Combustion Synthesis of UHTC Composites from Ti–B4C Solid State Reaction with Addition of VIb Transition Metals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
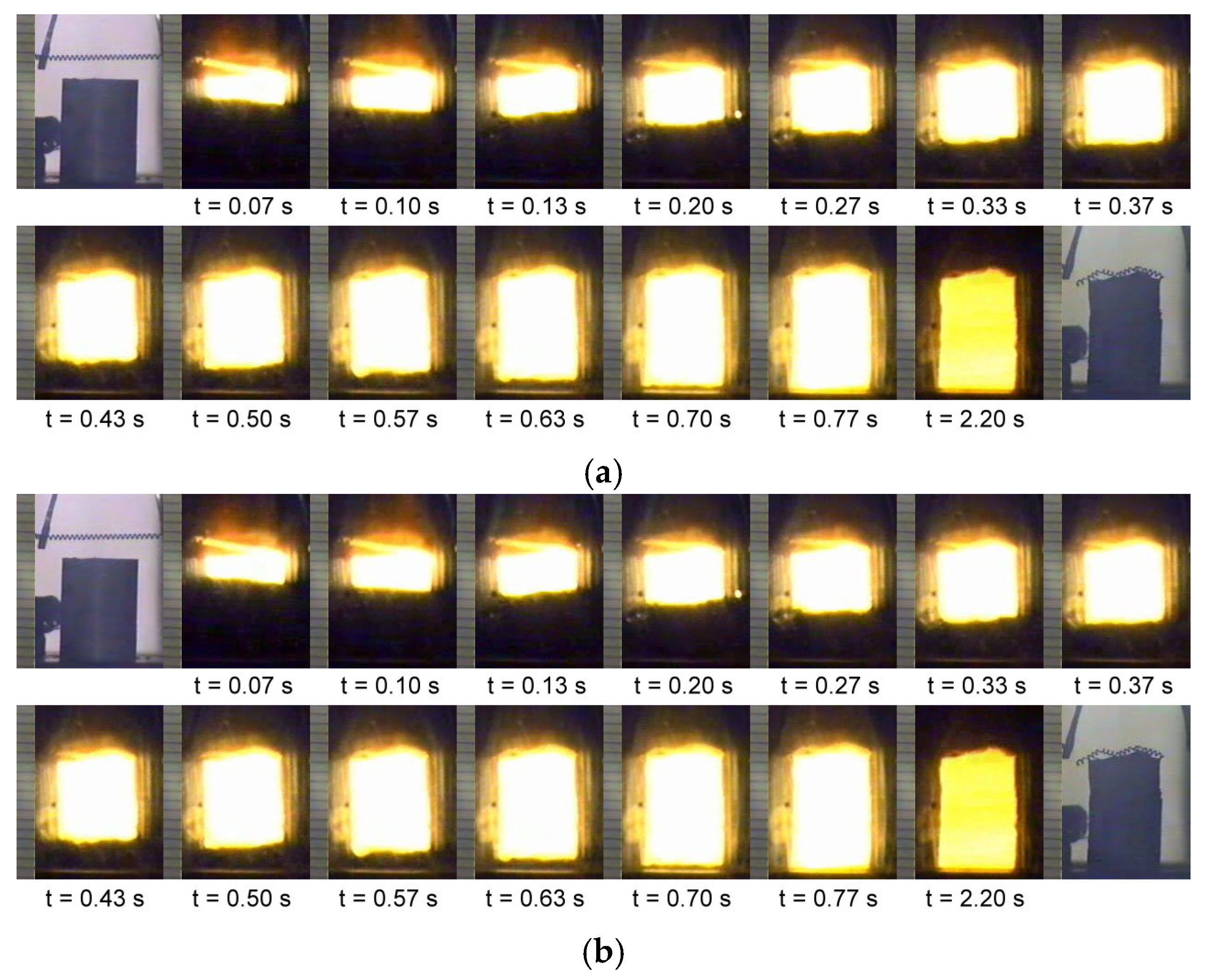
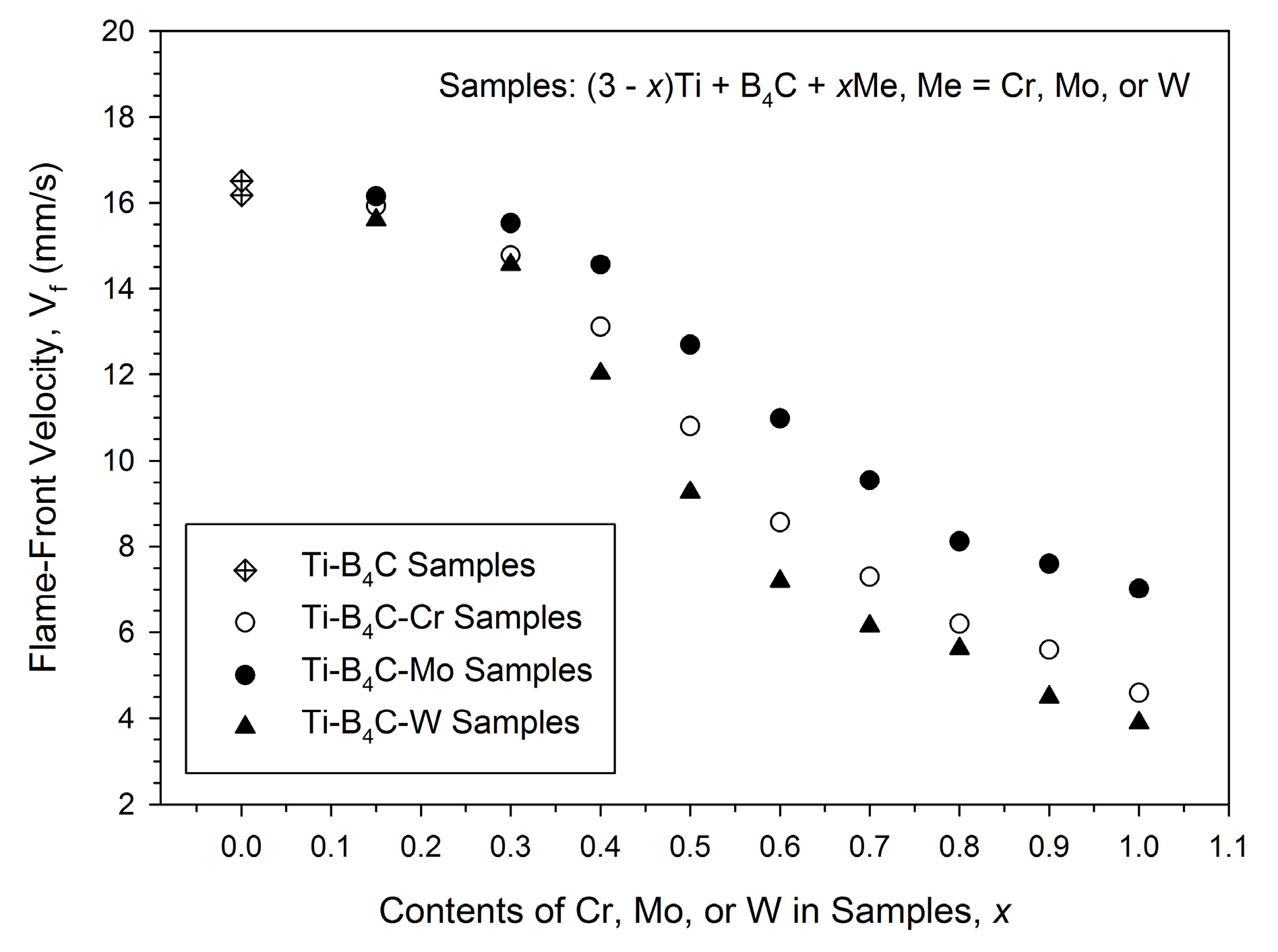
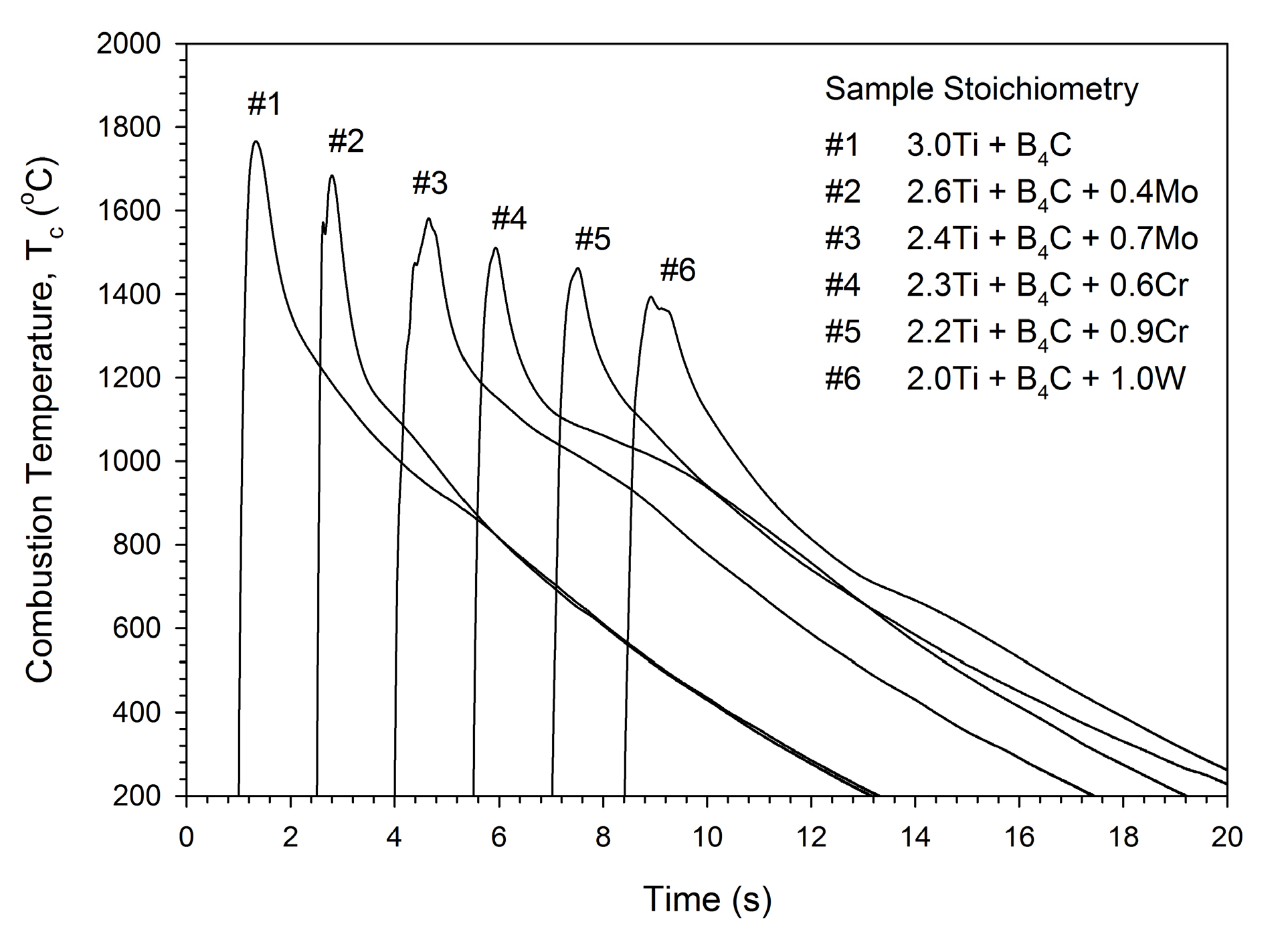
3.1. Combustion Front Velocity and Combustion Temperature
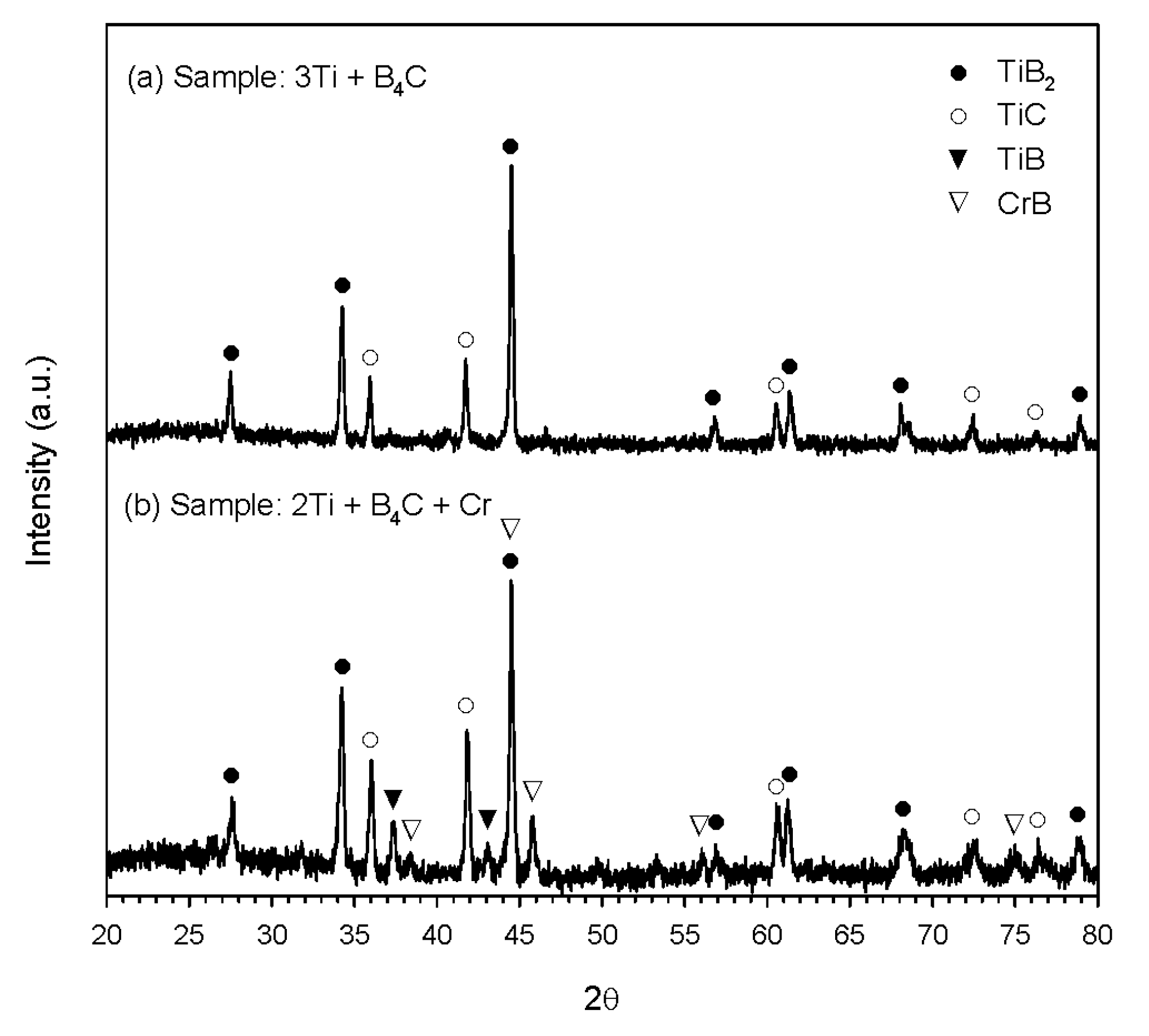
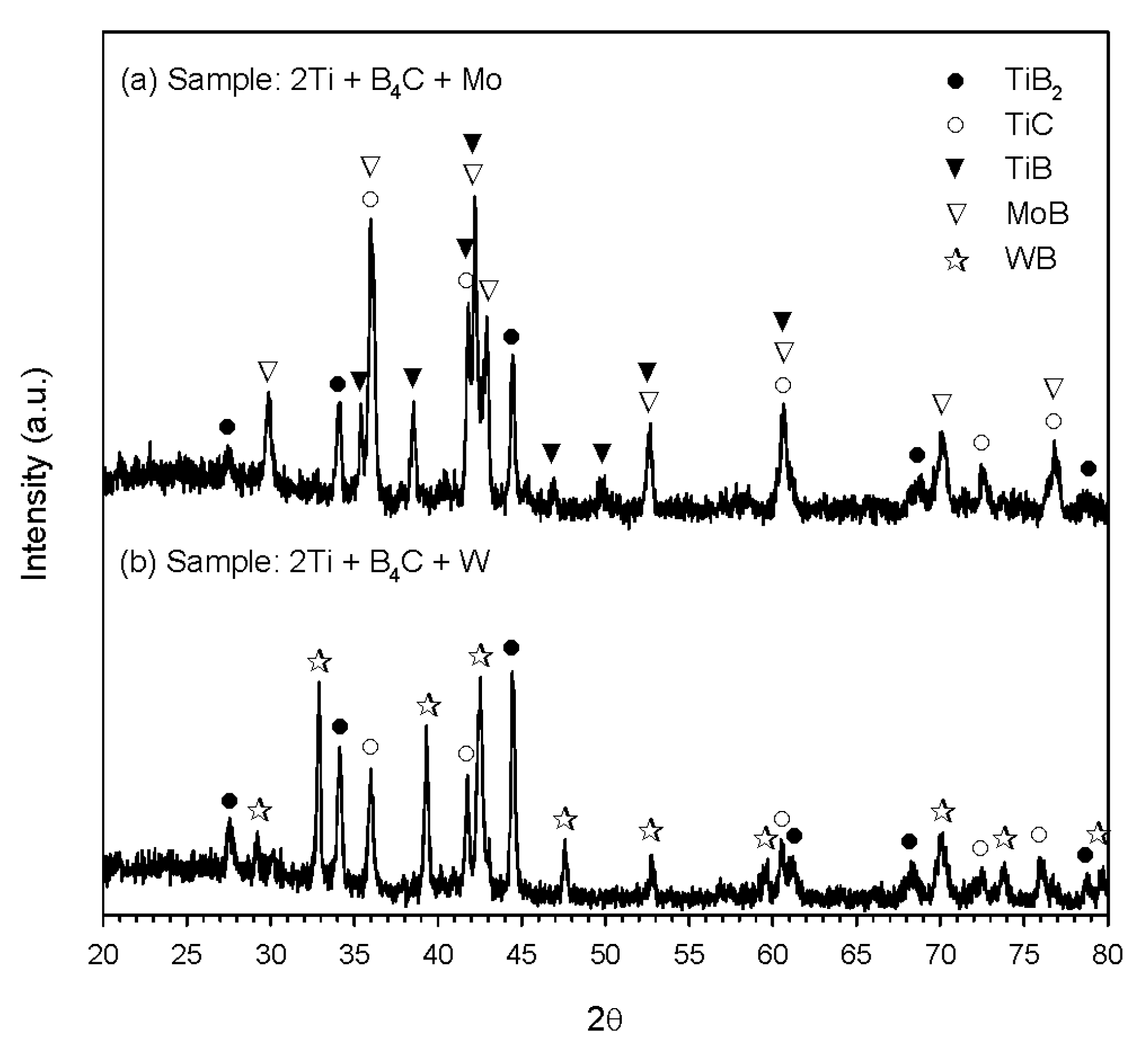
3.2. Phase Constituents of Synthesized Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Chakraborty, S.; Debnath, D.; Mallick, A.R.; Das, P.K. Mechanical and thermal properties of hot pressed ZrB2 system with TiB2. Int. J. Refract. Met. Hard Mater. 2014, 46, 35–42. [Google Scholar] [CrossRef]
- Gürcan, K.; Ayas, E. In-situ synthesis and densification of HfB2 ceramics by the spark plasma sintering technique. Ceram. Int. 2017, 43, 3547–3555. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Huang, C.; Liu, X.; Zou, B.; Zhao, B. Microstructure and mechanical properties of TiC–TiB2 composite cermet tool materials at ambient and elevated temperature. Ceram. Int. 2016, 42, 2717–2723. [Google Scholar] [CrossRef]
