Studies on the Effect of Arc Current Mode and Substrate Rotation Configuration on the Structure and Corrosion Behavior of PVD TiN Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Coating Design and Deposition
2.3. Coating Characterisation
2.4. Corrosion Testing
3. Results
3.1. Coating Thickness Results
3.2. Surface Roughness Results
3.3. Phase Composition Results
3.4. Potentiodynamic Scanning Results
3.5. Tafel Extrapolation Results
3.6. Structural and Morphological Characterisation of Corroded and Uncorroded Coatings
3.6.1. SEM Image of TiN Fracture Cross Sections
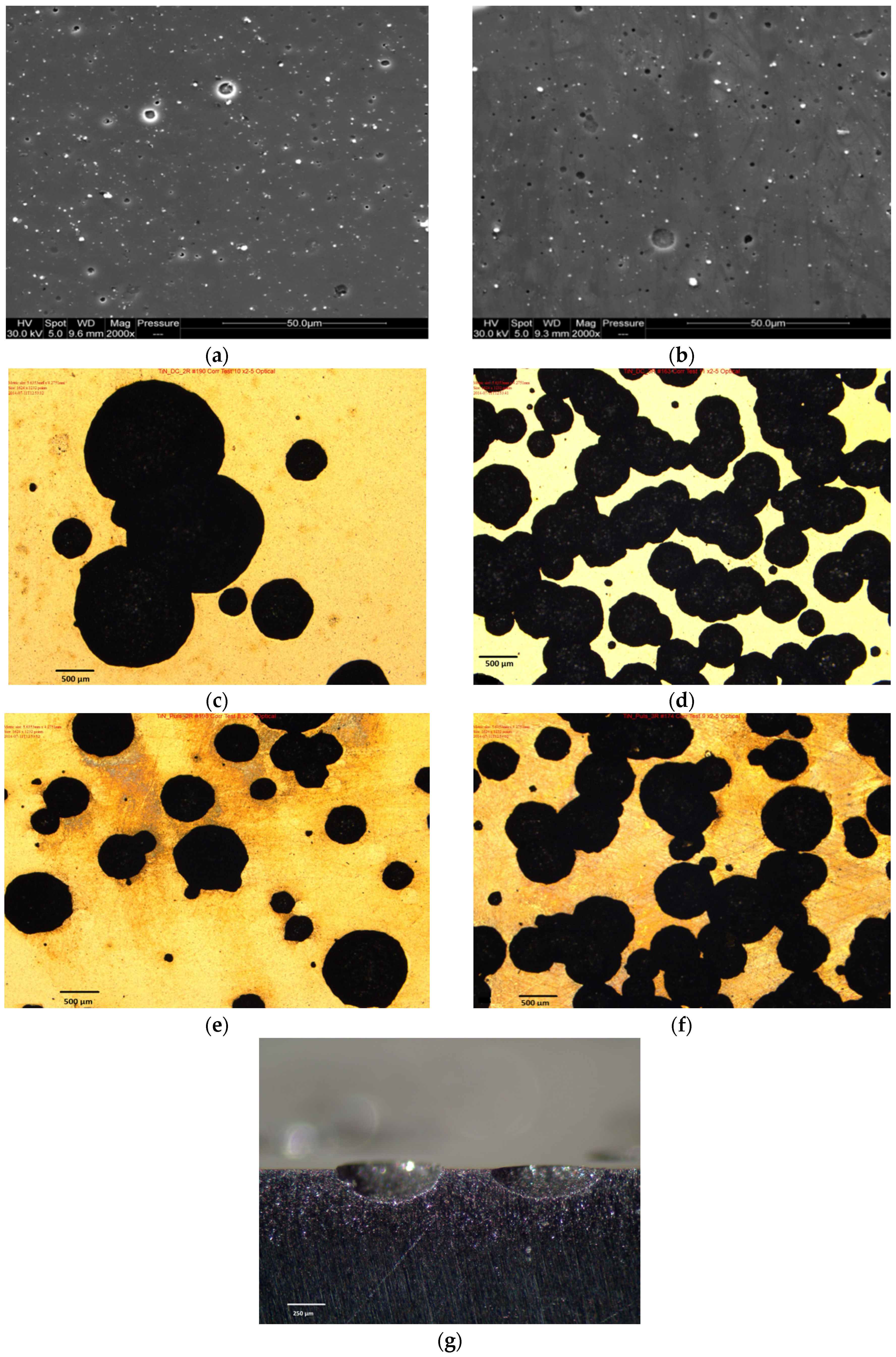
3.6.2. SEM and Optical Images of Corroded TiN Coatings
4. Discussion
4.1. Correlations Between the Process Deposition Conditions, Film Thickness and Surface Roughness of TiN Coatings
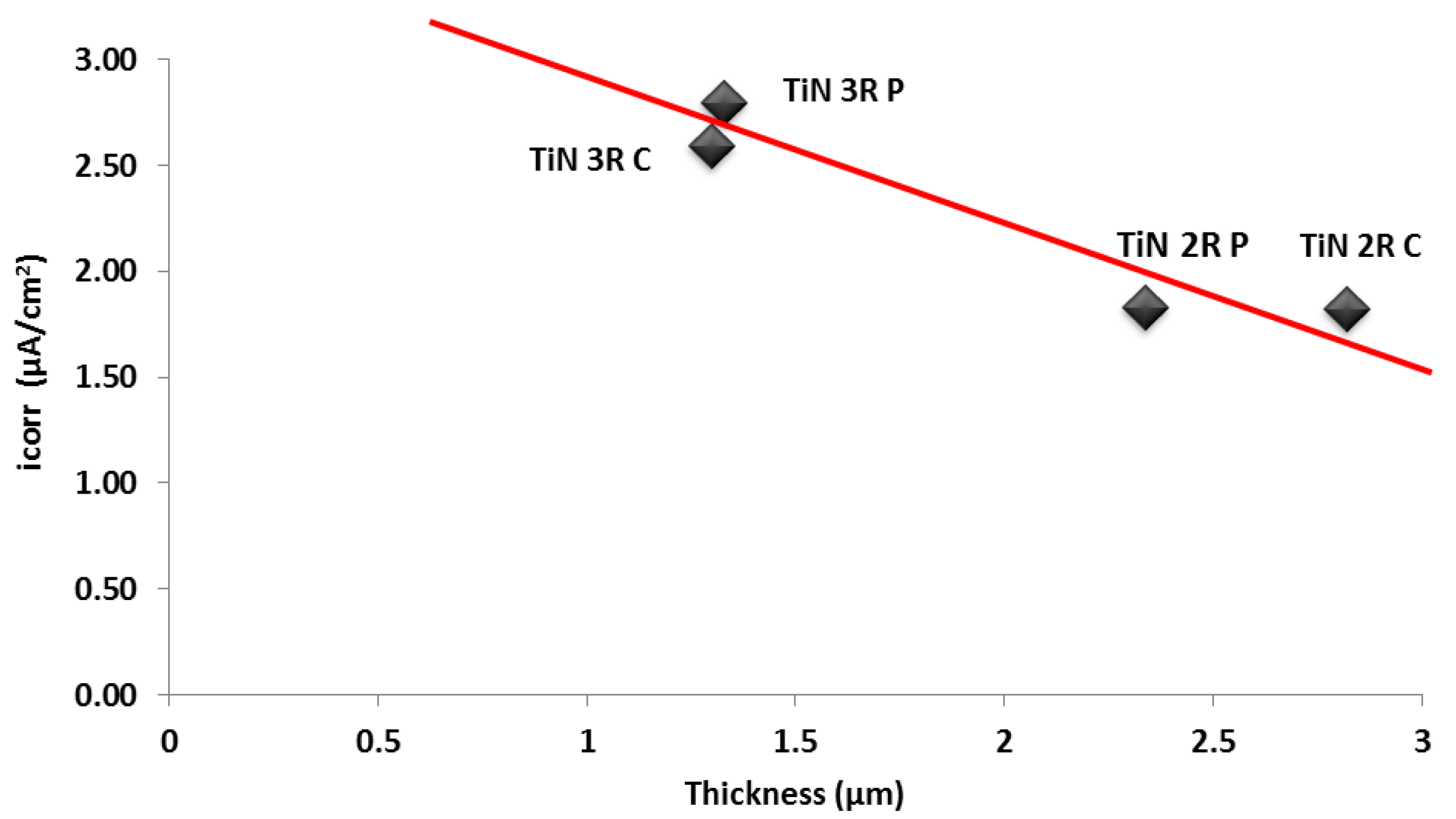
4.2. Corrosion Behaviour of TiN Coatings
4.3. Morphological Effects on the Corrosion Behaviour of TiN Coatings
5. Conclusions
- The deposition of TiN coatings resulted in less negative Ecorr and lower Icorr values, determined from Tafel Extrapolation, when compared with the mild steel substrate after potentiodynamic corrosion testing in 3.5% NaCl.
