Corrosion Protection of Steel by Epoxy-Organoclay Nanocomposite Coatings
Abstract
:1. Introduction
2. Materials and Methods
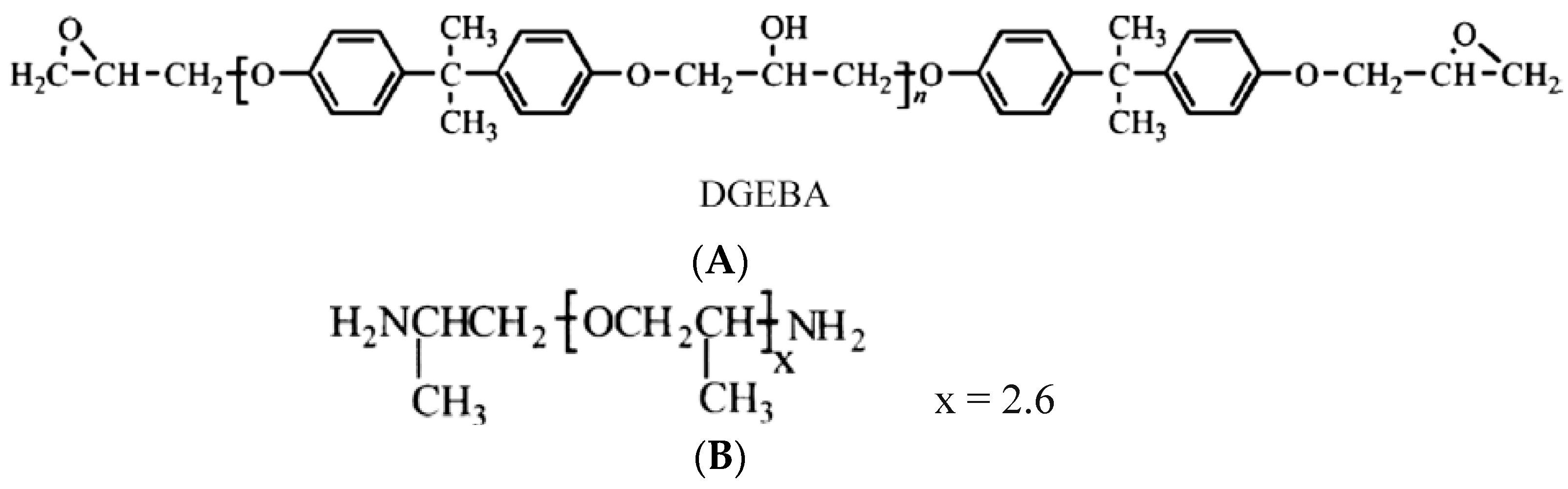
2.1. Materials and Epoxy Coatings
2.2. Characterization and Testing of Epoxy Polymer and Nanocomposites
2.3. Characterization of Coated Specimens and Protective Properties
3. Results and Discussion
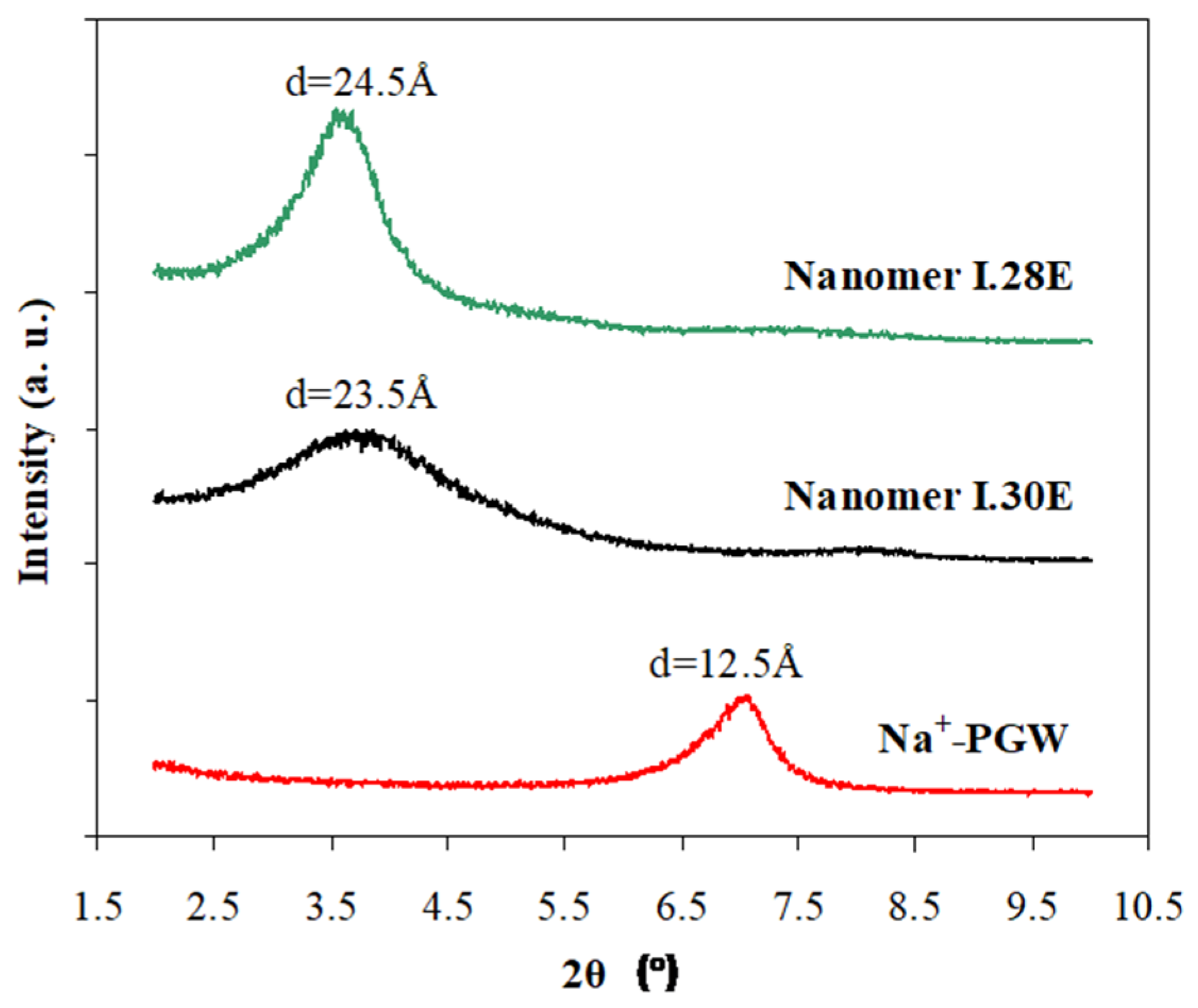
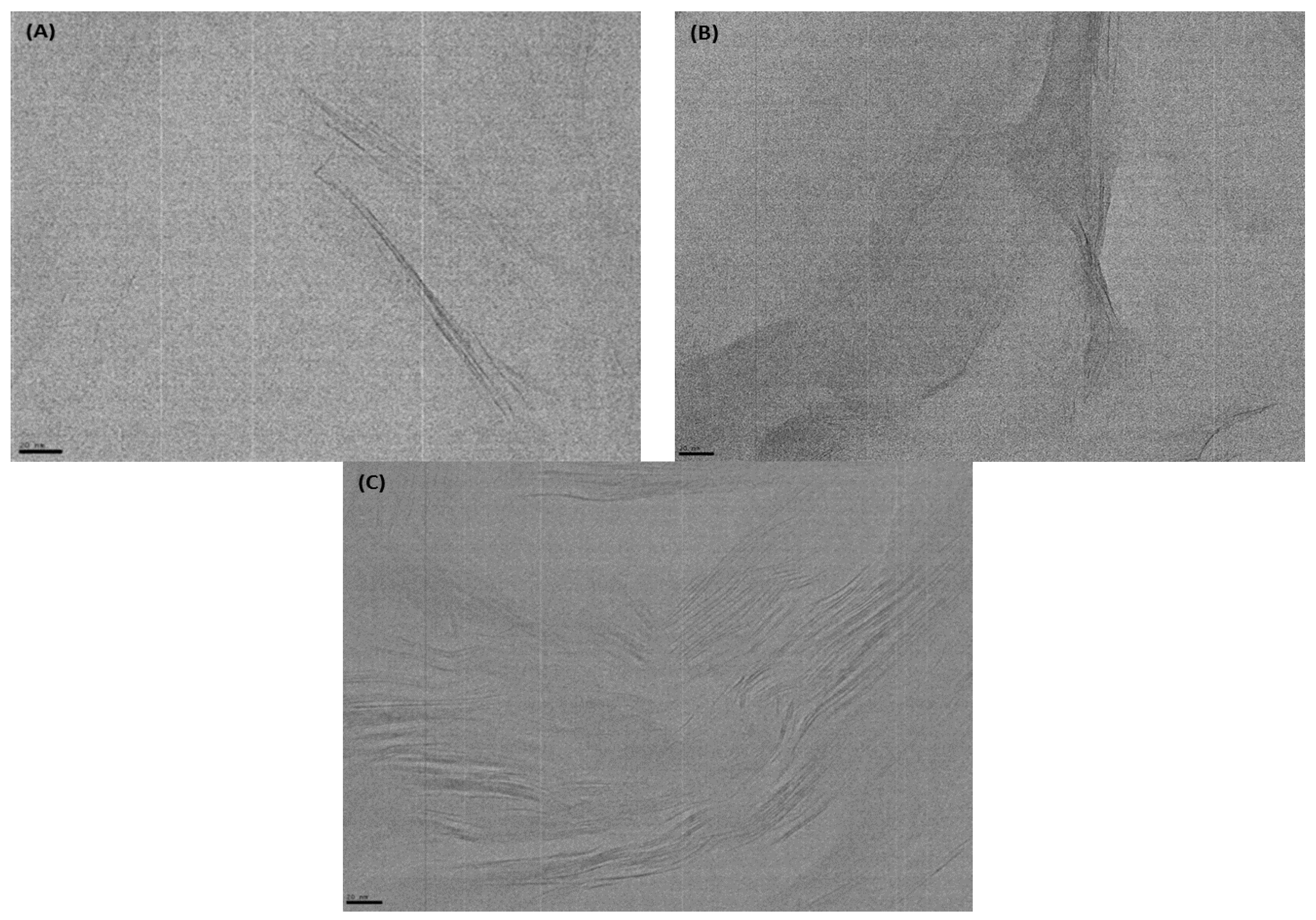
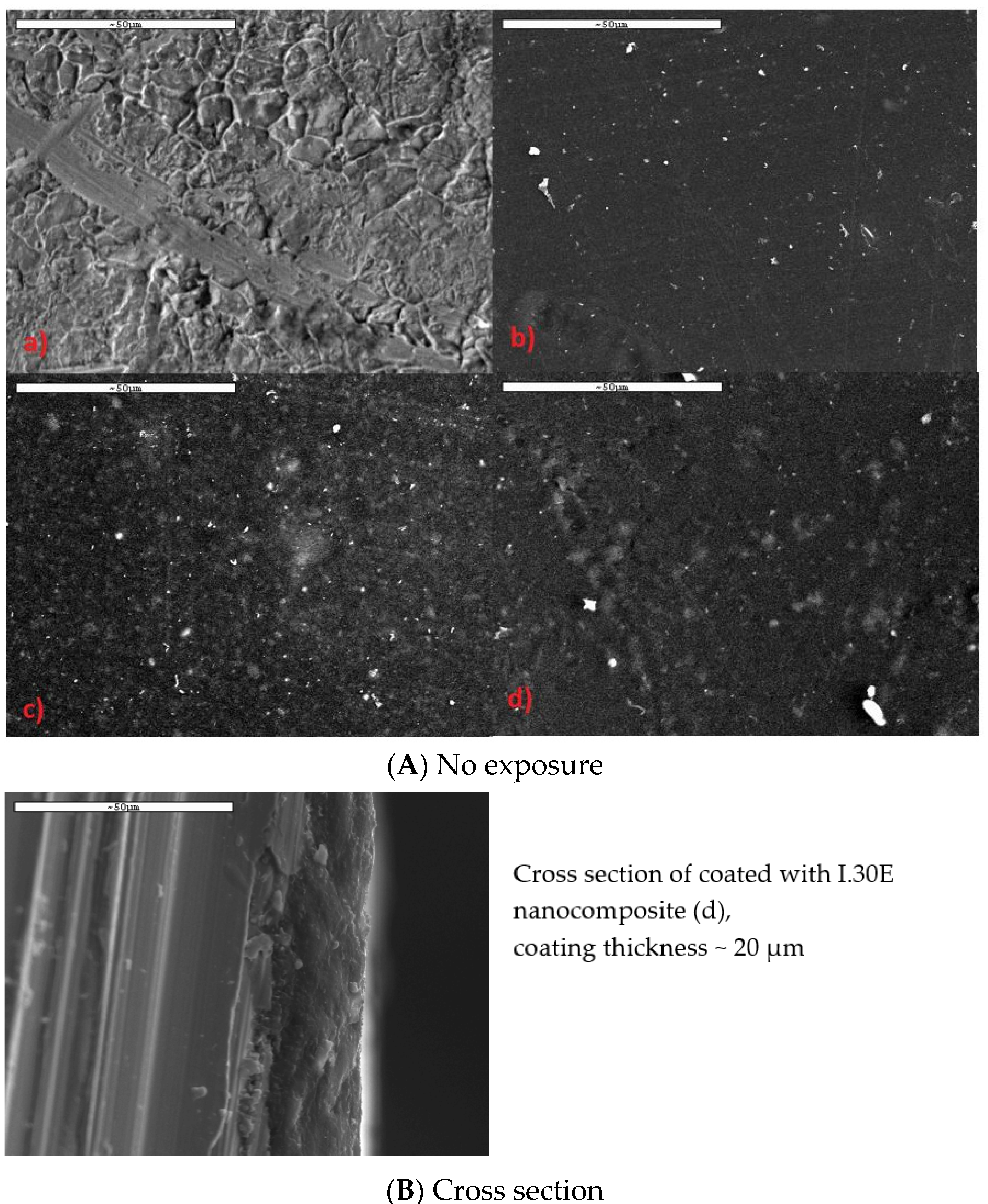
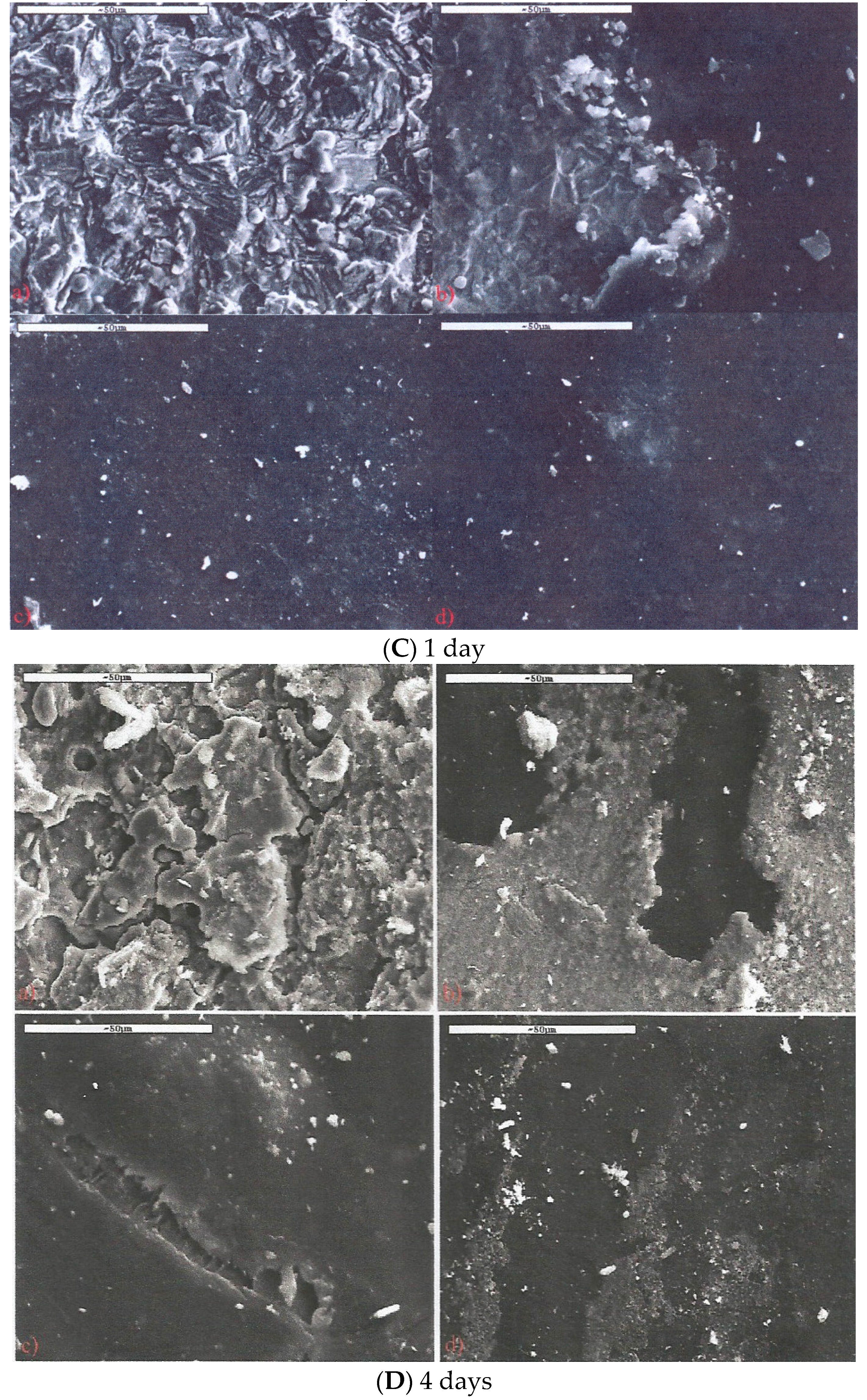
3.1. Structure and Morphology of Organo-Clays and Epoxy-Clay Nanocomposites
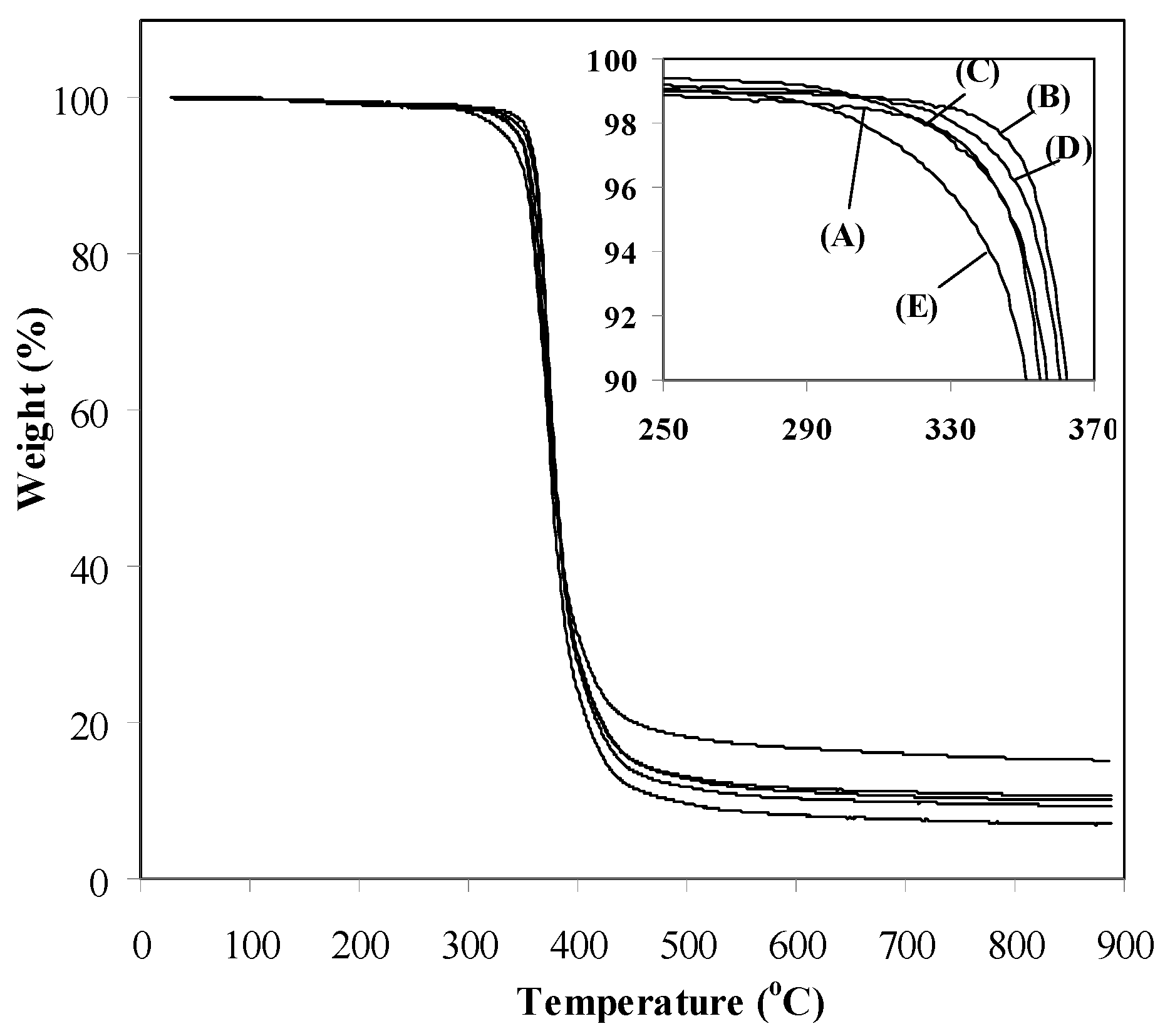
3.2. Properties of Epoxy-Clay Nanocomposites
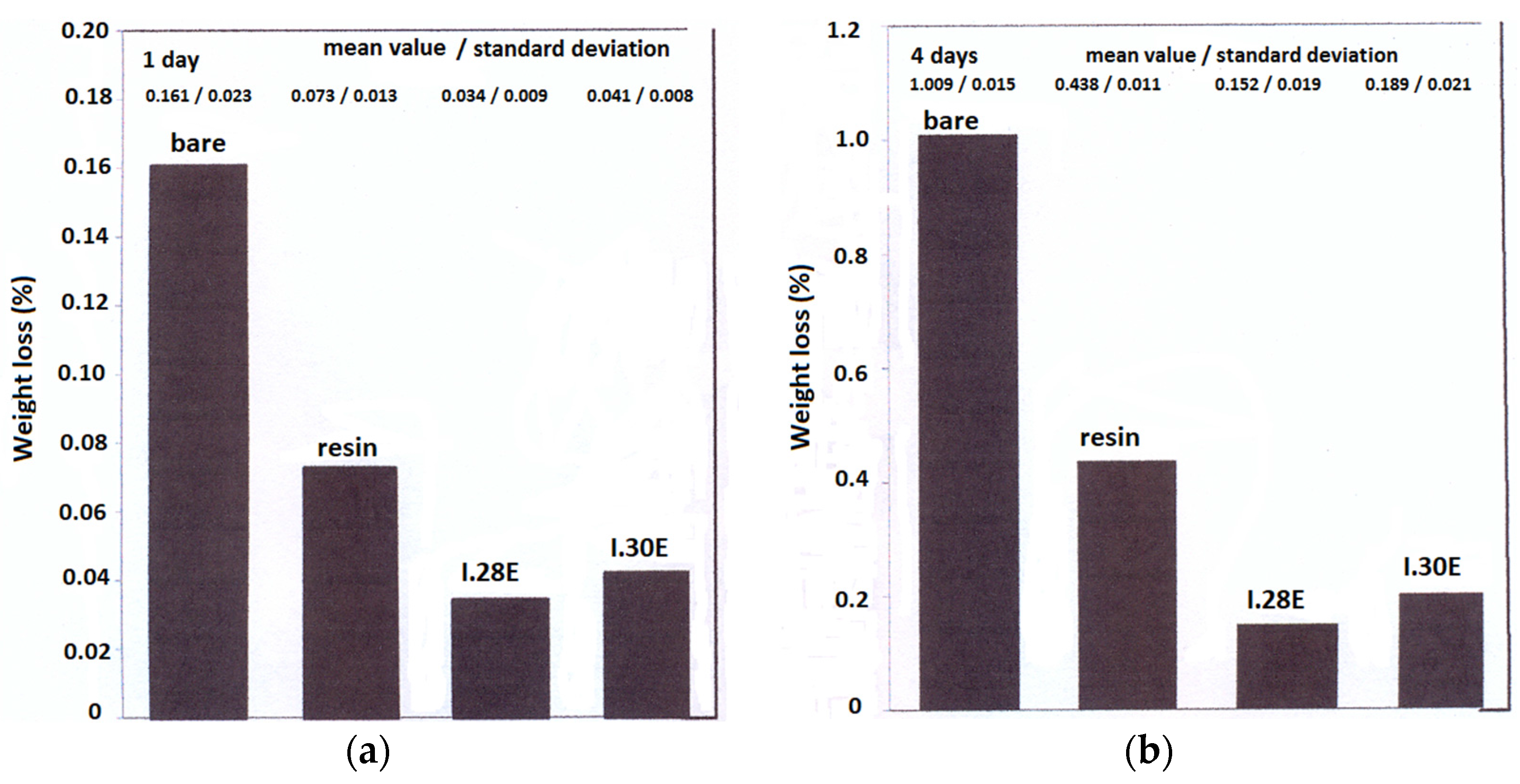
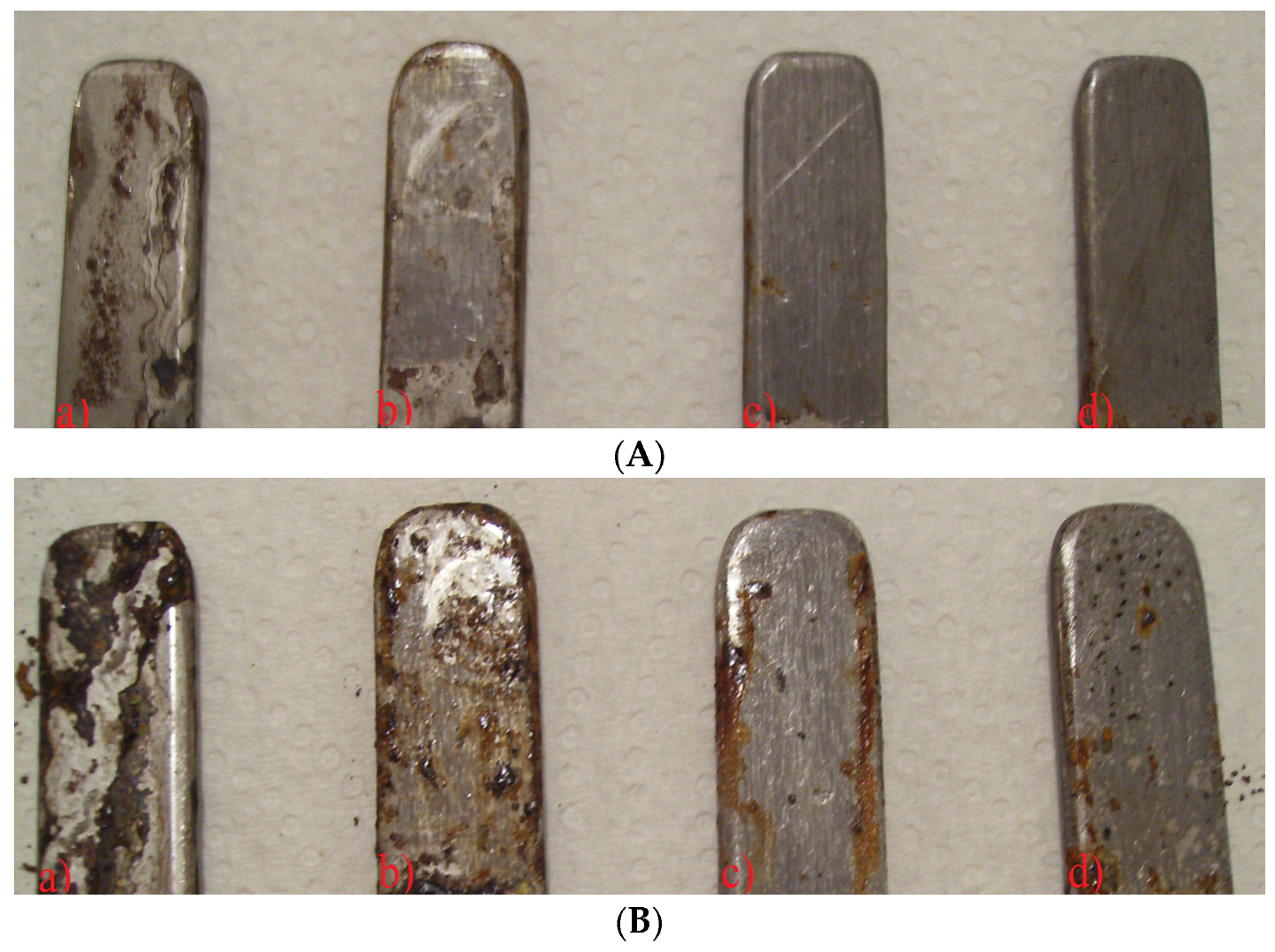
3.3. Salt Spray Corrosion Tests
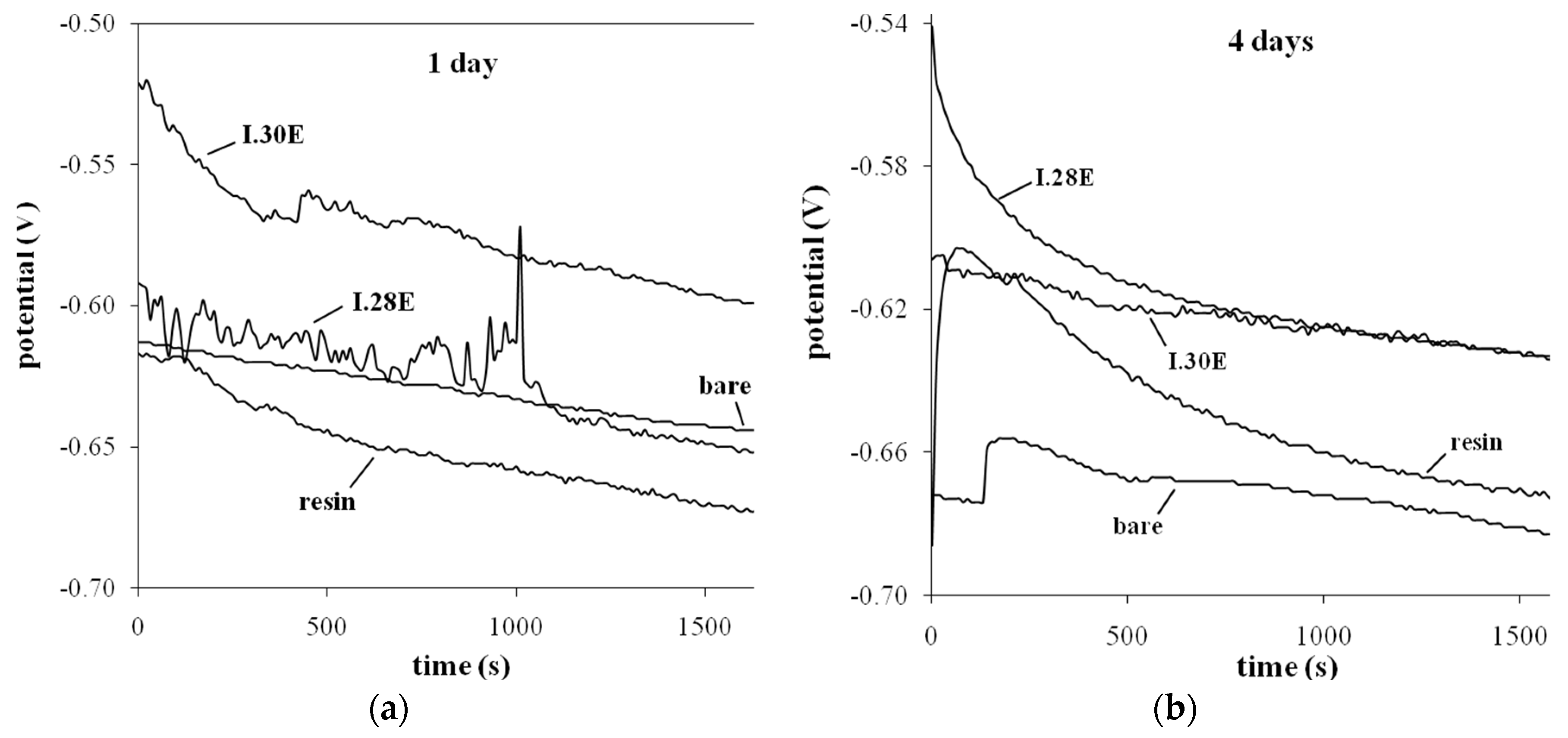
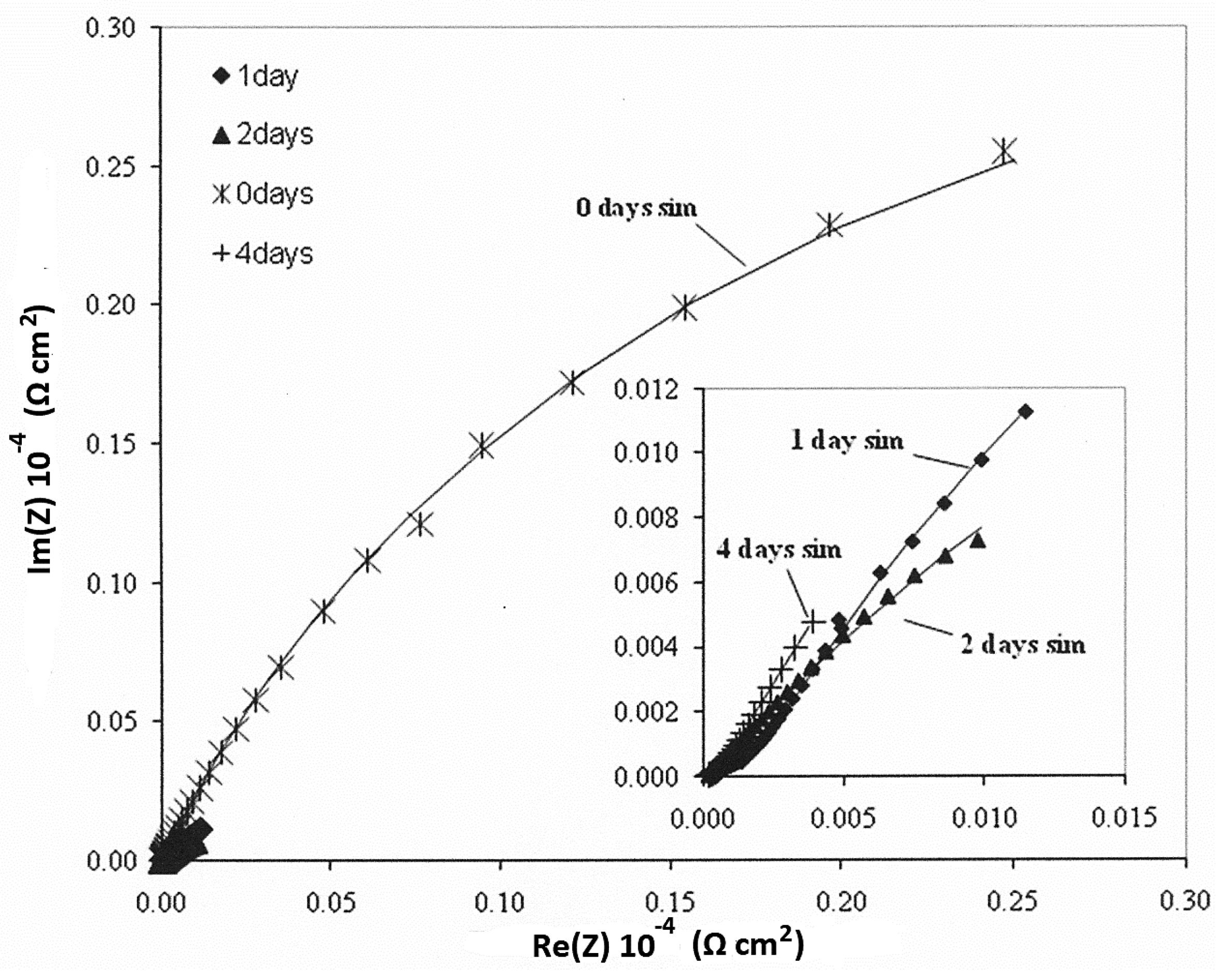
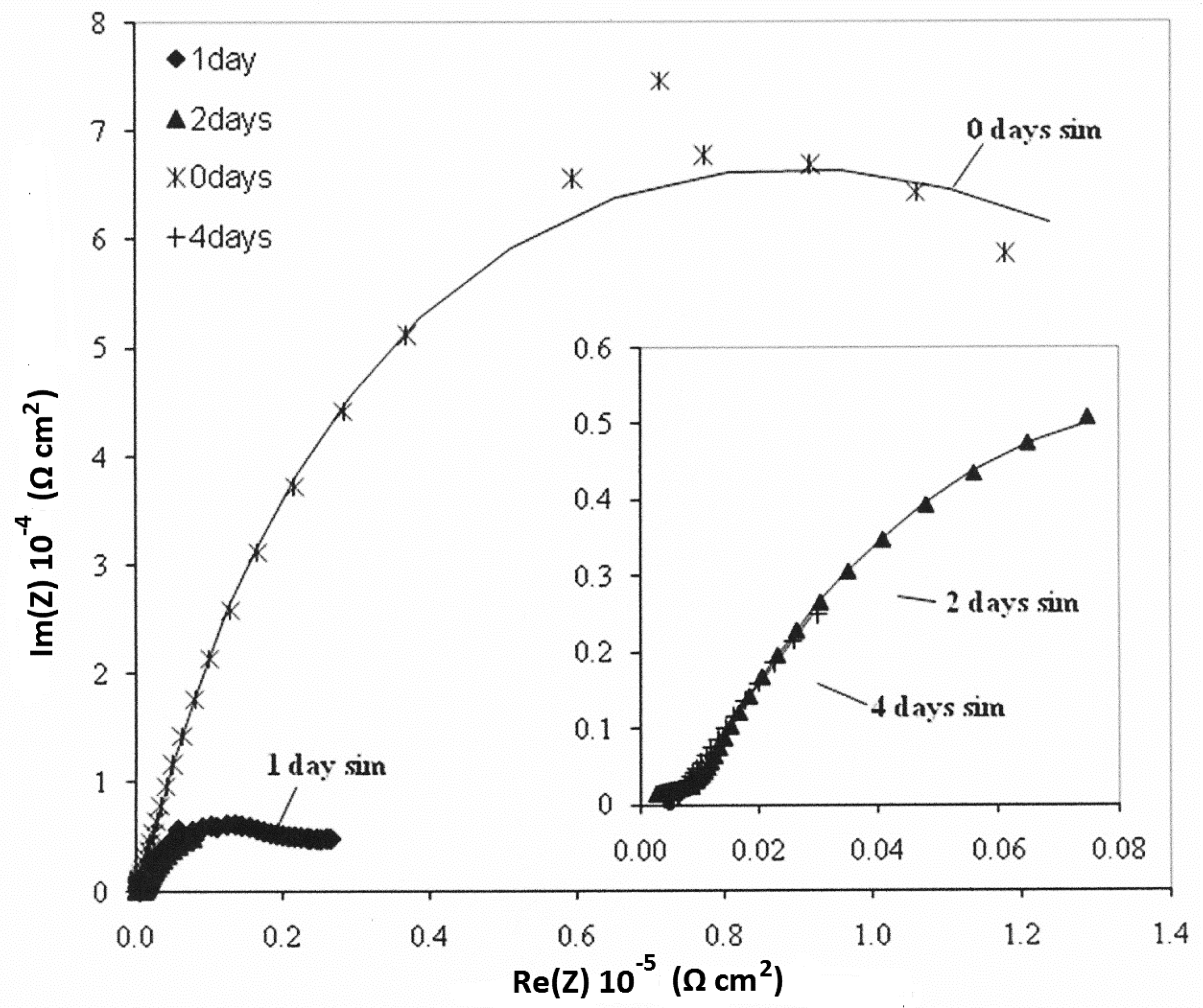
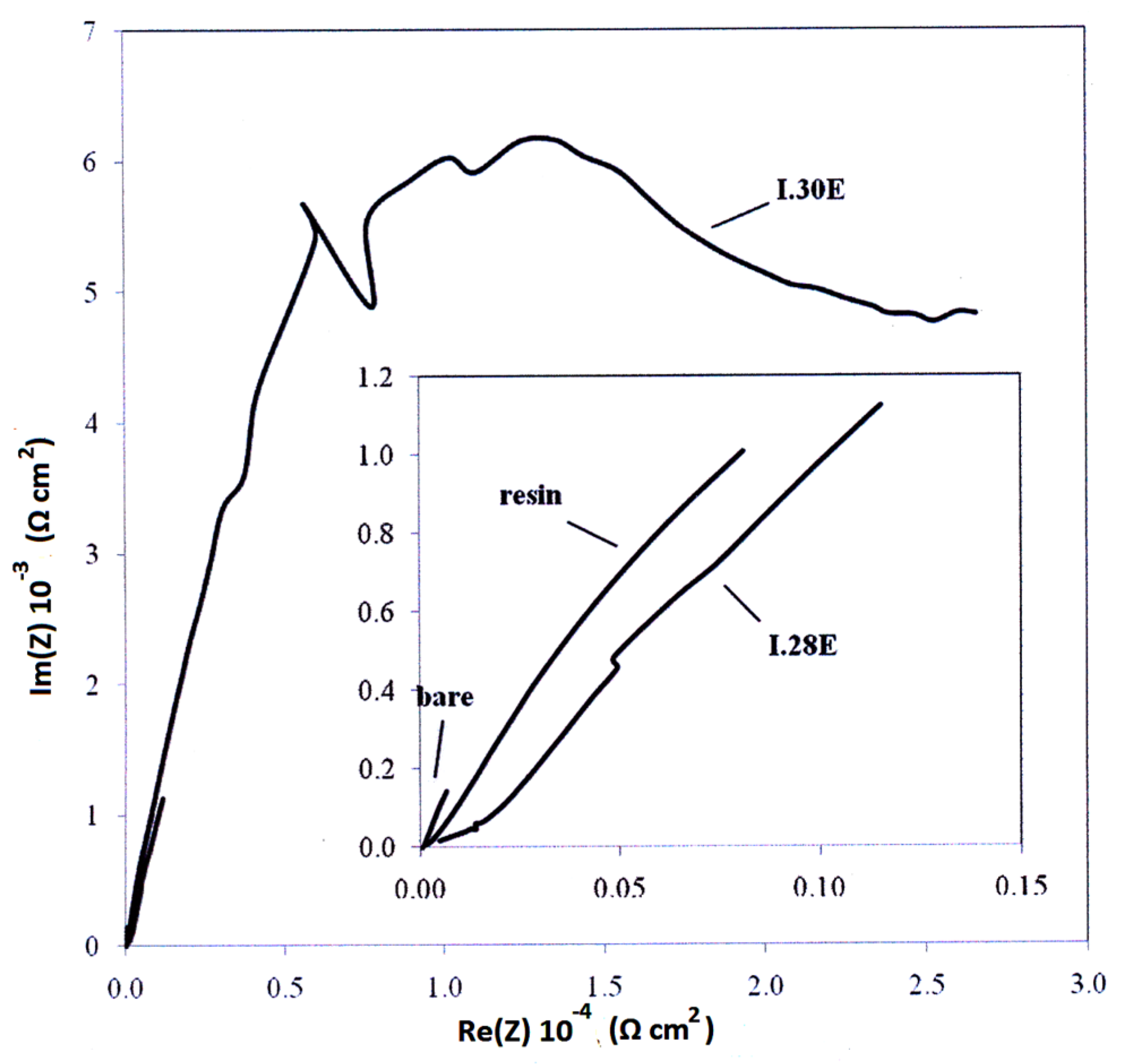
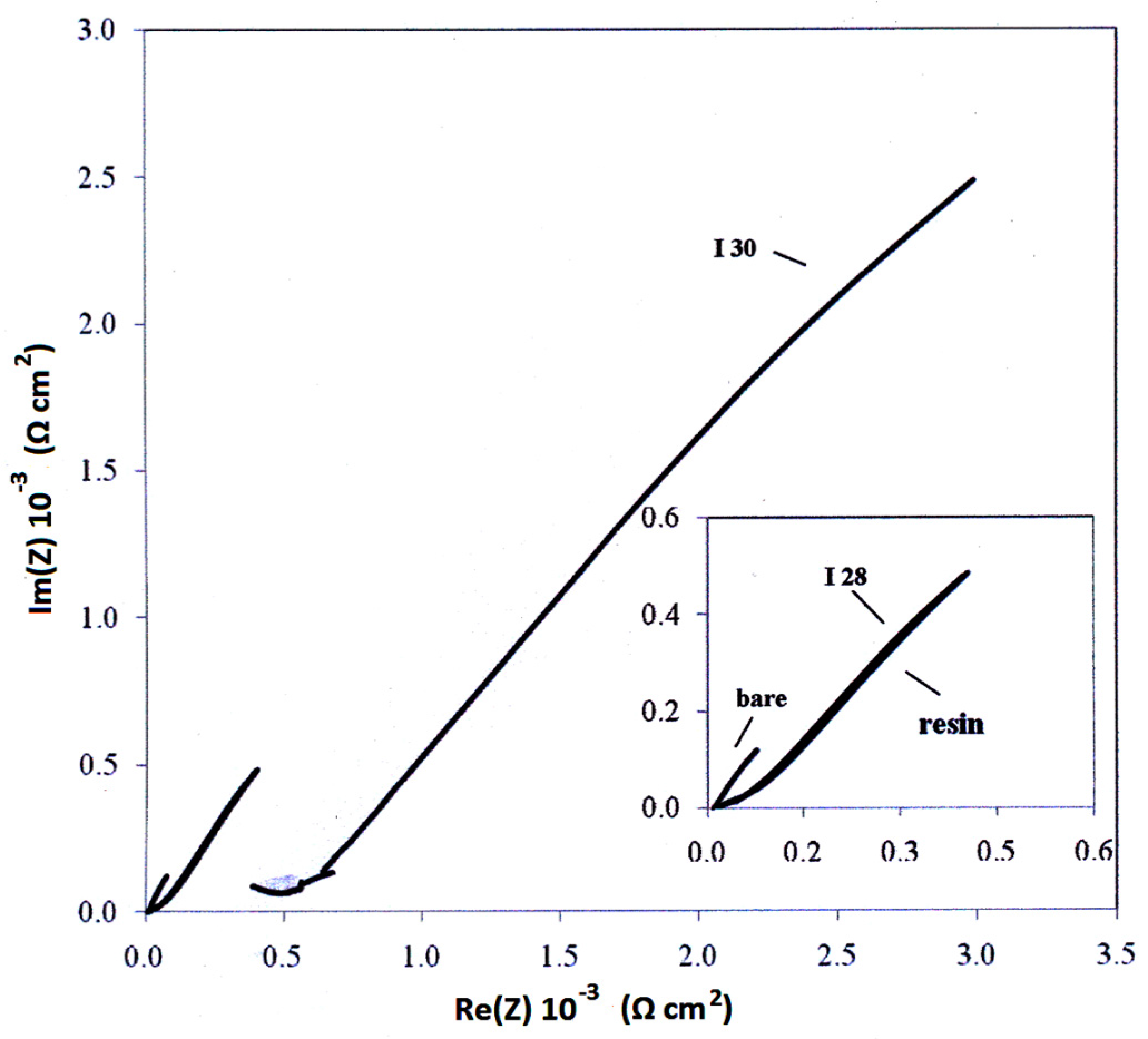