- Guo, W.M.; Yang, Z.G.; Zhang, G.J. Comparison of ZrB2–SiC ceramics with Yb2O3 additive prepared by hot pressing and spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2011, 29, 452–455. [Google Scholar] [CrossRef]
- Demirskyi, D.; Vasylkiv, O. Flexural strength behavior of a ZrB2–TaB2 composite consolidated by non-reactive spark plasma sintering at 2300 °C. Int. J. Refract. Met. Hard Mater. 2017, 66, 31–35. [Google Scholar] [CrossRef]
- Demirskyi, D.; Vasylkiv, O. Mechanical properties of SiC–NbB2 eutectic composites by in situ spark plasma sintering. Ceram. Int. 2016, 42, 19372–19385. [Google Scholar] [CrossRef]
- Murthy, T.S.R.Ch.; Sonber, J.K.; Subramanian, C.; Fotedar, R.K.; Gonal, M.R.; Suri, A.K. Effect of CrB2 addition on densification, properties and oxidation resistance of TiB2. Int. J. Refract. Met. Hard Mater. 2009, 27, 976–984. [Google Scholar] [CrossRef]
- Momozawa, A.; Telle, R. Controlled precipitation of W2B4 platelets and of β-WB nanolaminates for the in situ reinforcement of ternary TiB2–W2B4–CrB2 ceramics. J. Eur. Ceram. Soc. 2012, 32, 85–95. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Combustion and explosion processes in physical chemistry and technology of inorganic materials. Russ. Chem. Rev. 2003, 72, 289–310. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Chen, K. Combustion synthesis of refractory and hard materials: A review. Int. J. Refract. Met. Hard Mater. 2013, 39, 90–102. [Google Scholar] [CrossRef]
- Yeh, C.L. Combustion synthesis: Principles and applications. In Reference Module in Materials Science and Materials Engineering; Hashmi., S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–8. [Google Scholar]
- Radev, D.D.; Klissurski, D. Mechanochemical synthesis and SHS of diborides of titanium and zirconium. J. Mater. Synth. Process. 2001, 9, 131–136. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. A comparative study on combustion synthesis of Nb-B compounds. J. Alloy. Compd. 2006, 422, 78–85. [Google Scholar] [CrossRef]
- Yeh, C.L.; Wang, H.J. A comparative study on combustion synthesis of Ta-B compounds. Ceram. Int. 2011, 37, 1569–1573. [Google Scholar] [CrossRef]
- Dunmead, S.D.; Readey, D.W.; Semler, C.E.; Holt, J.B. Kinetics of combustion synthesis in the Ti-C and Ti-C-Ni systems. J. Am. Ceram. Soc. 1989, 72, 2318–2324. [Google Scholar] [CrossRef]
- Yeh, C.L.; Liu, E.W. Combustion synthesis of tantalum carbides TaC and Ta2C. J. Alloy. Compd. 2006, 415, 66–72. [Google Scholar] [CrossRef]
- Vallauri, D.; Atías Adrián, I.C.; Chrysanthou, A. TiC-TiB2 composites: A review of phase relationships, processing and properties. J. Eur. Ceram. Soc. 2008, 28, 1697–1713. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Sun, S.; Zhu, X.; Tu, G. Fabrication and characterization of TiB2/TiC composites. Int. J. Refract. Met. Hard Mater. 2014, 45, 95–101. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, Y.L. Combustion synthesis of TiC-TiB2 composites. J. Alloy. Compd. 2008, 463, 373–377. [Google Scholar] [CrossRef]
- Shen, P.; Zou, B.; Jin, S.; Jiang, Q. Reaction mechanism in self-propagating high temperature synthesis of TiC-TiB2/Al composites from an Al-Ti-B4C system. Mater. Sci. Eng. A 2007, 454–455, 300–309. [Google Scholar] [CrossRef]