- Coatings deposited under double rotation (2R) configuration showed more noble (more positive Ecorr values) characteristics than those deposited under triple rotation (3R) configuration. This was attributed to the increased film thickness of the 2R coatings. Any correlations between substrate rotation configuration, arc current mode and observed Icorr values were inconclusive from this study and further work is required in this area.
- At high anodic potentials approaching +500 mV, the corrosion behaviour of most coating systems (except 2R C) was characteristic of uncoated mild steel. This suggests that the limiting factors controlling the corrosion behaviour of the coating system at lower anodic potentials were the presence of defects, pinholes and macro-particles. The initiation, formation and growth of large pitted regions with further coating spallation resulted in underlying substrate corrosion which became the dominant factors at higher anodic potentials.
- An increased number of pits were observed for thinner coatings deposited by triple rotation (both 3R C and 3R P). Fewer, but larger, pits were observed for 2R C coatings, indicating an increased resistance to breakdown at higher anodic potentials.
- Surface roughness from FVM measurements was found to increase with increasing film thickness, although no correlation was found with the type of arc current (continuous or pulsed) in the study.
- The effects of coating microstructure, composition, residual stress and film thickness on the reduced corrosion performance of coatings deposited using pulsed arc current techniques requires further investigation. This will include the deposition of coatings with similar film thicknesses to determine the effects of arc current mode and substrate rotation configuration on the resultant properties, with a focus on coating formation, growth and distribution of defects and their influence on the corrosion behavior.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ward, L.P. Studies on the Corrosion behaviour of a selection of metal nitride coatings and the effect of surface modification. In Proceedings of the Corrosion and Prevention Conference 2009 (CAP09), Coffs Harbour, NSW, Australia, 15–18 November 2009. [Google Scholar]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corr. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Navinsek, B.; Panjan, P.; Milosev, I. PVD coatings as an environmentally clean alternative to electroplating and electroless processes. Surf. Coat. Technol. 1999, 116–119, 476–487. [Google Scholar] [CrossRef]
- Cunha, L.; Andritschky, M.; Rebouta, L.; Pischow, K. Corrosion of CrN and TiAlN coatings in chloride-containing atmospheres. Surf. Coat. Technol. 1999, 116–119, 1152–1160. [Google Scholar] [CrossRef]
- Cunha, L.; Andritschky, M.; Pischow, K.; Wang, Z. Microstructure of CrN coatings produced by PVD techniques. Thin Solid Films 1999, 355–356, 465–470. [Google Scholar] [CrossRef]
