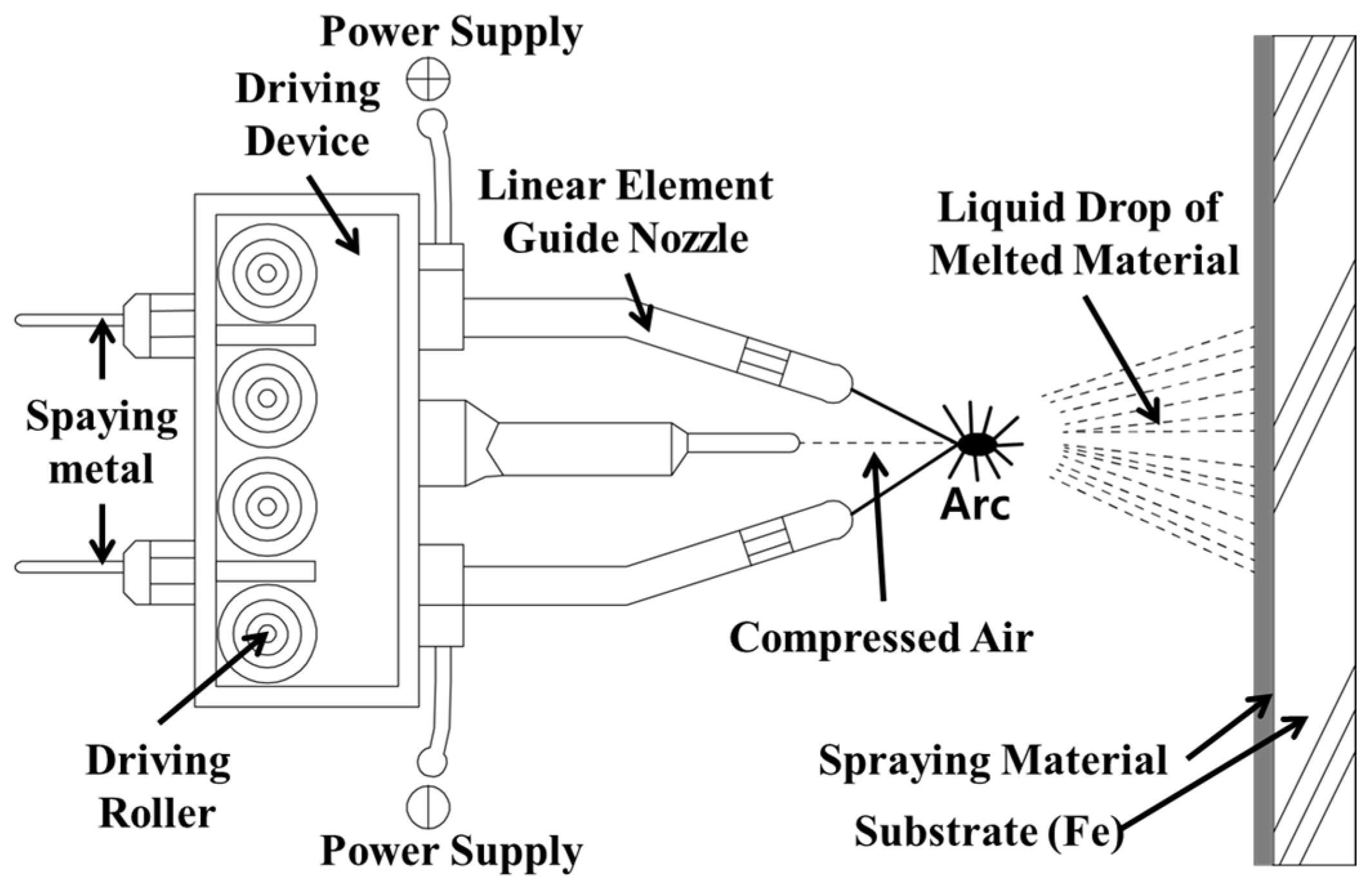
2.3. Specimen Production
2.4. Method
2.4.1. Weight Reduction after Ozone Treatment
2.4.2. Appearance after Ozone Treatment
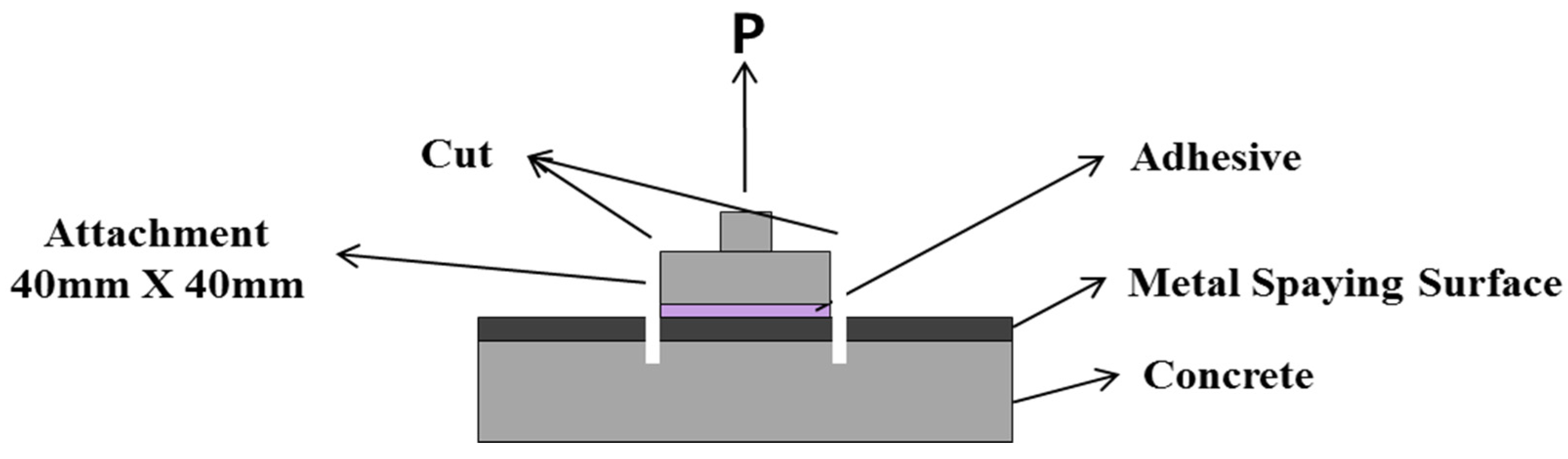