- Liang, Y.H.; Wang, H.Y.; Yang, Y.F.; Zhao, R.Y.; Jiang, Q.C. Effect of Cu content on the reaction behaviors of self-propagating high-temperature synthesis in Cu-Ti-B4C system. J. Alloy. Compd. 2008, 462, 113–118. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhao, R.Y.; Liang, Y.H.; Jiang, Q.C. Effect of Ni content on the reaction behaviors of self-propagating high-temperature synthesis in the Al-Ti-B4C system. Int. J. Refract. Met. Hard Mater. 2008, 26, 77–83. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Zhang, L.; Wu, J. The effects of ultra-high-gravity field on phase transformation and microstructure evolution of the TiC-TiB2 ceramics fabricated by combustion synthesis. Int. J. Refract. Met. Hard Mater. 2014, 43, 1–6. [Google Scholar] [CrossRef]
- Deorsola, F.A.; Atias Adrian, I.C.; Ortigoza Villalba, G.A.; DeBenedatti, B. Nanostructured TiC-TiB2 composites obtained by adding carbon nanotubes into the self-propagating high-temperature synthesis process. Mater. Res. Bull. 2011, 46, 995–999. [Google Scholar] [CrossRef]
- Talako, T.; Ilyuschenko, A.; Letsko, A. SHS powders for thermal spray coating. KONA Powder Part. J. 2009, 27, 55–72. [Google Scholar] [CrossRef]