- Constantin, R.; Miremad, B. Performance of hard coatings, made by balanced and unbalanced magnetron sputtering, for decorative applications. Surf. Coat. Technol. 1999, 120–121, 728–733. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Zhou, Z.; Li, L.K.-Y.; Yan, J. Electrochemical performance of TiCN coatings with low carbon concentration in simulated body fluid. Surf. Coat. Technol. 2014, 253, 199–204. [Google Scholar] [CrossRef]
- Antunes, R.A.; de Assis, S.L.; Lorenzetti, S.G.; Higa, O.Z.; Costa, I. Comparison of in vitro corrosion behaviour and biocompatibility of Ti-13Zr-13Nb and passivated 316L stainless steel coated with TiCN. In Proceedings of the COBEM, 18th International Conference of Mechanical Engineering, Ouro Preto, MC, Brazil, 6–11 November 2005. [Google Scholar]
- Massiani, Y.; Medjahed, A.; Crousier, JP.; Gravier, P.; Rebatel, I. Corrosion of sputtered titanium nitride films deposited on iron and stainless steel. Surf. Coat. Technol. 1991, 45, 115–120. [Google Scholar] [CrossRef]
- Piippo, J.; Elsener, B.; Bohni, H. Electrochemical characterisation of TiN coatings. Surf. Coat. Technol. 1993, 61, 43–46. [Google Scholar]
- Brown, R.; Alias, M.N.; Fontana, R. Effect of composition and thickness on corrosion behaviour of TiN and ZrN thin films. Surf. Coat. Technol. 1993, 62, 467–473. [Google Scholar] [CrossRef]
- Kazuhisa, A.; Shigeyo, W.; Masahiro, S.; Isao, S.; Yokio, I.; Patrick, J.; William, S. Characterization of anodic oxide film formed on TiN coating in neutral borate buffer solution. Corr. Sci. 1998, 40, 1363–1377. [Google Scholar]