2.4.3. Bond Strength Depending on the Surface Treatment Method
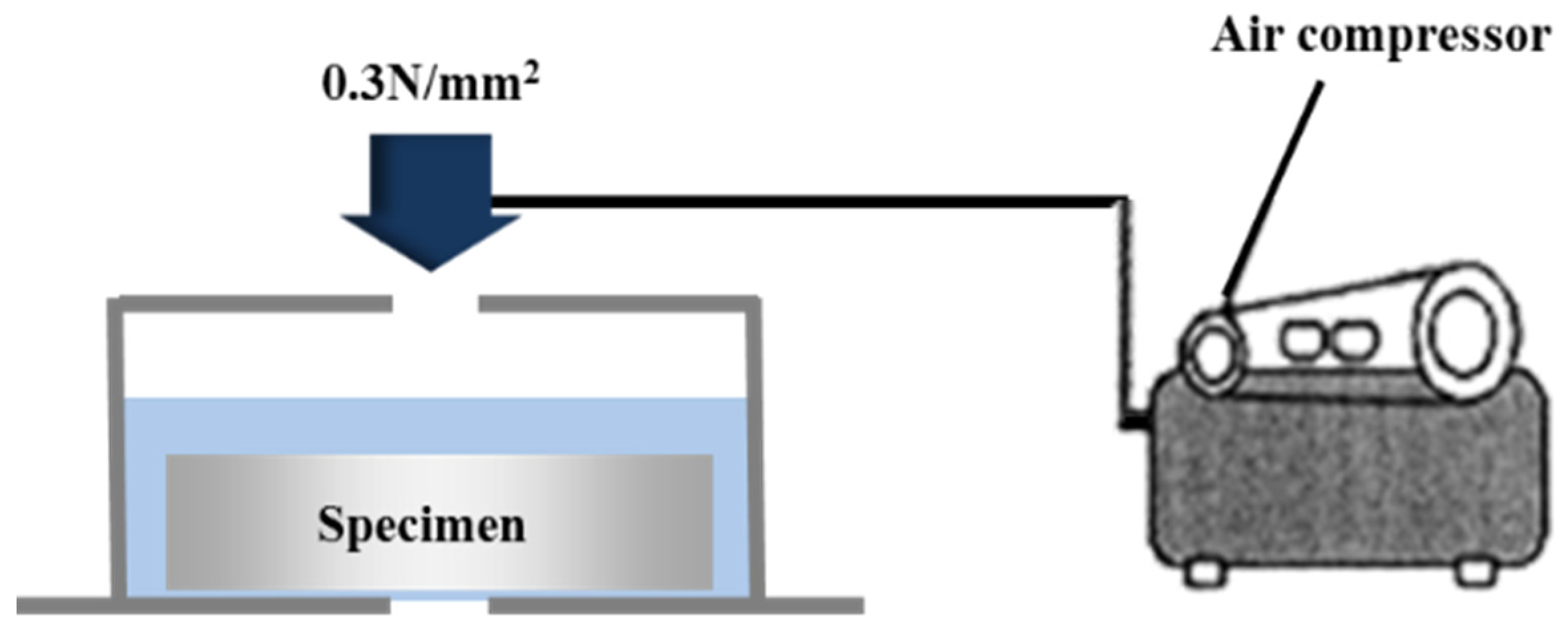
2.4.4. Impermeability Depending on the Surface Treatment Method
3. Results and Discussion
