Electrodeposition of Vanadium Oxides at Room Temperature as Cathodes in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
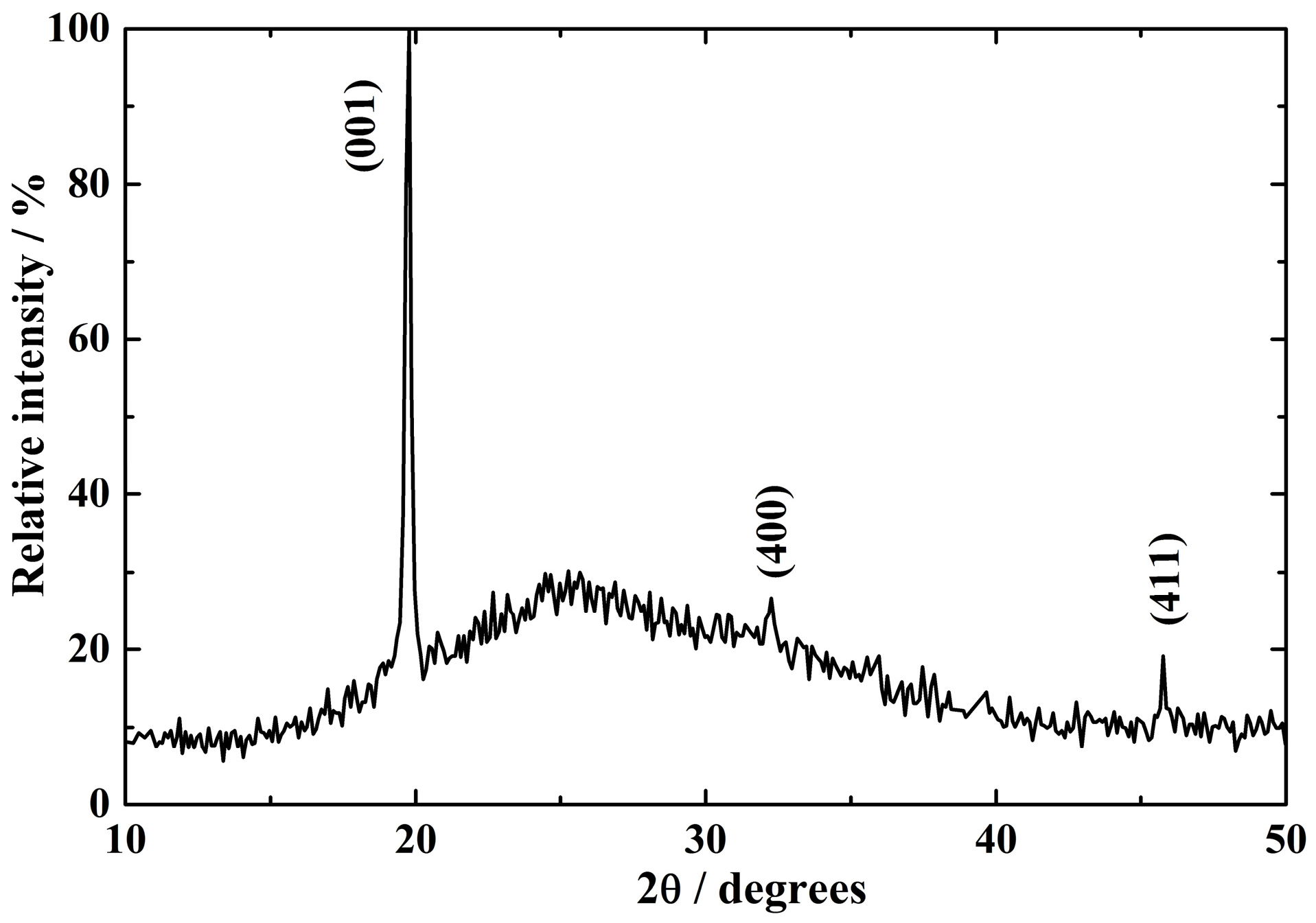
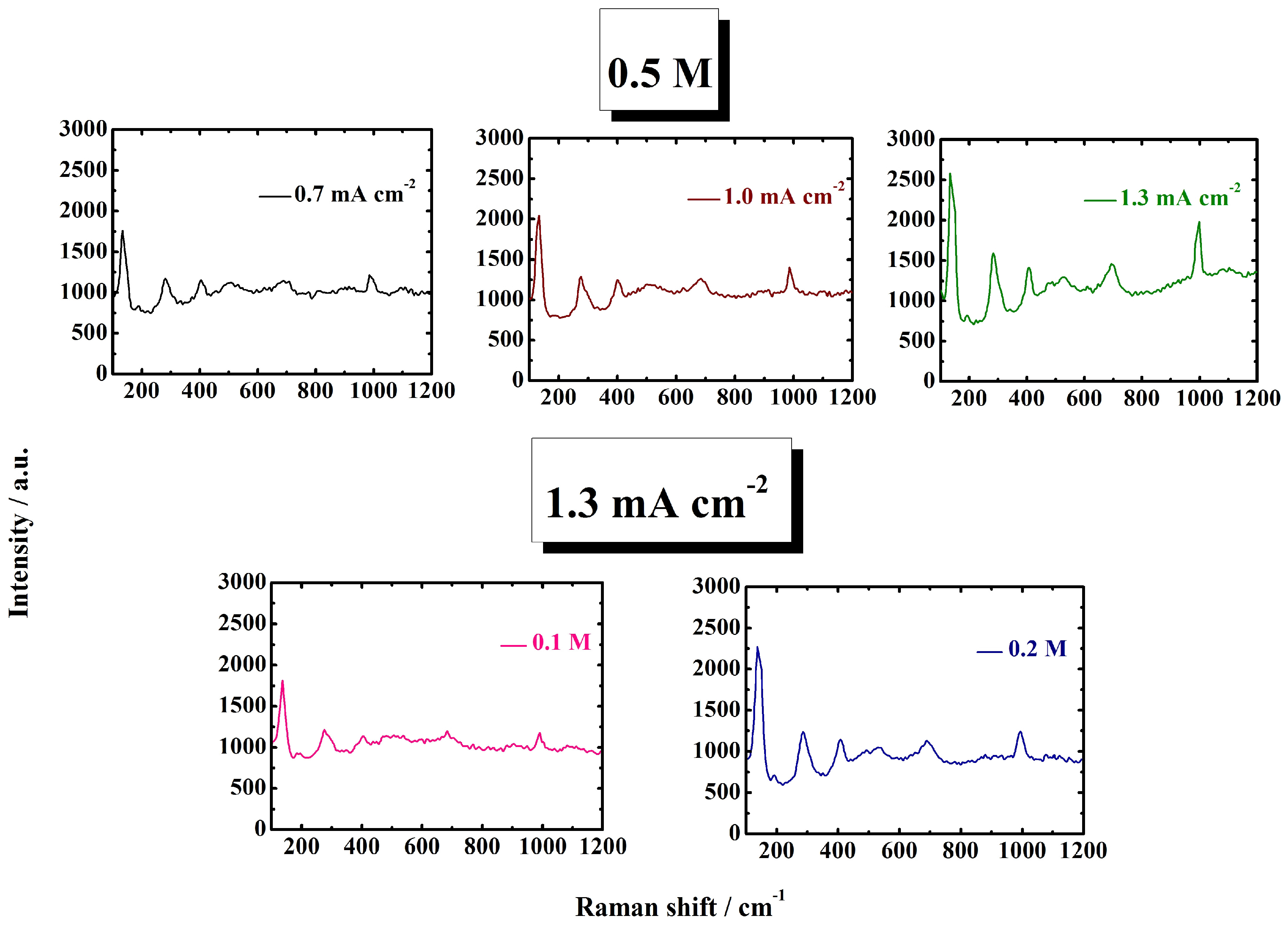
3.1. Structure
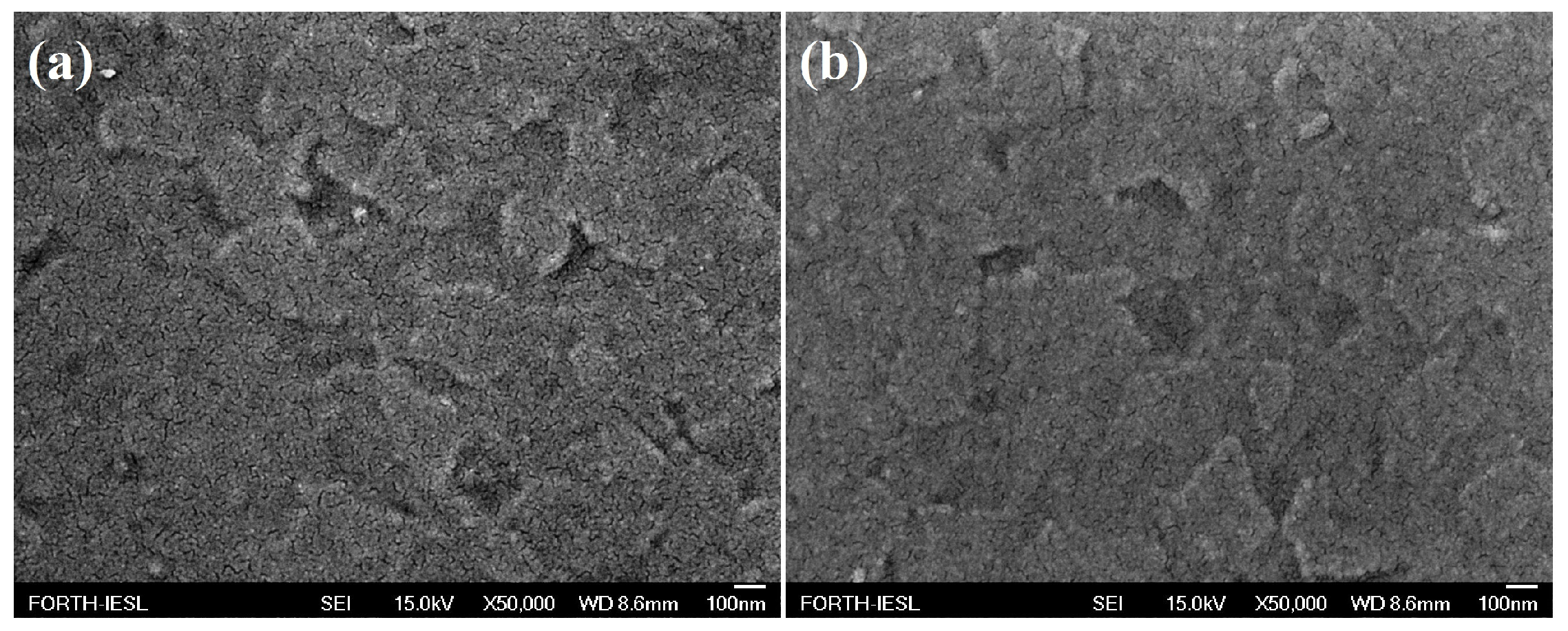
3.2. Morphology
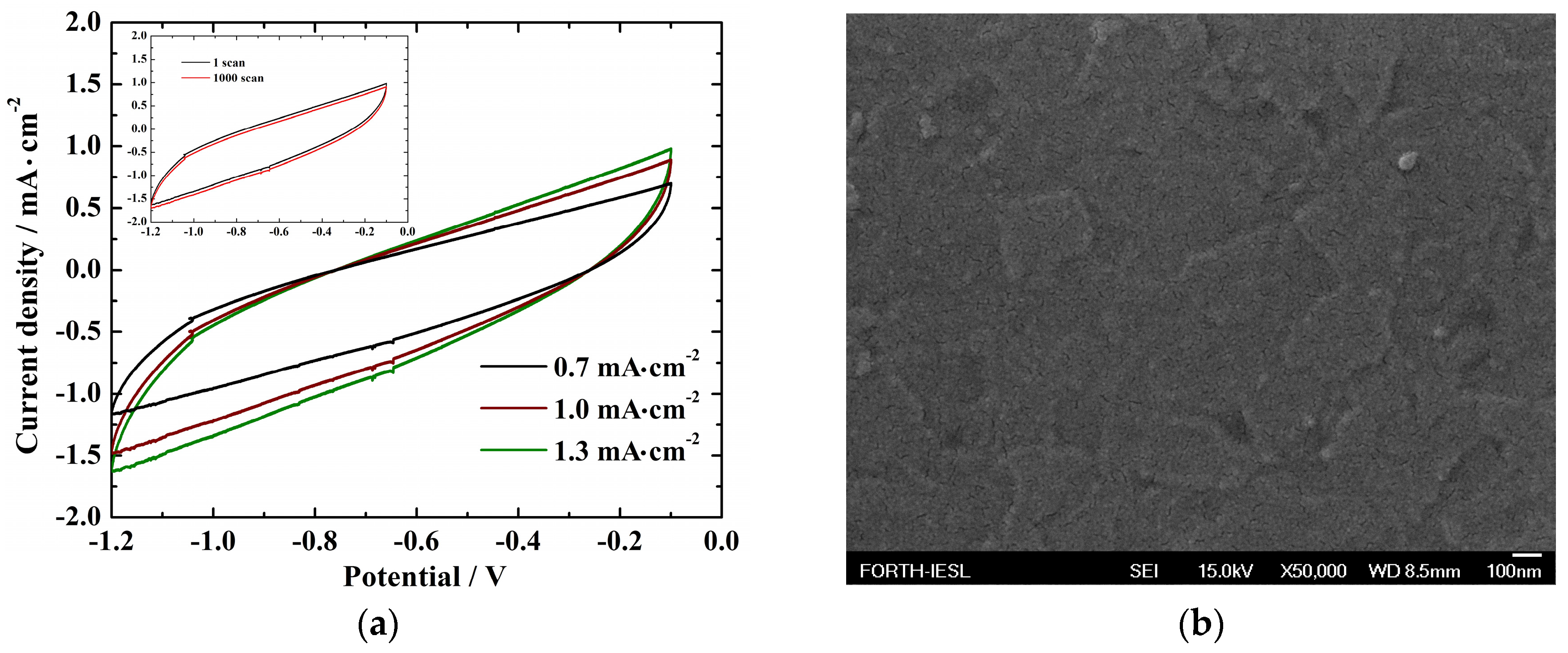
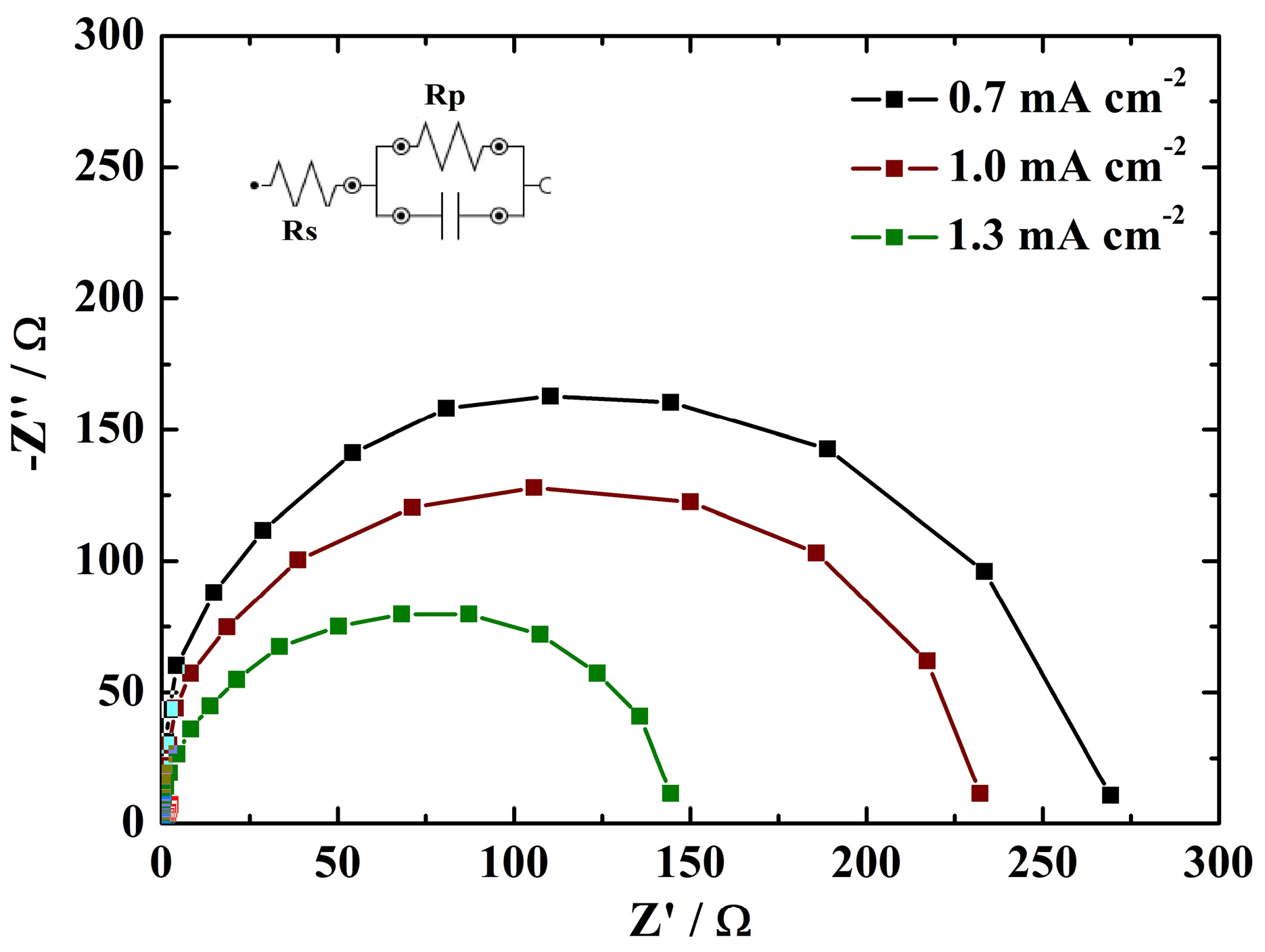
3.3. Electrochemical Performance
Cyclic Voltammetry
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Aberg, B.; Carrizosa, S.B.; Dimakis, N. Vanadium pentoxide nanobelt-reduced graphene oxide nanosheet as high-performance pseudocapacitive electrodes: AC impedance spectroscopy data modeling and theoretical calculations. Materials 2016, 9, 615. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Liu, X.; Sun, Q.; Djuri, A.B.; Xie, M.; Mei, Y.; Tang, C.Y.; Shih, K. Template-free synthesis of hierarchical hollow V2O5 microspheres with highly stable lithium storage capacity. RSC Adv. 2017, 7, 2480–2485. [Google Scholar] [CrossRef]