- Massiani, Y.; Medjahed, A.; Gravier, P.; Argeme, L.; Fedrizzi, L. Electrochemical study of titanium nitride films obtained by reactive sputtering. Thin Solid Films 1990, 191, 305–316. [Google Scholar] [CrossRef]
- Wang, H.W.; Stack, M.M.; Lyon, S.B.; Hovsepian, P.; Munz, W.-D. Preferential wear of droplet defects for combined cathodic arc-unbalanced magnetron sputtering CrN/NbN superlattice coatings during erosion in alkaline slurries. J. Mater. Sci. Lett. 2001, 20, 1995–1997. [Google Scholar] [CrossRef]
- Pilkington, A. Development and Evaluation of Plasma-Assisted Alumina Based Coatings. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2014. [Google Scholar]
- Büschel, M.; Grimm, W. Influence of the pulsing of the current of a vacuum arc on rate and droplets. Surf. Coat. Technol. 2001, 142–144, 665–668. [Google Scholar] [CrossRef]
- Ward, L.P.; Pilkington, A.; Dowey, S.J.; Doyle, E.D. Corrosion Properties of Cathodic Arc Evaporated Coatings. In Proceedings of the Corrosion and Prevention Conference 2014, CAP14, Darwin, NT, Australia, 21–24 September 2014. [Google Scholar]
- Ward, L.P.; Pilkington, A.; Dowey, S.J.; Toton, J.T.; Doyle, E.D. Studies on the corrosion behaviour of AlCrON coatings. In Proceedings of the Corrosion and Prevention Conference 2013, CAP13, Brisbane, QLD, Australia, 12–15 Nobember 2013. [Google Scholar]
- Hu, Y.; Li, L.; Dai, H.; Li, X.; Cai, X.; Chu, P.K. Effects of pulse parameters on macro-particle production in pulsed cathodic vacuum arc deposition. Surf. Coat. Technol. 2007, 201, 6542–6544. [Google Scholar] [CrossRef]