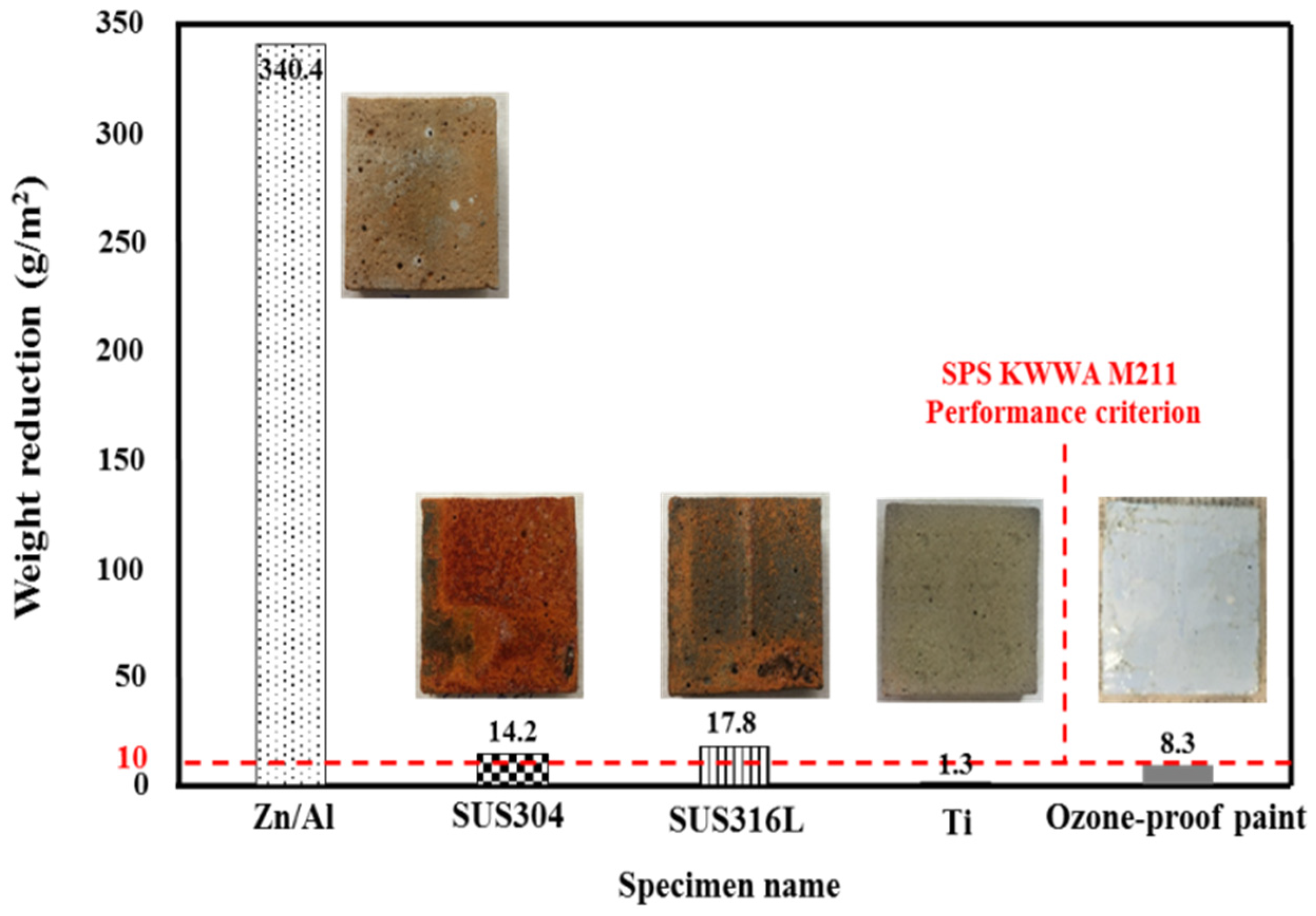
3.1. Weight Reduction after Ozone Treatment
3.2. Change in Appearance after Ozone Treatment
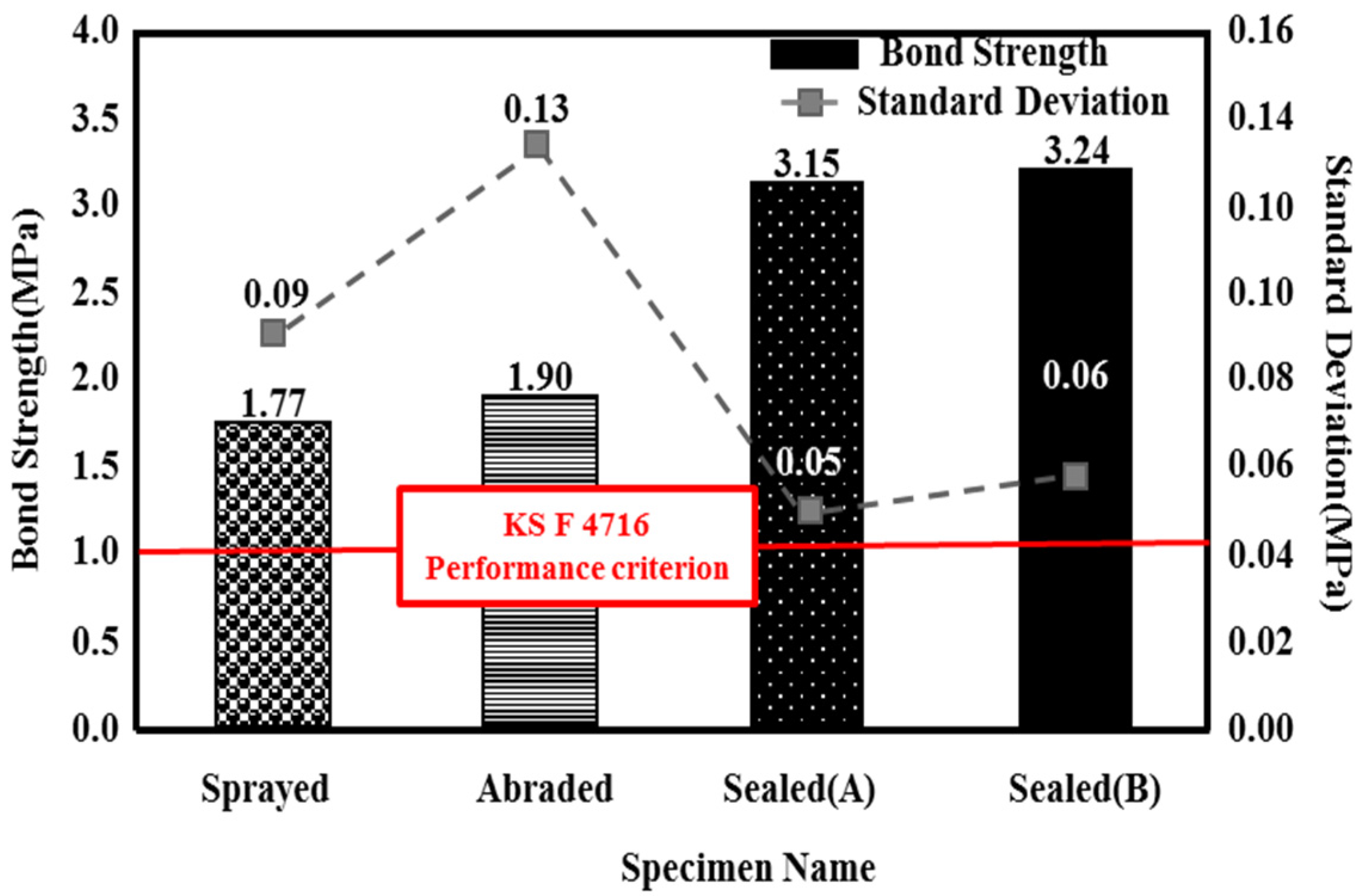
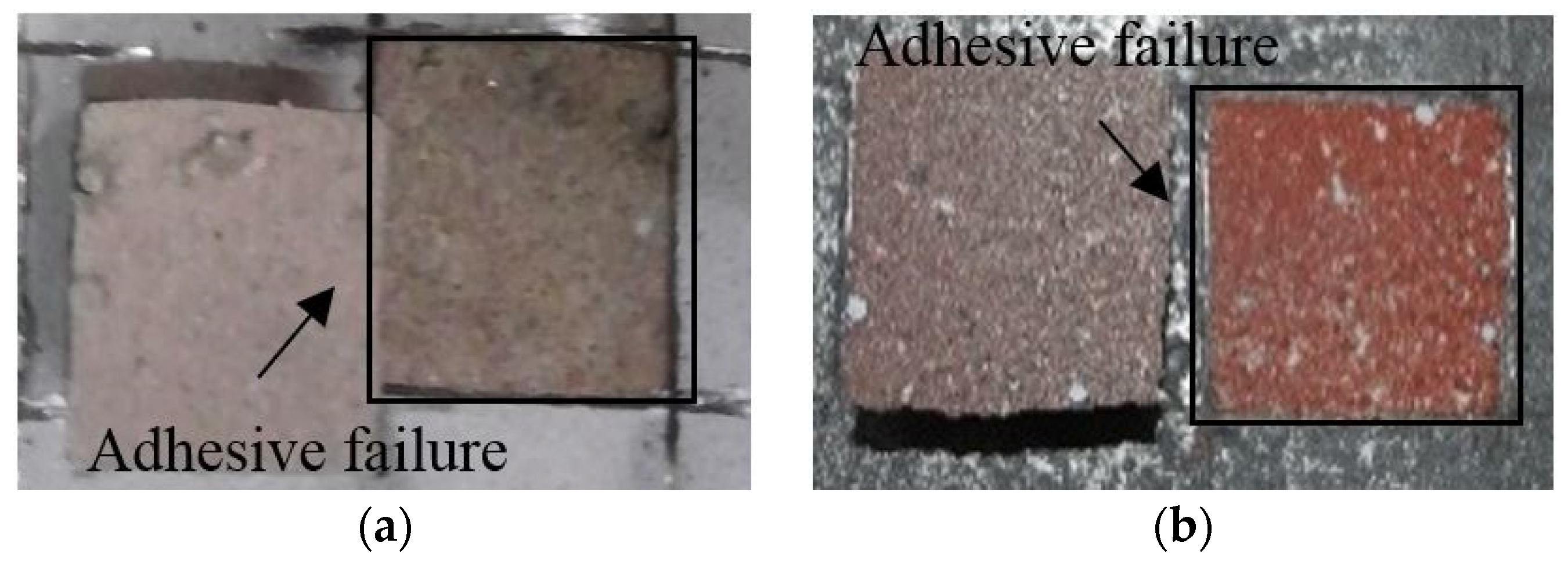