- Cao, A.M.; Hu, J.S.; Liang, H.P.; Wan, L.J. Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries. Angew. Chem. Int. Ed. 2005, 44, 4391–4395. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; McNulty, D.; Geaney, H.; O’Dwyer, C. Electrodeposited structurally stable V2O5 inverse opal networks as high performance thin film lithium batteries. ACS Appl. Mater. Inter. 2015, 7, 27006–27015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qi, L. Recent progress in self-supported metal oxide nanoarray electrodes for advanced lithium-ion batteries. Adv. Sci. 2016, 3, 1600049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, D.; Wang, L.; Zhang, X. Facile and low-cost synthesis of large-area pure V2O5 nanosheets for high-capacity and high-rate lithium storage over a wide temperature range. Chem. Plus. Chem. 2012, 77, 124–128. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Uchaker, E.; Yang, J.; Huang, Y.; Zhang, M.; Cao, G. Leaf-like V2O5 nanosheets fabricated by a facile green approach as high energy material for lithium-ion batteries. Adv. Energy Mater. 2013, 3, 1171–1175. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Burke, D.M.; Gabriel, T.; O’Regan, C.; O’Dwyer, C.; Petkov, N.; Holmes, J.D. Carbon nanocage supported synthesis of V2O5 nanorods and V2O5/TiO2 nanocomposites for Li-ion batteries. J. Mater. Chem. A 2013, 1, 12568–12578. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Pamesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Xiong, W.L. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Liu, Y.Y.; Garcia, B.B.; Zhang, Q.F.; Pan, A.Q.; Jeong, Y.H.; Cao, G.Z. V2O5 xerogel electrodes with much enhanced lithium-ion intercalation properties with N2 annealing. J. Mater. Chem. 2009, 19, 8789–8795. [Google Scholar] [CrossRef]
- Khoo, E.; Wang, J.M.; Ma, J.; Lee, P.S. Electrochemical energy storage in a β-Na0.33V2O5 nanobelt and its application for supercapacitors. J. Mater. Chem. 2010, 20, 8368–8374. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, C.-K.; Lee, S.-W.; Li, H.-Y.; Chang, J.-K.; Deng, M.-J. Nanostructured Na-doped vanadium oxide synthesized using an anodic deposition technique for supercapacitor applications. J. Alloy Compd. 2012, 536, S428–S431. [Google Scholar] [CrossRef]
- Drosos, H.; Sapountzis, A.; Koudoumas, E.; Katsarakis, N.; Vernardou, D. Effect of deposition current density on electrodeposited vanadium oxide coatings. J. Electrochem. Soc. 2012, 159, E145–E147. [Google Scholar] [CrossRef]
- Vernardou, D.; Sapountzis, A.; Spanakis, E.; Kenanakis, G.; Koudoumas, E.; Katsarakis, N. Electrochemical activity of electrodeposited V2O5 coatings. J. Electrochem. Soc. 2013, 160, D6–D9. [Google Scholar] [CrossRef]
- Louloudakis, D.; Vernardou, D.; Spanakis, E.; Katsarakis, N.; Koudoumas, E. Electrochemical properties of vanadium oxide coatings grown by APCVD on glass substrates. Surf. Coat. Technol. 2013, 230, 186–189. [Google Scholar] [CrossRef]
- Vernardou, D.; Louloudakis, D.; Spanakis, E.; Katsarakis, N.; Koudoumas, E. Electrochemical properties of vanadium oxide coatings grown by hydrothermal synthesis on FTO substrates. New J. Chem. 2014, 38, 1959–1964. [Google Scholar] [CrossRef]
- Vernardou, D.; Apostolopoulou, M.; Louloudakis, D.; Katsarakis, N.; Koudoumas, E. Hydrothermally grown β-V2O5 electrode at 95 °C. J. Colloid. Interf. Sci. 2014, 424, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Huang, C.K.; Wang, Y.; Fan, Y.C.; Lu, B.A.; Lan, W.; Wang, Y.Y.; Liu, X.Q. Formation of vanadium oxides with various morphologies by chemical vapor deposition. J. Alloys Comp. 2009, 475, 518–523. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cheong, H.M.; Je Seong, M.; Liu, P.; Tracy, C.E.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman spectroscopic studies of amorphous vanadium oxide thin films. Solid State Ionics 2003, 165, 111–116. [Google Scholar] [CrossRef]
- Abello, L.; Husson, E.; Repelin, Y.; Lucazeau, G. Vibrational spectra and valence force field of crystalline V2O5. Spectrochim. Acta A-M. 1983, 39, 641–651. [Google Scholar] [CrossRef]
- Julien, C.; Nazri, G.A.; Bergström, O. Raman scattering studies of microcrystalline V6O13. Phys. Status Solidi 1997, 201, 319–326. [Google Scholar] [CrossRef]
- Jehng, J.M.; Hardcastle, F.D.; Wachs, I.E. The interaction of V2O5 and Nb2O5 with oxide surface. Solid State Ionics 1989, 32/33, 904–910. [Google Scholar] [CrossRef]
- Ng, S.H.; Chew, S.Y.; Wang, J.; Wexler, D.; Tournayre, Y.; Konstantinov, K.; Liu, H.K. Synthesis and electrochemical properties of V2O5 nanostructures prepared via a precipitation process for lihium-ion battery cathodes. J. Power Sources 2007, 174, 1032–1035. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Y.; Qiu, H.; Gao, Y.; Wang, C.; Du, F.; Wei, Y.; Chen, G. Facile synthesis of V2O5 nanoparticles as a capable cathode for high energy lithium-ion batteries. J. Alloy Comp. 2015, 650, 370–373. [Google Scholar] [CrossRef]
- Uchaker, E.; Zhou, N.; Li, Y.; Cao, G. Polyol-mediated solvothermal synthesis and electrochemical performance of nanostructured V2O5 hollow microspheres. J. Phys. Chem. C 2013, 117, 1621–1625. [Google Scholar] [CrossRef]
- Tartaj, P.; Amarilla, J.M.; Vazquez-Santos, M.B. Surfactant-free vanadium oxides from reverse micelles and organic oxidants: Solution processable nanoribbons with potential applicability as battery insertion electrodes assembled in different configurations. Langmuir 2015, 31, 12489–12496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Zhang, Q.; Zhou, N.; Uchaker, E.; Cao, G. Porous nanostructured V2O5 film electrode with excellent Li-ion intercalation properties. Electrochem. Commun. 2011, 13, 1276–1279. [Google Scholar] [CrossRef]