3.4. Electrochemical—Open Circuit Potential Measurements
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hang, T.T.X.; Truc, T.A.; Olivier, M-G.; Vandermiers, C.; Guérit, N.; Pébère, N. Corrosion protection of carbon steel by an epoxy resin containing organically modified clay. Surf. Coat. Technol. 2007, 201, 7408–7415. [Google Scholar] [CrossRef]
- Yeh, J.M.; Huang, H.Y.; Chen, C.L.; Su, W.F.; Yu, Y.H. Siloxane-modified epoxy resin—Clay nanocomposite coatings with advanced anticorrosive properties prepared by a solution dispersion approach. Surf. Coat. Technol. 2006, 200, 2753–2763. [Google Scholar] [CrossRef]
- Kouloumbi, N.; Ghivalos, L.G.; Pantazopoulou, P. Determination of the performance of epoxy coatings containing feldspars filler. Pigment Resin Technol. 2005, 34, 148–153. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; LeBaron, P.C.; Park, I.; Pinnavaia, T.J. Epoxy-clay fabric film composites with unprecedented oxygen-barrier properties. Chem. Mater. 2006, 18, 4393–4398. [Google Scholar] [CrossRef]
- Pinnavaia, T.G.; Beall, G.W. (Eds.) Polymer–Clay Nanocomposites, Wiley Series in Polymer Science; John Wiley & Sons: Oxford, UK, 2000. [Google Scholar]
- Vaia, R.A.; Giannelis, E.P. Polymer Nanocomposites: Status and Opportunities. MRS Bull. 2001, 26, 394–401. [Google Scholar] [CrossRef]
- Allie, L.; Thorn, J.; Aglan, H. Evaluation of nanosilicate filled poly (vinyl chloride-co-vinyl acetate) and epoxy coatings. Corros. Sci. 2008, 50, 2189–2196. [Google Scholar] [CrossRef]
- Dai, C.-F.; Li, P.-R.; Yeh, J.M. Comparative studies for the effect of intercalating agent on the physical properties of epoxy resin-clay based nanocomposite materials. Eur. Polym. J. 2008, 44, 2439–2447. [Google Scholar] [CrossRef]
- Yu, H.J.; Wang, L.; Shi, Q.; Jiang, G.H.; Zhao, Z.R.; Dong, X.C. Study on nano-CaCO3 modified epoxy powder coatings. Prog. Org. Coat. 2006, 55, 296–300. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Raghibi-Boroujeni, M.; Ahadzadeh, I.; Najjar, R.; Seyed Dorraji, M.S. Effect of polypyrrole—Montmorillonite nanocomposites powder addition on corrosion performance of epoxy coatings on Al 5000. Prog. Org. Coat. 2009, 66, 321–327. [Google Scholar] [CrossRef]
- Rezaul Karim, M.; Hyun Yeum, J. In situ intercalative polymerization of conducting polypyrrole/montmorillonite nanocomposites. J. Polym. Sci. Part B 2008, 46, 2279–2285. [Google Scholar] [CrossRef]
- Pinnavaia, T.J. Intercalated Clay Catalysts. Science 1983, 220, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, C.S.; LeBaron, P.C.; Pinnavaia, T.J. Thermoset Epoxy—Clay Nanocomposites: The Dual Role of α, ω-Diamines as Clay Surface Modifiers and Polymer Curing Agents. J. Solid State Chem. 2002, 167, 354–362. [Google Scholar] [CrossRef]
- Triantafillidis, C.S.; LeBaron, P.C.; Pinnavaia, T.J. Homostructured mixed inorganic-organic ion clays: A new approach to epoxy polymer-exfoliated clay nanocomposites with a reduced organic modifier content. Chem. Mater. 2002, 14, 4088–4095. [Google Scholar] [CrossRef]