3.3. Adhesion Performance of the Metal Spray Depending on the Surface Treatment Method
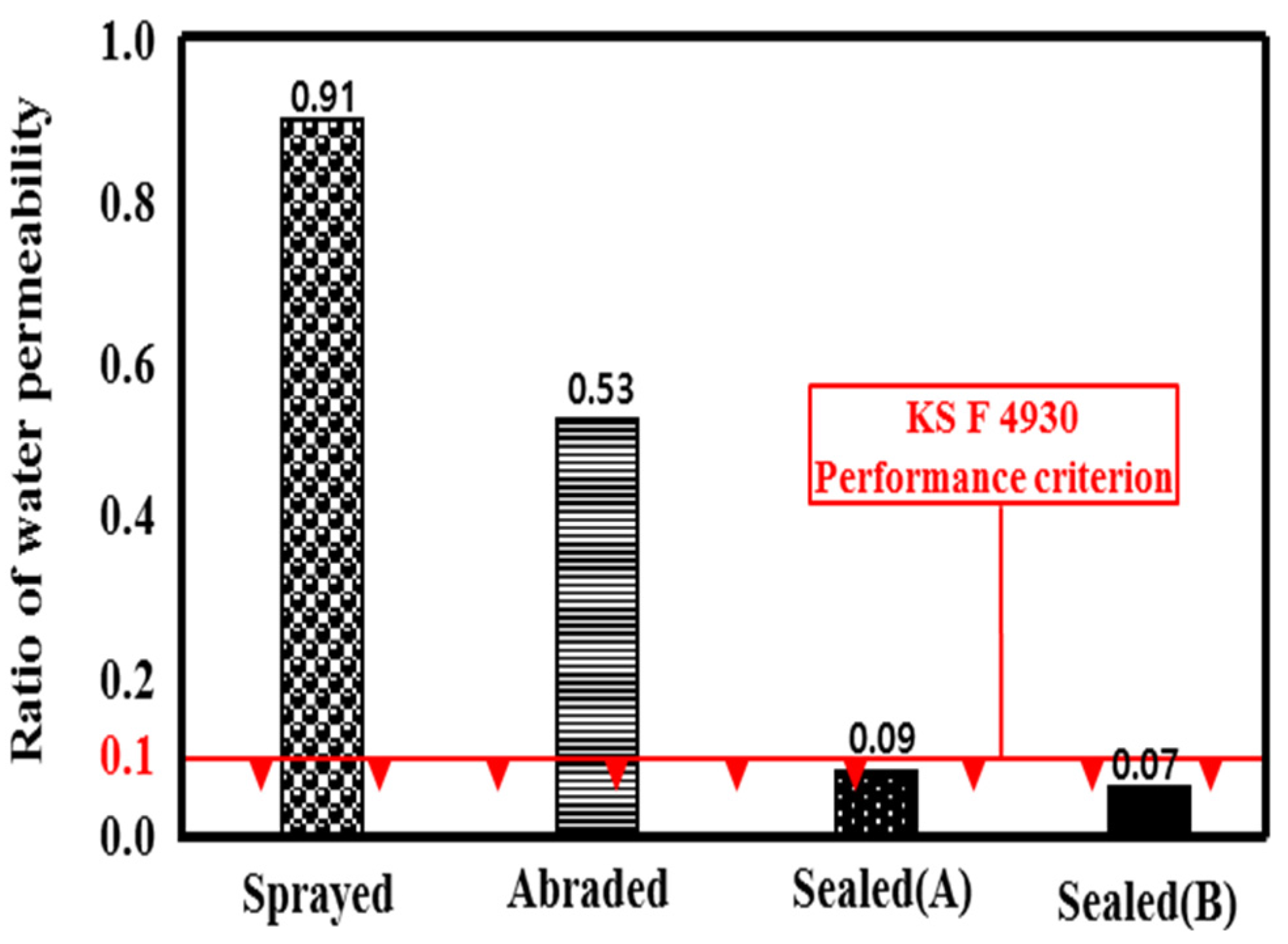
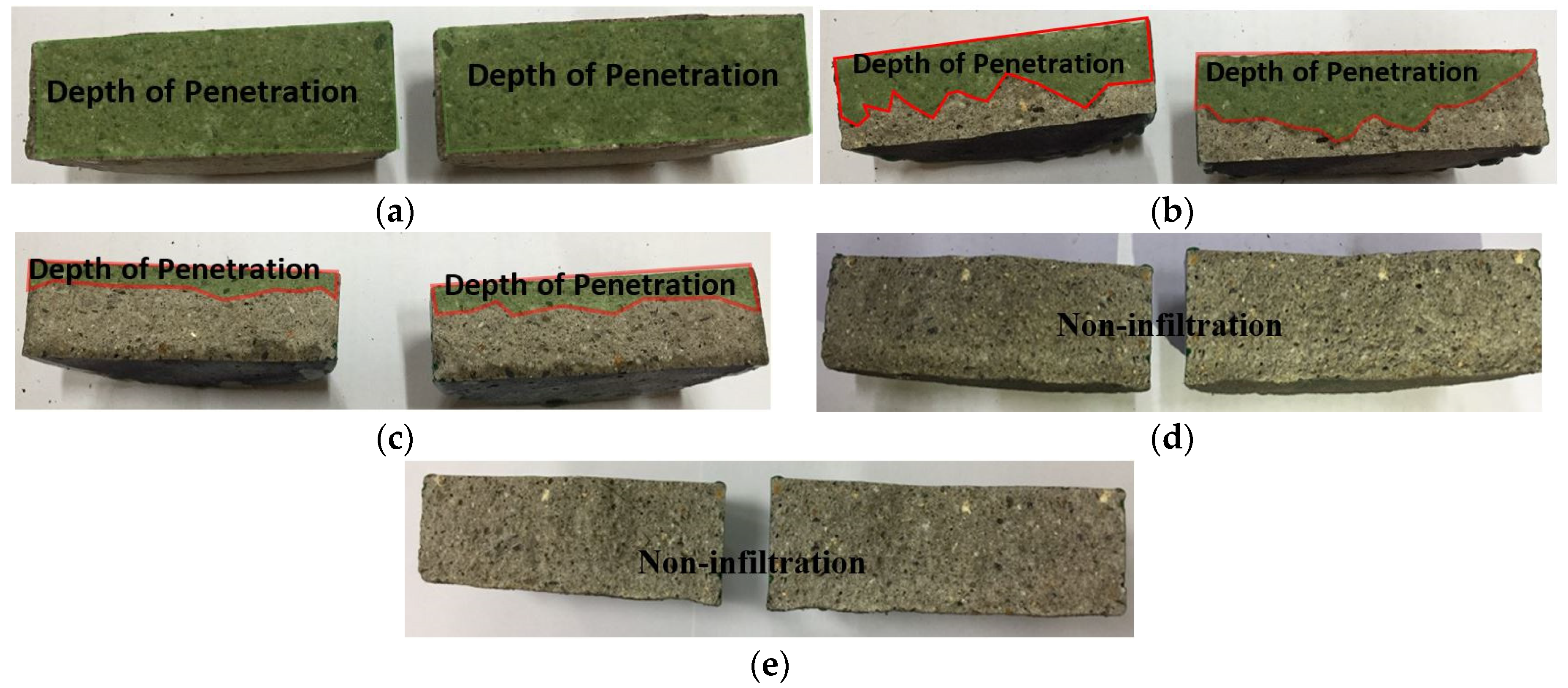