- Qin, J.; Lv, Z.; Li, Z.; Li, B.; Kang, F.; Yang, Q.-H. An interlaced silver vanadium oxide-graphene hybrid with high structural stability for use in lithium ion batteries. Chem. Commun. 2014, 50, 13447–13450. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, P.; Zhao, X.; Reddy, M.V.; Peng, S.; Chowdari, B.V.R.; Ramakrishna, S. Highly improved rechargeable stability for lithium/silver vanadium oxide battery induced via electrospinning technique. J. Mater. Chem. A 2013, 1, 852–859. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasoulis, M.; Vernardou, D. Electrodeposition of Vanadium Oxides at Room Temperature as Cathodes in Lithium-Ion Batteries. Coatings 2017, 7, 100. https://doi.org/10.3390/coatings7070100
Rasoulis M, Vernardou D. Electrodeposition of Vanadium Oxides at Room Temperature as Cathodes in Lithium-Ion Batteries. Coatings. 2017; 7(7):100. https://doi.org/10.3390/coatings7070100
Chicago/Turabian StyleRasoulis, Michalis, and Dimitra Vernardou. 2017. "Electrodeposition of Vanadium Oxides at Room Temperature as Cathodes in Lithium-Ion Batteries" Coatings 7, no. 7: 100. https://doi.org/10.3390/coatings7070100