- Kouloumbi, N.; Moundoulas, P. Anticorrosive performance of organic coatings on steel surfaces exposed to deionized water. Pigment Resin Technol. 2002, 4, 206–215. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Hsieh, C.-F.; Jaw, J.-H.; Kuo, T.-H.; Huang, H.-Y.; Lin, C.-L.; Hsu, M.-Y. Organo-Soluble Polyimde (ODA-BSAA)/Montmorillonite Nanocomposite Materials Prepared by Solution Dispersion Technique. J. Appl. Polym. Sci. 2005, 95, 1082–1090. [Google Scholar] [CrossRef]
- Sung, J.H.; Choi, H.J. Effect of pH on physical characteristics of conducting poly (O-ethoxyaniline) nanocomposites. J. Macromol. Sci. Part B 2005, 44, 365–375. [Google Scholar] [CrossRef]
- Xidas, P.I.; Triantafyllidis, K.S. Effect of the type of alkylammonium ion clay modifier on the structure and thermal/mechanical properties of glassy and rubbery epoxy-clay nanocomposites. Eur. Polym. J. 2010, 46, 404–417. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Xidas, P.I.; Pinnavaia, T.J. Alternative Synthetic Routes to Epoxy Polymer—Clay Nanocomposites using Organic or Mixed-Ion Clays Modified by Protonated Di/Triamines (Jeffamines). Macromol. Symp. 2008, 267, 41–46. [Google Scholar] [CrossRef]
- ASTM A366/A366M-97e1 Standard Specification for Commercial Steel (CS) Sheet, Carbon (0.15 Maximum Percent) Cold-Rolled (Withdrawn 2000); ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM D638-14 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D3985-05(2010)e1 Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor; ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM B117 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2003.
- Baboian, R. Corrosion Tests and Standards: Application and Interpretation, 2nd ed.; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- ASTM G106 Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements; ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM B457 Standard Test Method for Measurement of Impedance of Anodic Coatings on Aluminum; ASTM International: West Conshohocken, PA, USA, 2003.