3.4. Impermeability of the Metal Spray Depending on the Surface Treatment Method
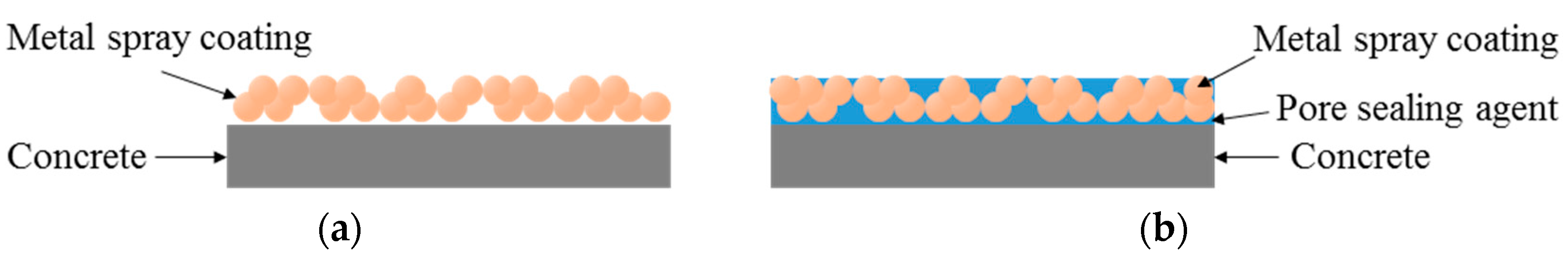
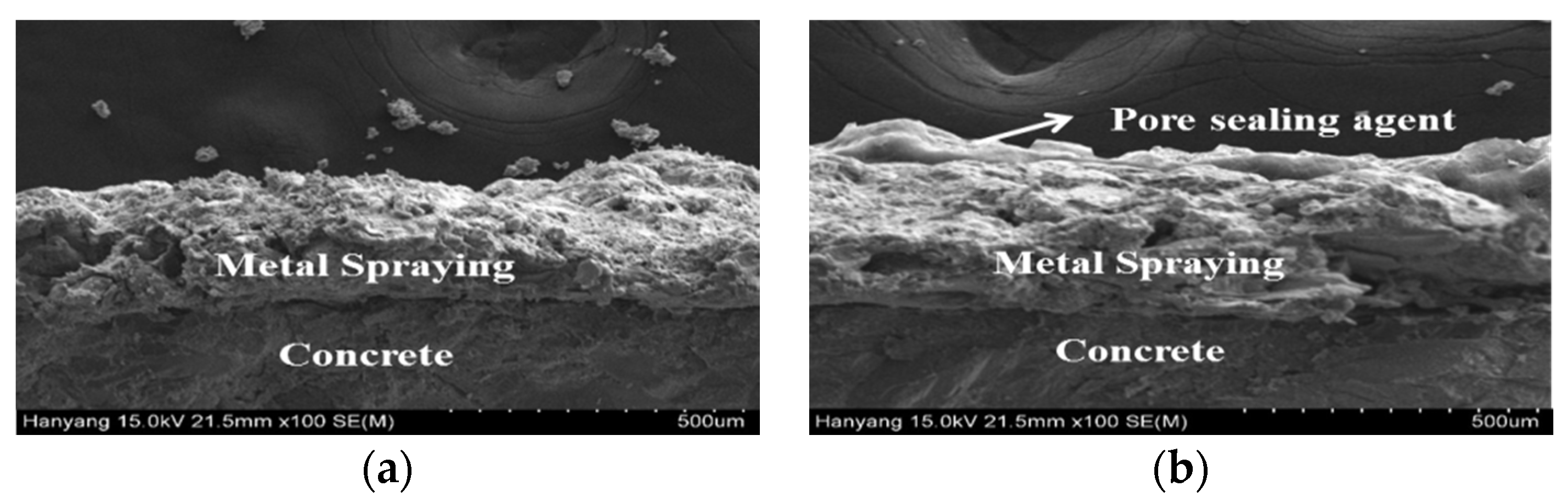
3.5. Analysis of the Surface and Cross-Section of the Sprayed Metal Depending on the Surface Treatment Method
4. Conclusions
- With regard to the ozone resistance, Ti showed the smallest weight reduction of 1.3 g/m2 among the metal sprays and no observable deterioration.