- Ramm, J.; Ante, M.; Bachmann, T.; Widrig, B.; Brändle, H.; Döbeli, M. Pulse enhanced electron emission (P3e™) arc evaporation and the synthesis of wear resistant Al–Cr–O coatings in corundum structure. Surf. Coat. Technol. 2007, 202, 876–883. [Google Scholar] [CrossRef]
- Fuchs, H.; Engers, B.; Hettkamp, E.; Mecke, H.; Schultz, J. Deposition rate and thickness uniformity of thin films deposited by a pulsed cathodic arc process. Surf. Coat. Technol. 2001, 142–144, 655–660. [Google Scholar] [CrossRef]
- Devia, D.M.; Restrepo-Parra, E.; Arango, P.J. Comparative study of titanium carbide and nitride coatings grown by cathodic vacuum arc technique. App. Surf. Sci. 2011, 258, 1164–1174. [Google Scholar] [CrossRef]
- Ward, L.P.; Biddle, G.; Hinton, B.; Gerrard, D. Characterisation and evaluation of the corrosion behaviour of modified HVOF sprayed WC based coatings deposited on AISI 1020 mild steel substrates. In Proceedings of the Corrosion and Prevention Conference 2006 (CAP06), Hobart, Tasmania, Australia, 19–22 November 2006. [Google Scholar]
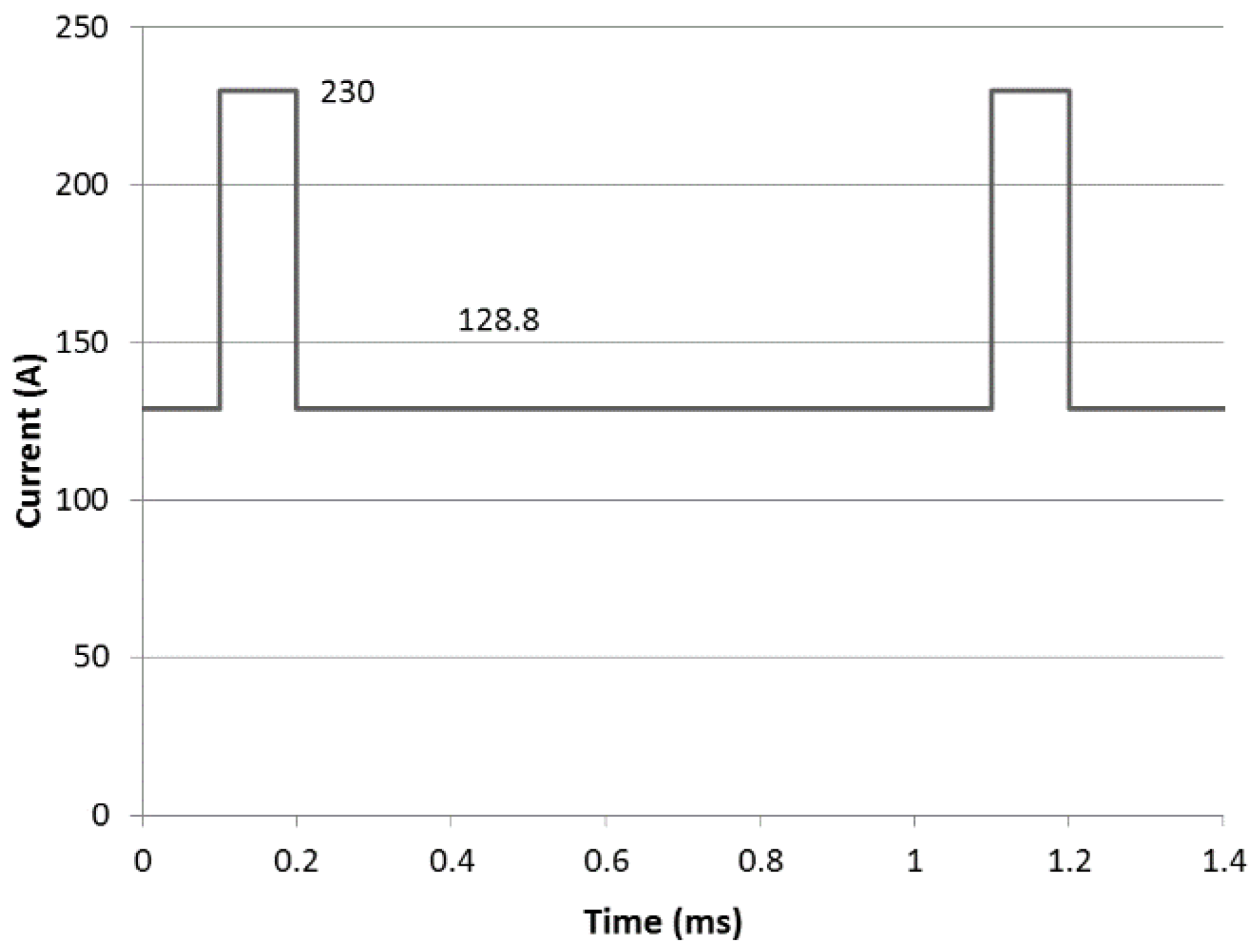
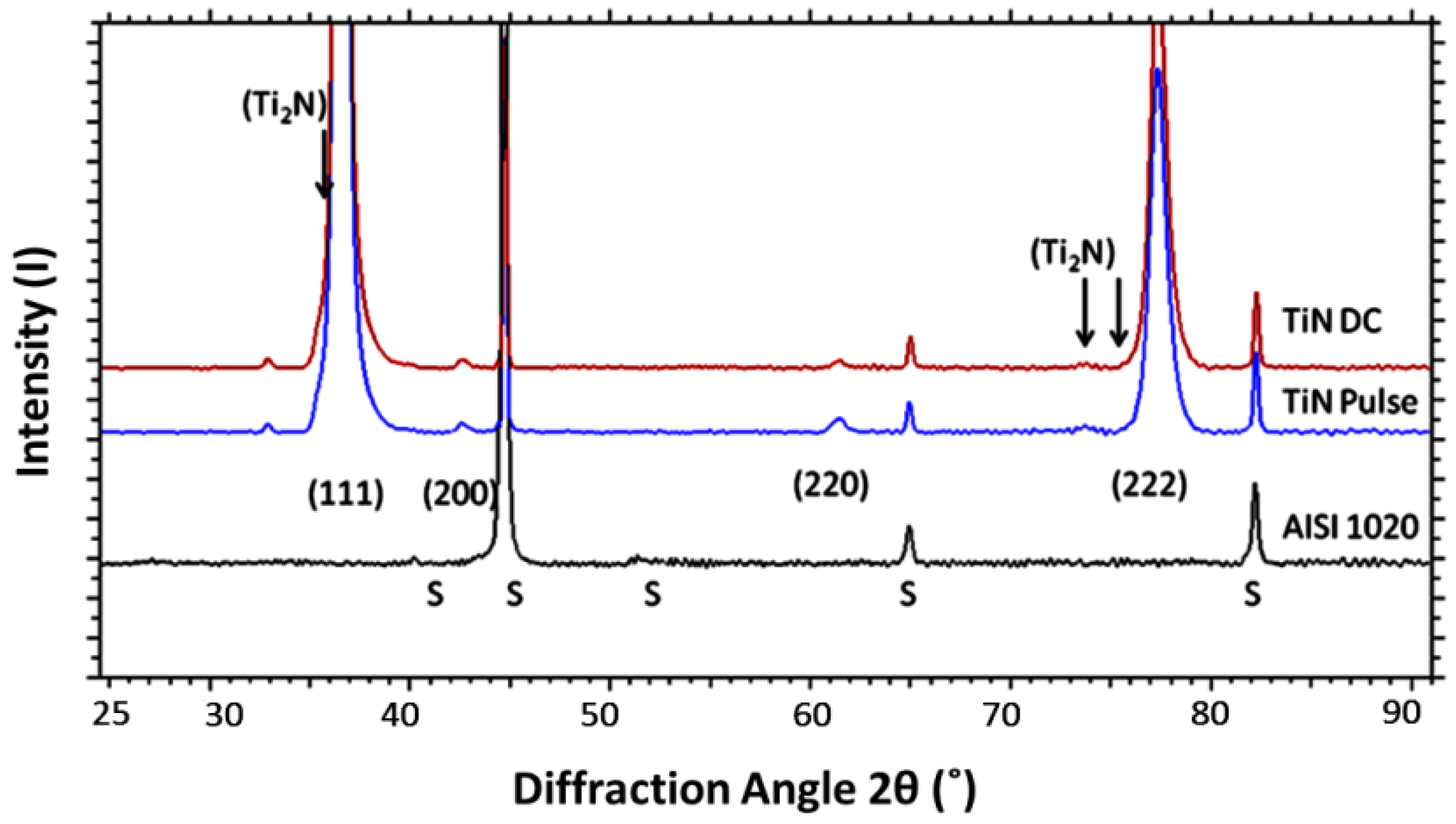
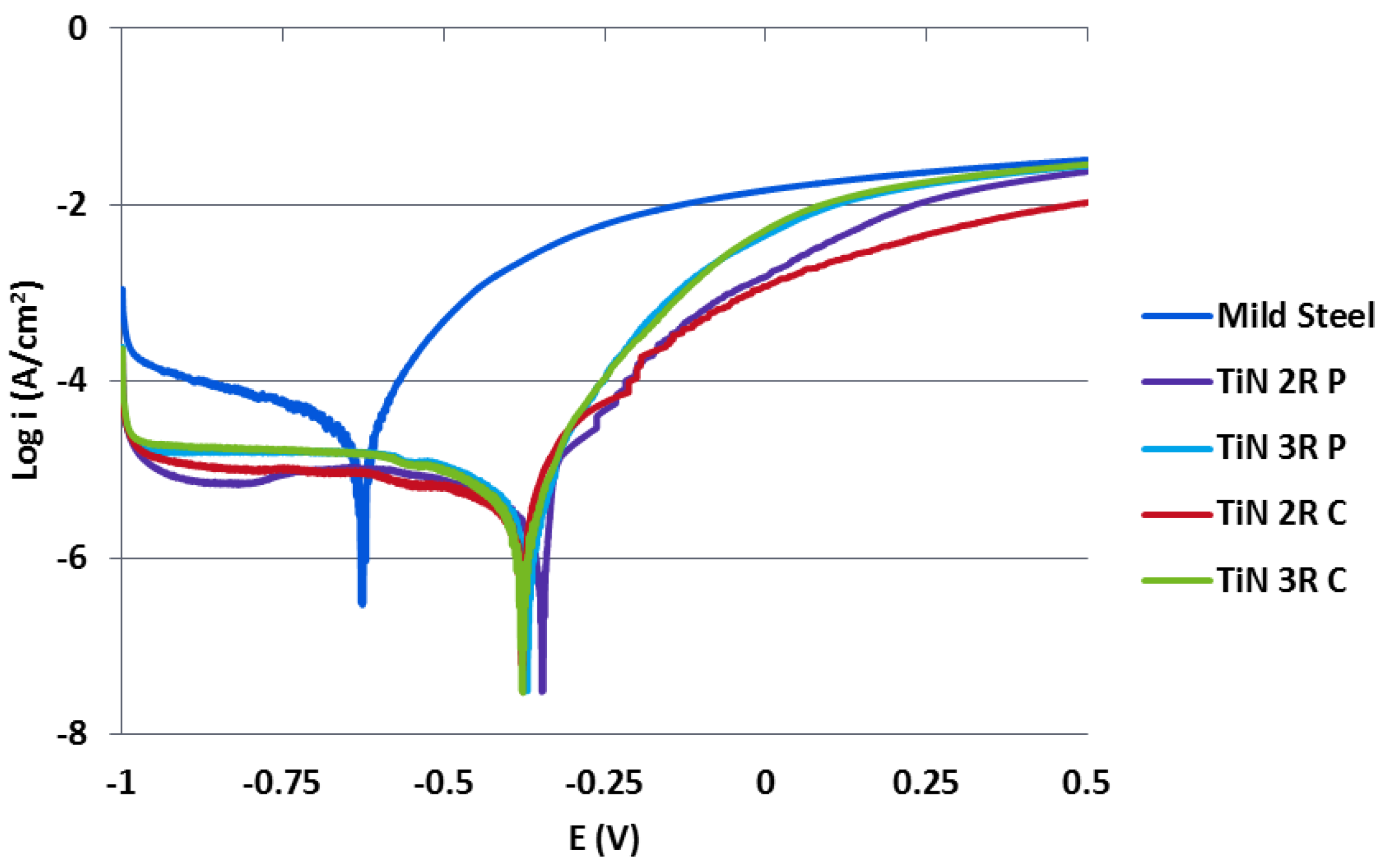
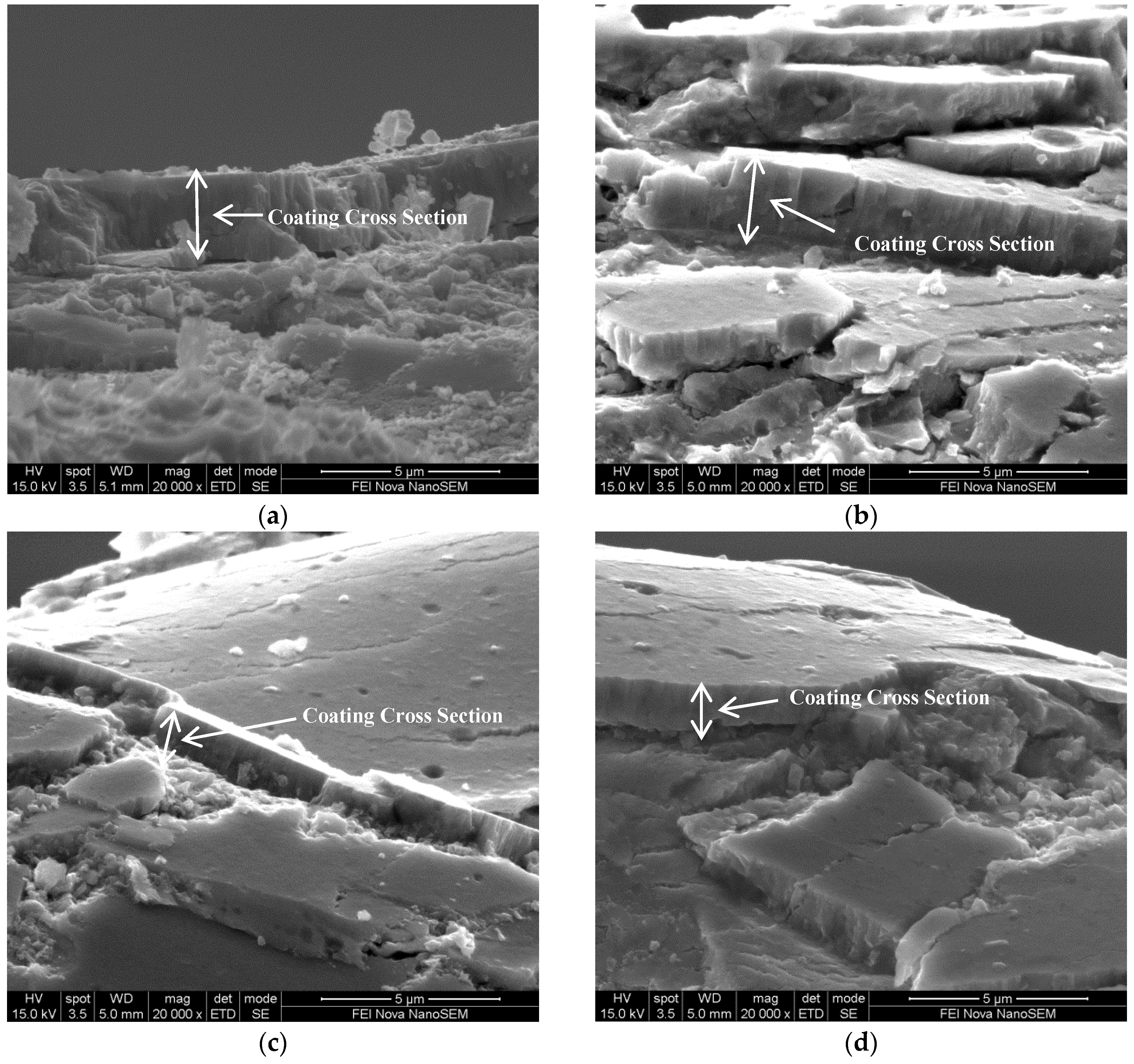
| Coating | Arc Current (A) | Pulse Duty Cycle, Frequency |
|---|---|---|
| TiN 2R C | 180 | n/a |
| TiN 3R C | 180 | n/a |
| TiN 2R P | 230/128.8 | 10%, 1 kHz |
| TiN 3R P | 230/128.8 | 10%, 1 kHz |
| Coating | Coating Thickness (μm) | Deposition Rate (nm/s) | Evaporation Rate (mg/s) | Evaporation Efficiency (g/C) | Surface Roughness Sq (nm) | Surface Roughness S10z (μm) |