- Becker, O.; Simon, G.P.; Dusek, K. Inorganic Polymeric Nanocomposites and Membranes; Springer: Berlin, Germany, 2005. [Google Scholar]
- Ho, M.-W.; Lam, C.-K.; Lau, K.-T.; Ng, D.H.L.; Hui, D. Mechanical properties of epoxy-based composites using nanoclays. Compos. Struct. 2006, 75, 415–421. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Oxford, UK, 2001. [Google Scholar]
- Orazem, M.; Tribollet, B. Electrochemical Impedance Spectroscopy; the Electrochemical Society Series; John Wiley & Sons: Oxford, UK, 2008. [Google Scholar]
- Perez, N. Electrochemistry and Corrosion Science; Kluwer Academic Publishers: New York, NY, USA, 2004. [Google Scholar]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1994. [Google Scholar]

| Samples Were Calcined at 600 °C | Aluminum % (Al) | Calcium % (Ca) | Iron % (Fe) | Potassium % (K) | Magnesium % (Mg) | Sodium % (Na) |
|---|---|---|---|---|---|---|
| Na+-PGW | 12.02 | 0.36 | 1.57 | 0.16 | 2.3 | 3.31 |
| I.30E | 12.48 | 0.28 | 1.7 | 0.17 | 2.37 | 0.32 |
| I.28E | 12.17 | 0.22 | 1.74 | 0.23 | 2.3 | 0.08 |
| Property | Na+-PGW | I.30E | I.28E |
|---|---|---|---|
| Color | White Powder | White Powder | White Powder |
| Cation Exchange Capacity, CEC (meq/100 g) ± 10% | 145 | – | – |
| Mean Dry Particle Size (μm) | ~2 (0.5–10) | 8–10 | 8–10 |
| Aspect Ratio | 200–400 | – | – |
| +325 Mesh Residue (%) | – | 0.1 | 0.1 |
| Specific Gravity | 2.6 | 1.71 | 1.9 |
| Max Moisture (%) | 12 | 3 | 3 |
| pH (5% dispersion) | 9.5–10.5 | – | – |
| Bulk Density (pounds/ft3) (gm/cc) | – | 250.41 | 260.42 |
| Mineral Purity (min %) | – | 98.5 | 98.5 |
| Sample | Tensile Properties | Dynamic Mechanical Analysis | ||||
|---|---|---|---|---|---|---|
| Stress at Break (MPa) | Elongation at Break (%) | Elastic Modulus (MPa) | Storage Modulus at 40 °C (MPa) | Storage Modulus at 100 °C (MPa) | Tg (°C) | |
| Pristine | 62.5 | 7.9 | 3026 | 1660 | 16 | 84.5 |
| 3% Na+-PGW | 64.9 | 7.3 | 3139 | 1750 | 19 | 86.9 |
| 3% I.30E | 69.7 | 6.4 | 3315 | 2847 | 26 | 82.6 |
| 3% I.28E | 57.5 | 6.0 | 3030 | 2540 | 27 | 85.5 |
| 6% I.30E | 55.8 | 3.7 | 3514 | 2635 | 34 | 78.5 |
| 6% I.28E | 47.3 | 3.4 | 3279 | 2395 | 31 | 81.3 |
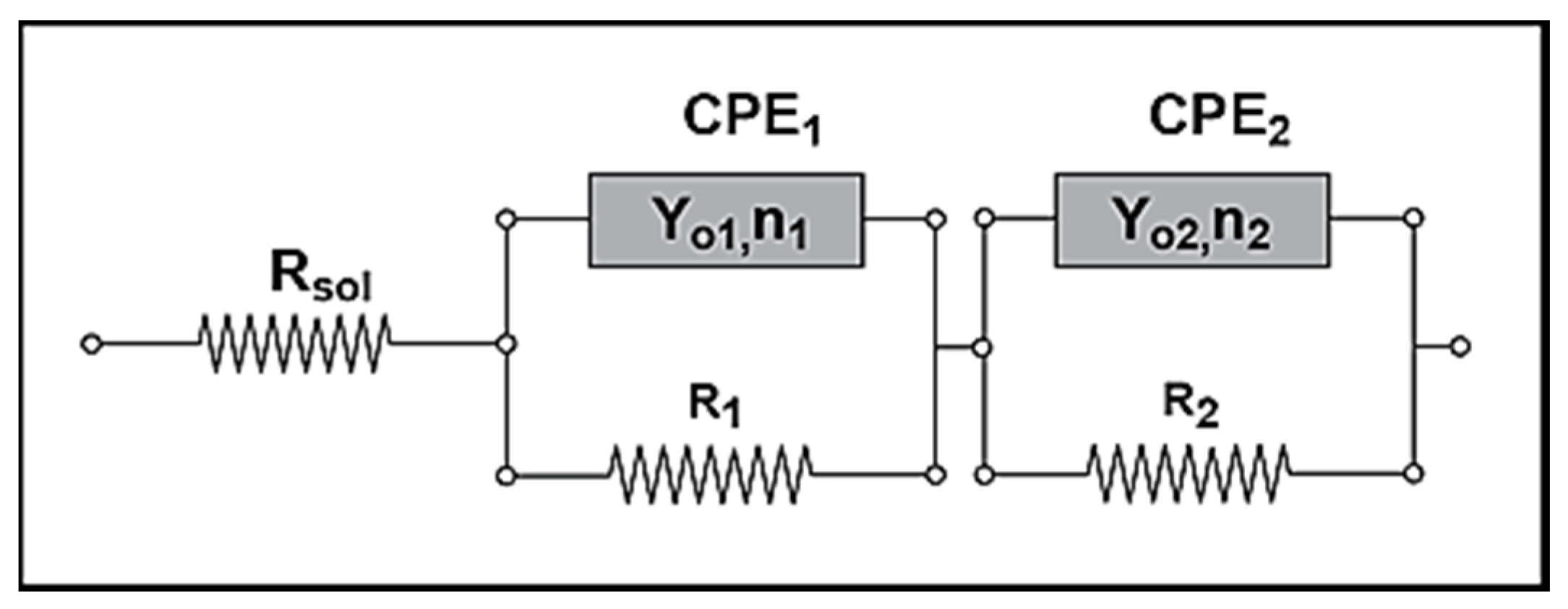
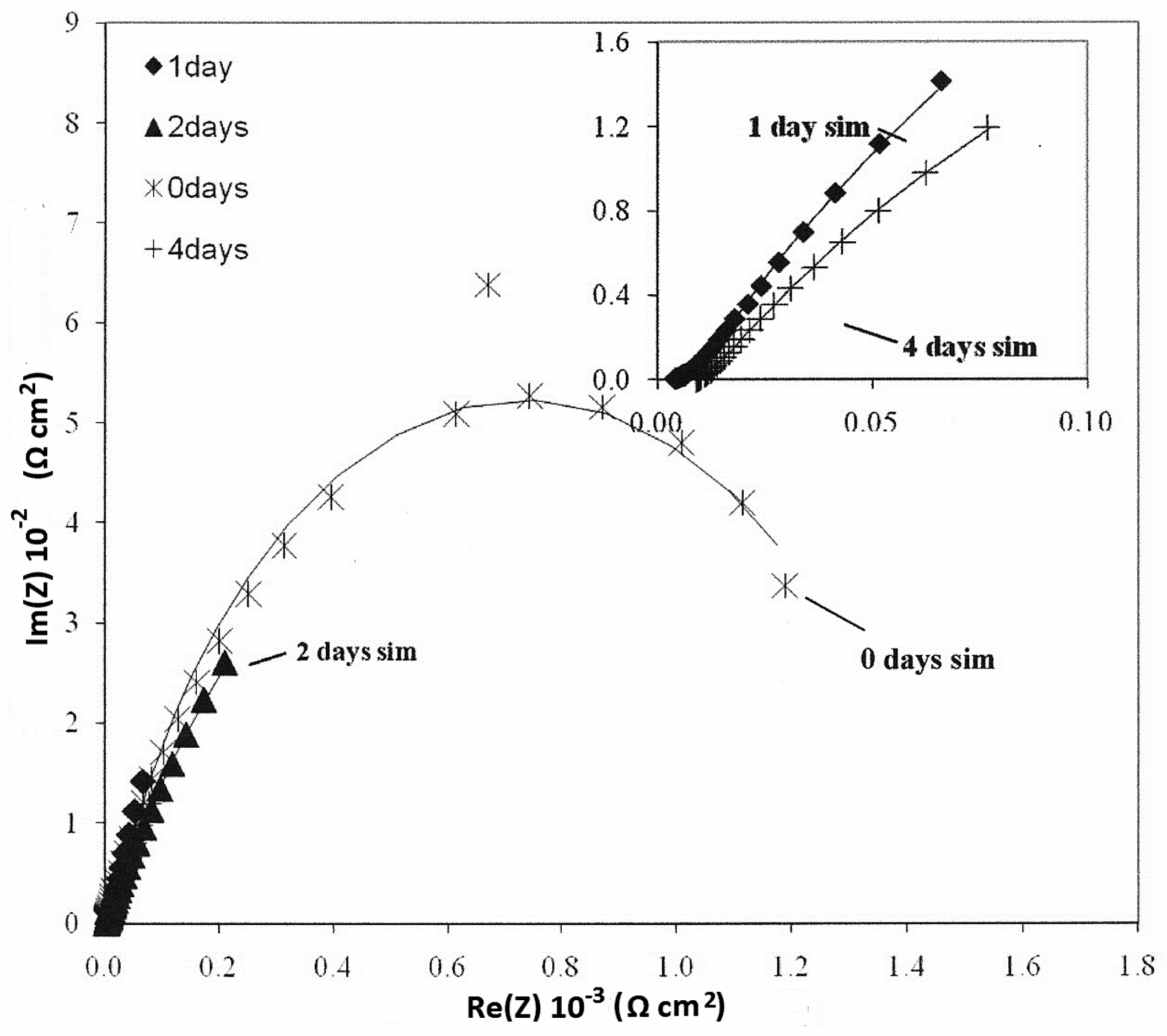
| Parameters of Equivalent Circuit | Bare— 0 day | Bare— 1 day | Bare— 2 days | Bare— 4 days | Epoxy— 0 day | Epoxy— 1 day | Epoxy— 2 days | Epoxy— 4 days |
|---|---|---|---|---|---|---|---|---|
| Rsol | 1.27 | 4.00 | 2.22 | 9.08 | 3.14 × 102 | 2.00 | 4.00 | 1.62 × 10 |
| R1 | 1.42 × 103 | 9.93 × 102 | 6.55 × 102 | 3.67 | 2.15 × 103 | 5.43 × 10 | 1.87 × 10 | 5.27 × 103 |