- Licheri, R.; Orrù, R.; Cao, G.; Crippa, A.; Scholz, R. Self-propagating combustion synthesis and plasma spraying deposition of TiC-Fe powders. Ceram. Int. 2003, 29, 519–526. [Google Scholar] [CrossRef]
- Xu, J.; Zou, B.; Zhao, S.; Hui, Y.; Huang, W.; Zhou, X.; Wang, Y.; Cai, X.; Cao, X. Fabrication and properties of ZrC–ZrB2/Ni cermet coatings on a magnesium alloy by atmospheric plasma spraying of SHS powders. Ceram. Int. 2014, 40, 15537–15544. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Marinou, A.; Vekinis, G.; Lekatou, A.; Vardavoulias, M. Ni-Al and NiO-Al composite coatings by combustion-assisted flame spraying. Coatings 2014, 4, 231–252. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Marinou, A.; Karanasios, K.; Vekinis, G. Combustion synthesis during flame spraying (“CAFSY”) for the production of catalysts on substrates. Coatings 2017, 7, 14. [Google Scholar] [CrossRef]
- Contreras, L.; Turrillas, X.; Vaughan, G.B.M.; Kvick, Å.; Rodríguez, M.A. Time-resolved XRD study of TiC-TiB2 composites obtained by SHS. Acta Mater. 2004, 52, 4783–4790. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, Y.L. An experimental study on self-propagating high-temperature synthesis in the Ta-B4C system. J. Alloy Compd. 2009, 478, 163–167. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Wiley-VCH Verlag GmbH: Weinheim, NY, USA, 2002. [Google Scholar]
- Hildenbrand, D.L.; Hall, W.F. The decomposition pressure of boron carbide and the heat of sublimation of boron. J. Phys. Chem. 1964, 68, 989–993. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Lin, W.-Z. Combustion Synthesis of UHTC Composites from Ti–B4C Solid State Reaction with Addition of VIb Transition Metals. Coatings 2017, 7, 73. https://doi.org/10.3390/coatings7060073
Yeh C-L, Lin W-Z. Combustion Synthesis of UHTC Composites from Ti–B4C Solid State Reaction with Addition of VIb Transition Metals. Coatings. 2017; 7(6):73. https://doi.org/10.3390/coatings7060073
Chicago/Turabian StyleYeh, Chun-Liang, and Wei-Zuo Lin. 2017. "Combustion Synthesis of UHTC Composites from Ti–B4C Solid State Reaction with Addition of VIb Transition Metals" Coatings 7, no. 6: 73. https://doi.org/10.3390/coatings7060073