- The stainless steel family (SUS304 and SUS316L) showed large weight reductions and deterioration phenomena, such as rust and exfoliation, in contrast to Ti. These materials may have failed against ozone because the Fe–Ni–Cr structure was destroyed when they were heat-treated, due to the characteristics of the metal spray method. This caused the Fe component to move to the surface. Thus, Ti is the most suitable metal spray for finishing a water treatment facility.
- With regard to the bond strength depending on the surface treatment method, all of the specimens satisfied the KS standard. Using a pore sealing agent (i.e., sealed (A) and sealed (B)) produced the best adhesion performance. This may be because the pore sealing agent permeates the pores generated by the metal spray method and acts as an adhesive to enhance the adhesion performance.
- When the failure modes were compared, while the sprayed and abraded specimens had a relatively low bond strength and showed interfacial failure between the metal spray coating and concrete, the sealed (A) and sealed (B) specimens exhibited a high bond strength and showed non-interfacial failure that occurred in the concrete.
- With regard to the impermeability depending on the surface treatment method, the sprayed specimen showed the lowest impermeability, similar to the case of the bond strength. This may be because water permeates through the pores generated by the metal spray method.
- The sealed (A) and sealed (B) specimens showed that no water permeated the structure. This may be because the pore sealing agents formed a thin film on the metal spray coating that prevented water permeation.
- Surface analysis confirmed many pores on the surface of the sprayed specimen, while the pores were filled up to some extent, in the case of the abraded specimen, from polishing of the metal spray coating. However, polishing could not fill up the pores across the whole area and reduced the bond strength. Thus, applying a pore sealing agent is the most efficient method.
- Ti should be used with the proposed metal spray method to finish water treatment concrete structures, and a pore sealing agent is the most suitable surface treatment method to prevent water from permeating the concrete. However, because the main ingredient of the pore sealing agent of the sealed (A) specimen is epoxy, similar to paint, deterioration by ozonation is expected [17]. Accordingly, using a Teflon-based pore sealing agent is the most appropriate and efficient surface treatment method.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, H.S.; Park, J.H.; Singh, J.K.; Ismail, M.A. Protection of reinforced concrete structures of waste water treatment reservoirs with stainless steel coating using arc thermal spraying technique in acidified water. Materials 2016, 9, 753. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Krol, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard. Mater. 2008, 160, 47–255. [Google Scholar] [CrossRef] [PubMed]
- Dodo, M.C.; Buffle, M.O.; Ginten, U.V. Oxidation of antibacterial molecules by aqueous ozone: Moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef]
- Hirsch, R.; Ternes, T.A.; Haberer, K.; Kratz, K.L. Occurrence of antibiotics in the environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; Mcardell, C.S.; Joss, A.; Giger, W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Eichorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Hanson, M.L.; Sanderson, H.; Lam, M.W.; Young, C.; Mabury, S.A.; Sibley, P.K.; Solomon, K.R. Microcosm evaluation of the effects of an eight pharmaceutical mixture to the aquatic macrophytes Lemna gibba and Myriophyllum sibiricum. Aquat. Toxicol. 2004, 70, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Brain, R.A.; Sanderson, H.; Johnson, D.J.; Bestari, K.T.; Sibley, P.K.; Solomon, K.R. Structural and functional responses of plankton to a mixture of four tetracyclines in aquatic microcosms. Environ. Sci. Technol. 2004, 38, 6430–6439. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.S.; Shin, J.H. An experimental study on evaluation of bond strength of arc thermal metal spraying according to treatment method of water facilities concrete surface. J. Korea Inst. Build. Construct. 2016, 16, 107–115. [Google Scholar] [CrossRef]
- Lupsea, M.; Tiruta-Barna, L.; Schiopu, N. Leaching of hazardous substances from a composite construction product—An experimental and modelling approach for fibre-cement sheets. J. Hazard. Mater. 2014, 264, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q. Increases of lead and chromium in drinking water from using cement-mortar-lined pipes: Initial modeling and assessment. J. Hazard. Mater. 1997, 56, 181–213. [Google Scholar] [CrossRef]
- Jensen, H.S.; Lens, P.N.L.; Nielsen, J.L.; Bester, K.; Nielsen, A.; Haaning, H.-J.; Thorkild, V.J. Growth kinetics of hydrogen sulfide oxidizing bacteria in corroded concrete from sewers. J. Hazard. Mater. 2011, 189, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Owaki, E.; Okamoto, R.; Nagashio, D. Deterioration of concrete in an advanced water treatment plant. In Concrete Under Severe Condition: Environment and Loading; Gjorv, E., Odd, E., Sakai, K., Banthia, N., Eds.; E & FN Spon: London, UK, 1998; p. 438. [Google Scholar]
- Jang, B.H. A study on the ozone resistance test of concrete. Korea Soc. Civ. Eng. 2014, 10, 327–328. [Google Scholar]
- Swamy, R.N.; Tanikawa, S. An external surface coating to protect the concrete and steel from aggressive environments. Mater. Struct. 1993, 26, 465–478. [Google Scholar] [CrossRef]
- Vera, R.; Apablaza, J.; Carvajal, A.M.; Vera, E. Effect of surface coatings in the corrosion of reinforced concrete in acid environments. Int. J. Electrochem. Sci. 2013, 8, 11832–11846. [Google Scholar]
- Crowe, D.; Nixon, R. Corrosion of stainless steels in waste water applications. Available online: http://www.hwea.org/wp-content/uploads/2015/07/150204_Corrosion_of_Stainless_Steels_in_Wastewater_Applications.pdf (accessed on 19 January 2016).