| Y01 | 3.93 × 10−4 | 1.08 × 10−2 | 3.97 × 10−2 | 3.51 × 10−2 | 1.62 × 10−6 | 4.43 × 10−3 | 1.05 × 10−3 | 2.33 × 10−3 |
| n1 | 8.08 × 10−1 | 8.72 × 10−1 | 7.01 × 10−1 | 4.79 × 10−1 | 6.26 × 10−1 | 2.93 × 10−1 | 4.86 × 10−1 | 6.76 × 10−1 |
| R2 | 2.48 × 10 | – | – | 1.03 × 102 | 6.78 × 105 | 7.26 × 103 | 5.38 × 103 | 7.21 × 101 |
| Y02 | 1.26 × 10−3 | 5.92 × 10−2 | 4.22 × 10−2 | 9.99 × 10−2 | 2.10 × 10−6 | 1.03 × 10−3 | 2.31 × 10−3 | 1.34 × 10−3 |
| n2 | 7.10 × 10−1 | 3.37 × 10−1 | 3.17 × 10−1 | 7.61 × 10−1 | 5.68 × 10−1 | 6.93 × 10−1 | 6.19 × 10−1 | 4.60 × 10−1 |
| Rtot (R1 + R2) | 1.44 × 103 | 9.93 × 102 | 6.55 × 102 | 1.03 × 102 | 6.80 × 105 | 7.31 × 103 | 5.40 × 103 | 5.34 × 103 |
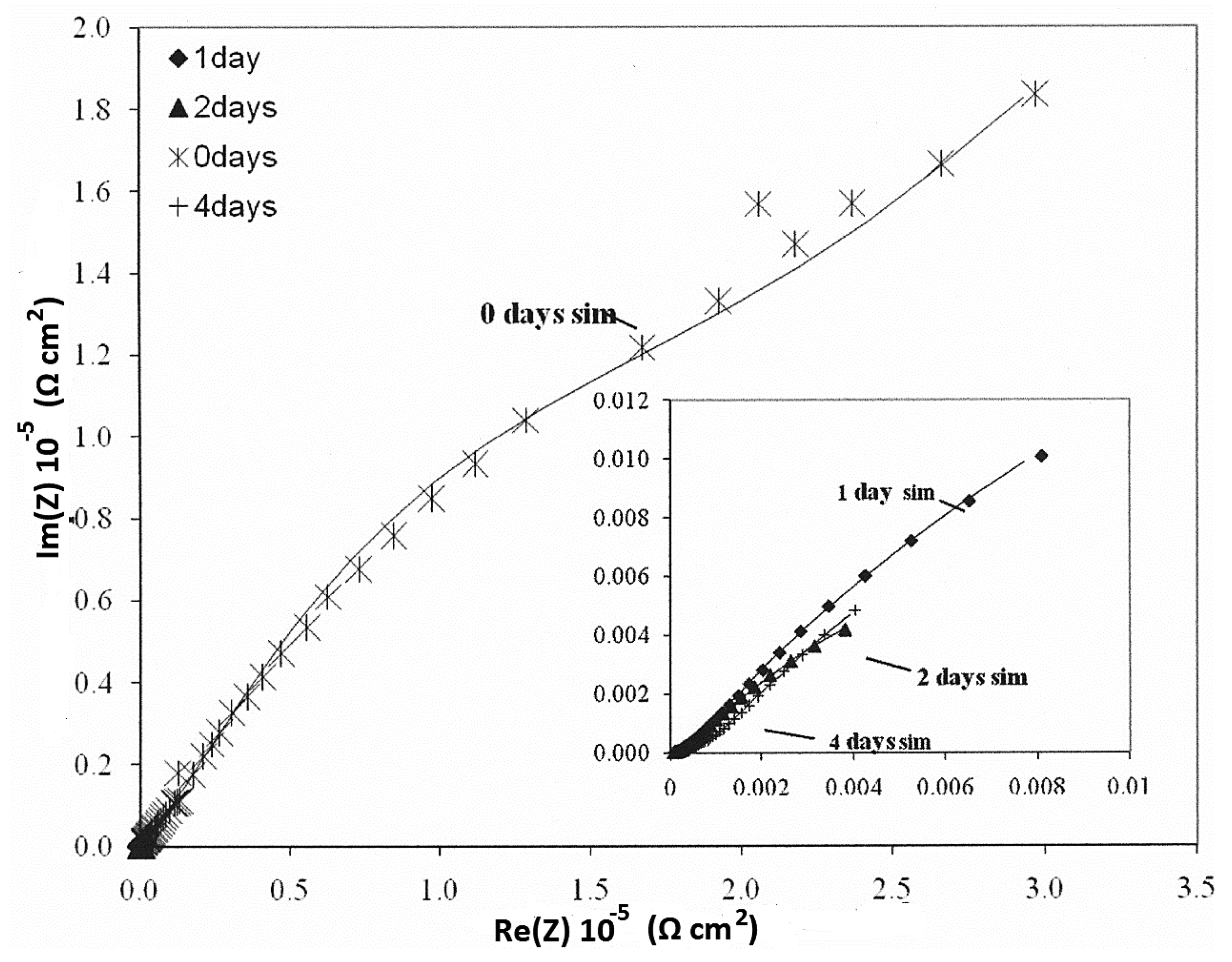
| Parameters of Equivalent Circuit | I.28E— 0 day | I.28E— 1 day | I.28E— 2 days | I.28E— 4 days | I.30E— 0 day | I.30E— 1 day | I.30E— 2 days | I.30E— 4 days |
| Rsol | 1.38 × 10 | 1.37 × 10 | 1.51 × 10 | 1.40 × 10 | 7.67 × 10 | 9.66 | 1.11 × 10 | 1.50 × 10 |
| R1 | 9.36 | 2.12 × 102 | 8.12 × 103 | 7.35 × 103 | 4.93 × 104 | 1.80 × 104 | 1.09 × 103 | 6.03 × 102 |
| Y01 | 1.37 × 10−4 | 7.18 × 10−4 | 8.98 × 10−4 | 2.33 × 10−3 | 9.74 × 10−6 | 2.63 × 10−6 | 4.08 × 10−5 | 4.26 × 10−5 |
| n1 | 1.00 | 3.15 × 10−1 | 4.94 × 10−1 | 6.38 × 10−1 | 1.00 | 6.53 × 10−1 | 3.79 × 10−1 | 2.96 × 10−1 |
| R2 | 7.99 × 104 | 8.99 × 103 | 6.24 | 4.81 × 10 | 1.54 × 105 | 2.63 × 104 | 3.02 × 104 | 2.90 × 104 |
| Y02 | 3.02 × 10−5 | 8.25 × 10−4 | 1.63 × 10−4 | 1.44 × 10−3 | 8.72 × 10−6 | 7.49 × 10−5 | 1.15 × 10−4 | 3.59 × 10−4 |
| n2 | 7.69 × 10−1 | 6.41 × 10−1 | 7.01 × 10−1 | 4.38 × 10−1 | 7.10 × 10−1 | 4.31 × 10−1 | 6.95 × 10−1 | 5.79 × 10−1 |
| Rtot (R1 + R2) | 7.99 × 104 | 9.21 × 103 | 8.12 × 103 | 7.40 × 103 | 2.03 × 105 | 4.43 × 104 | 3.13 × 104 | 2.96 × 104 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merachtsaki, D.; Xidas, P.; Giannakoudakis, P.; Triantafyllidis, K.; Spathis, P. Corrosion Protection of Steel by Epoxy-Organoclay Nanocomposite Coatings. Coatings 2017, 7, 84. https://doi.org/10.3390/coatings7070084
Merachtsaki D, Xidas P, Giannakoudakis P, Triantafyllidis K, Spathis P. Corrosion Protection of Steel by Epoxy-Organoclay Nanocomposite Coatings. Coatings. 2017; 7(7):84. https://doi.org/10.3390/coatings7070084
Chicago/Turabian StyleMerachtsaki, Domna, Panagiotis Xidas, Panagiotis Giannakoudakis, Konstantinos Triantafyllidis, and Panagiotis Spathis. 2017. "Corrosion Protection of Steel by Epoxy-Organoclay Nanocomposite Coatings" Coatings 7, no. 7: 84. https://doi.org/10.3390/coatings7070084