|---|---|---|---|---|---|---|
| AISI 1020 | – | – | – | – | 14.7 ± 3.9 | 0.234 ± 0.02 |
| TiN 2R C | 2.8 ± 0.1 | 0.78 | 3.47 | 2.17 × 10−5 | 180.83 ± 15.47 | 3.52 ± 0.39 |
| TiN 3R C | 1.3 ± 0.1 | 0.36 | – | – | 126.36 ± 12.93 | 1.93 ± 0.46 |
| TiN 2R P | 2.3 ± 0.1 | 0.64 | 2.97 | 2.05 × 10−5 | 187.37 ± 2.51 | 3.06 ± 0.41 |
| TiN 3R P | 1.3 ± 0.1 | 0.37 | – | – | 137.74 ± 4.33 | 1.91 ± 0.34 |
| Coating | Ecorr (mV) | Icorr (µA/cm2) |
|---|---|---|
| 1020 Carbon Steel | −626 ± 3 | 15.57 ± 6.04 |
| TiN 2R C | −350 ± 41 | 1.82 ± 1.08 |
| TiN 3R C | −399 ± 32 | 2.59 ± 1.18 |
| TiN 2R P | −357 ± 13 | 1.83 ± 0.36 |
| TiN 3R P | −376 ± 6 | 2.80 ± 0.99 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, L.; Pilkington, A.; Dowey, S. Studies on the Effect of Arc Current Mode and Substrate Rotation Configuration on the Structure and Corrosion Behavior of PVD TiN Coatings. Coatings 2017, 7, 50. https://doi.org/10.3390/coatings7040050
Ward L, Pilkington A, Dowey S. Studies on the Effect of Arc Current Mode and Substrate Rotation Configuration on the Structure and Corrosion Behavior of PVD TiN Coatings. Coatings. 2017; 7(4):50. https://doi.org/10.3390/coatings7040050
Chicago/Turabian StyleWard, Liam, Antony Pilkington, and Steve Dowey. 2017. "Studies on the Effect of Arc Current Mode and Substrate Rotation Configuration on the Structure and Corrosion Behavior of PVD TiN Coatings" Coatings 7, no. 4: 50. https://doi.org/10.3390/coatings7040050