- Bhalerao, B.B.; Arceivala, S.J. Application of corrosion control techniques in municipal water and waste water engineering. Available online: http://eprints.nmlindia.org/ 5825/1/129--139.PDF (accessed on 9 January 2016).
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Bettridge, D.F.; Ubank, R.G. Quality control of high-temperature protective coatings. Mater. Sci. Technol. 1986, 2, 232–242. [Google Scholar] [CrossRef]
- Jandin, G.; Liao, H.; Feng, Z.Q.; Coddet, C. Correlations between operating conditions, microstructure and mechanical properties of twin wire arc sprayed steel coatings. Mater. Sci. Eng. A 2003, 349, 298–305. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Vourlias, G.; Pavlidou, E.; Stergioudis, G.; Skolianos, S.; Chrissafis, K. High temperature oxidation and corrosion in marine environments of thermal spray deposited coatings. Appl. Surf. Sci. 2008, 255, 3104–3111. [Google Scholar] [CrossRef]
- Choi, H.-B.; Lee, H.-S.; Shin, J.-H. Experimental study on the electrochemical anti-corrosion properties of steel structures applying the arc thermal metal spraying method. Materials 2014, 7, 7722–7736. [Google Scholar] [CrossRef]
- Paredes, R.S.C.; Amico, S.C.; d’Oliveira, A.S.C.M. The effect of roughness and pre-heating of the substrate on the morphology of aluminium coatings deposited by thermal spraying. Surf. Coat. Technol. 2006, 200, 3049–3055. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C. Corrosion resistance properties of aluminum coating applied by arc thermal metal spray in SAE J2334 solution with exposure periods. Metals 2016, 6, 55. [Google Scholar] [CrossRef]
- Ozone Resistance Test Method of Material for Waterproof and Anticorrosion; SPS KWWA M211; Korea Water and Wastewater Works Association (KWWA): Seoul, Korea, 2015.
- Cement Filling Compound for Surface Preparation; Korean Standard KS K 4716; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2016.
- Cement-Polymer Modified Waterproof Coatings; Korean Standard KS K 4919; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2008.
- Filter Paper (for Chemical Analysis); Korean Standard KS M 7602; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2007.
- Penetrating Water Repellency of Liquid Type for Concrete Surface Application; Korean Standard KS F 4930; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2012.
- Lai, J.K.L. A review of precipitation behaviour in AISI type 316 stainless steel. Mater. Sci. Eng. 1983, 61, 101–109. [Google Scholar] [CrossRef]




| No. | Experimental Factor | Specimen | Measurement Catalog | Size |
|---|---|---|---|---|
| 1 | Metal sprays | Zn/Al | Ozone resistance (change in appearance, weight reduction) | 100 mm × 100 mm × 30 mm; 150 mm × 70 mm × 2 mm |
| 2 | SUS304 | |||
| 3 | SUS316L | |||
| 4 | Ti | |||
| 5 | Ozone-proof paint | |||
| 6 | Surface treatment method | Sprayed | Bond strength, water permeability | 300 mm × 300 mm × 50 mm; Ø100 mm × 30 mm |
| 7 | Abraded | |||
| 8 | Sealed (A) a | |||
| 9 | Sealed (B) b |
| Ozone Treatment | Zn/Al | SUS304 | SUS316L | Ti | Ozone-Proof Paint |
|---|---|---|---|---|---|
| Before |  |  |  |  |  |
| After |  |  |  |  |  |
| Phenomenon | Exfoliation, discoloration | Rust, discoloration | Rust, discoloration | No change | Discoloration |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Singh, J.K.; Lee, H.-S. Ozone Resistance, Water Permeability, and Concrete Adhesion of Metallic Films Sprayed on a Concrete Structure for Advanced Water Purification. Coatings 2017, 7, 41. https://doi.org/10.3390/coatings7030041
Park J-H, Singh JK, Lee H-S. Ozone Resistance, Water Permeability, and Concrete Adhesion of Metallic Films Sprayed on a Concrete Structure for Advanced Water Purification. Coatings. 2017; 7(3):41. https://doi.org/10.3390/coatings7030041
Chicago/Turabian StylePark, Jin-Ho, Jitendra Kumar Singh, and Han-Seung Lee. 2017. "Ozone Resistance, Water Permeability, and Concrete Adhesion of Metallic Films Sprayed on a Concrete Structure for Advanced Water Purification" Coatings 7, no. 3: 41. https://doi.org/10.3390/coatings7030041






