Oxidation Behavior and Mechanism of Al4SiC4 in MgO-C-Al4SiC4 System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Al4SiC4 Crystals
2.2. Evolution of Al4SiC4 in MgO-C-Al4SiC4 System
2.3. Characterization
3. Results and Discussion
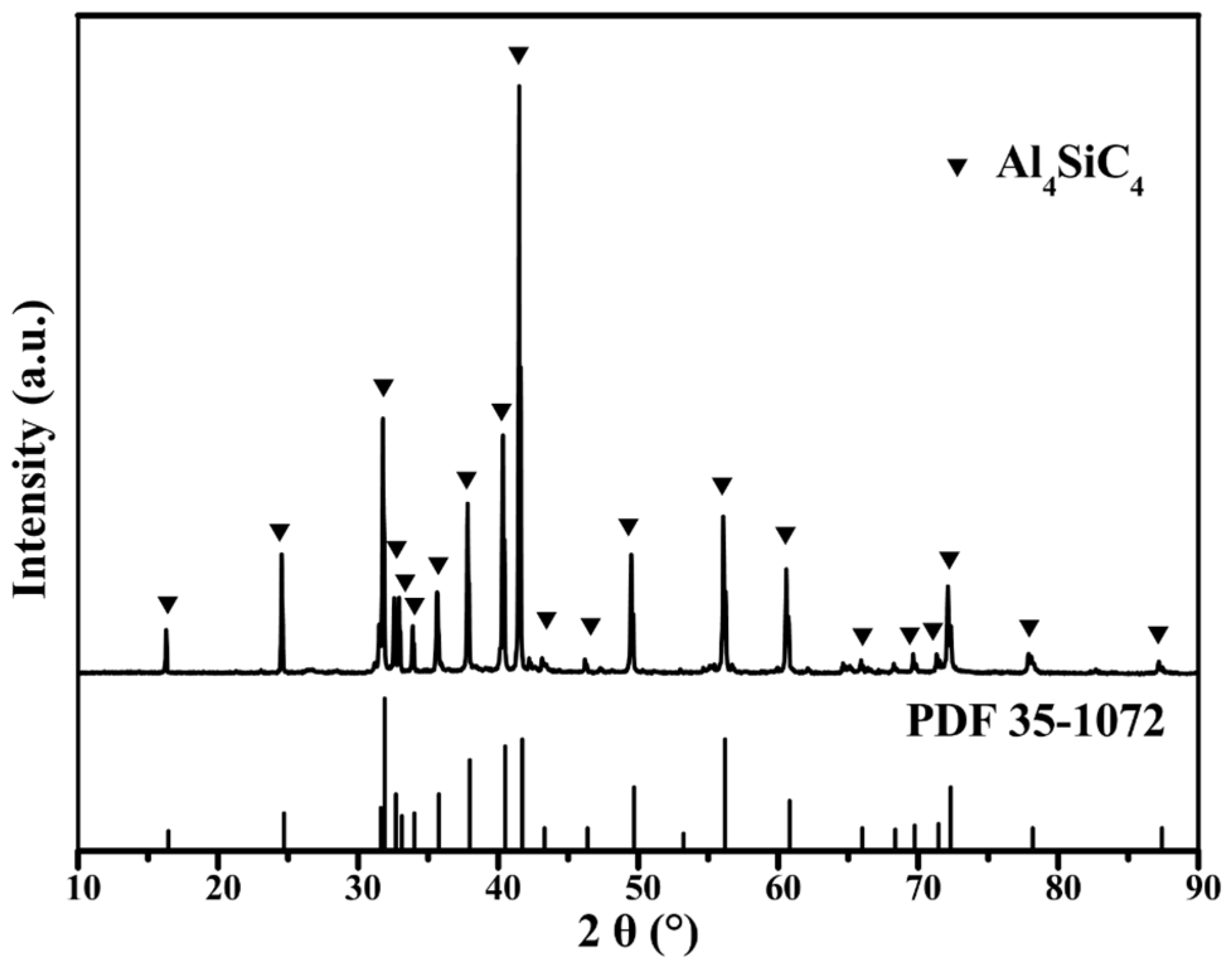
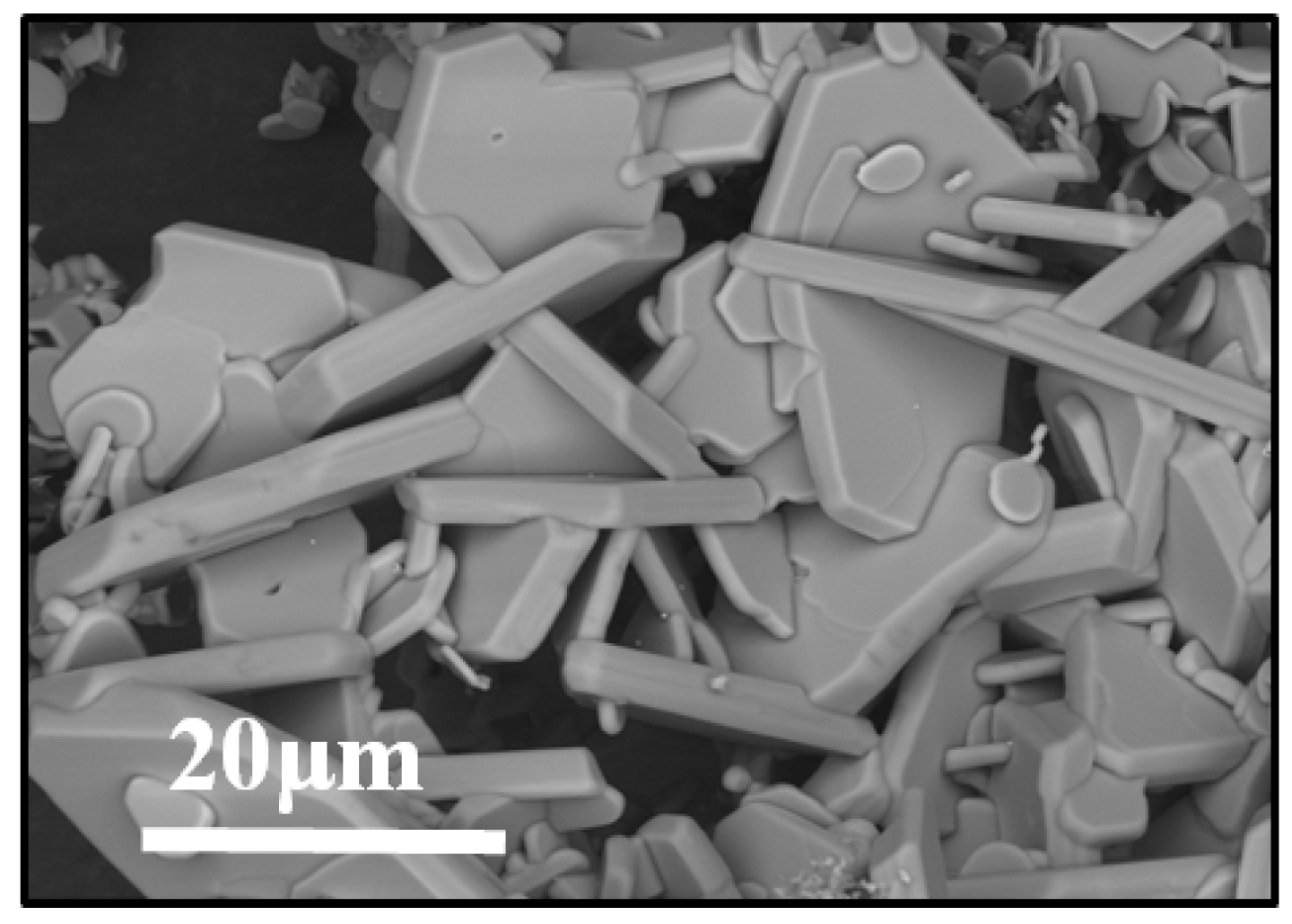
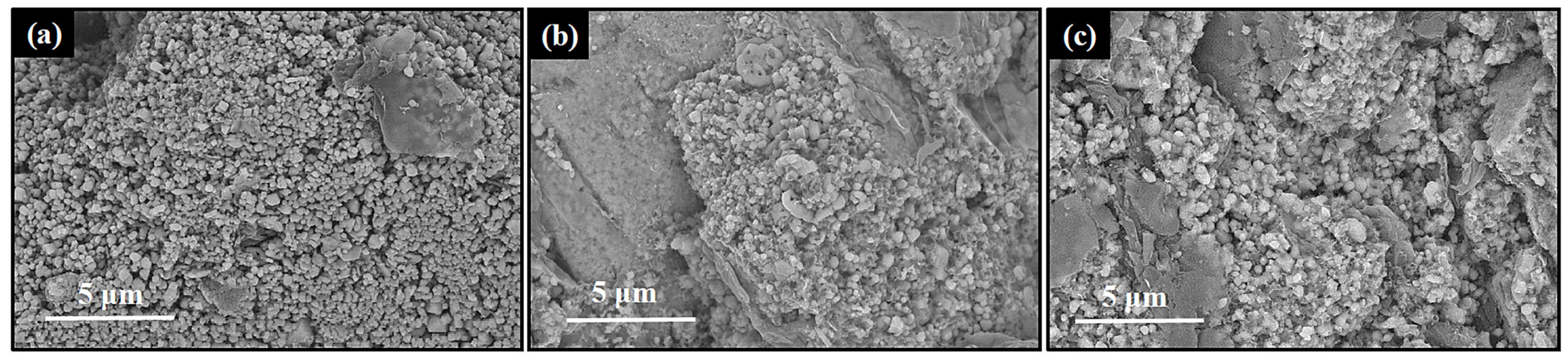
3.1. Characterization of Synthesized Al4SiC4 Crystals
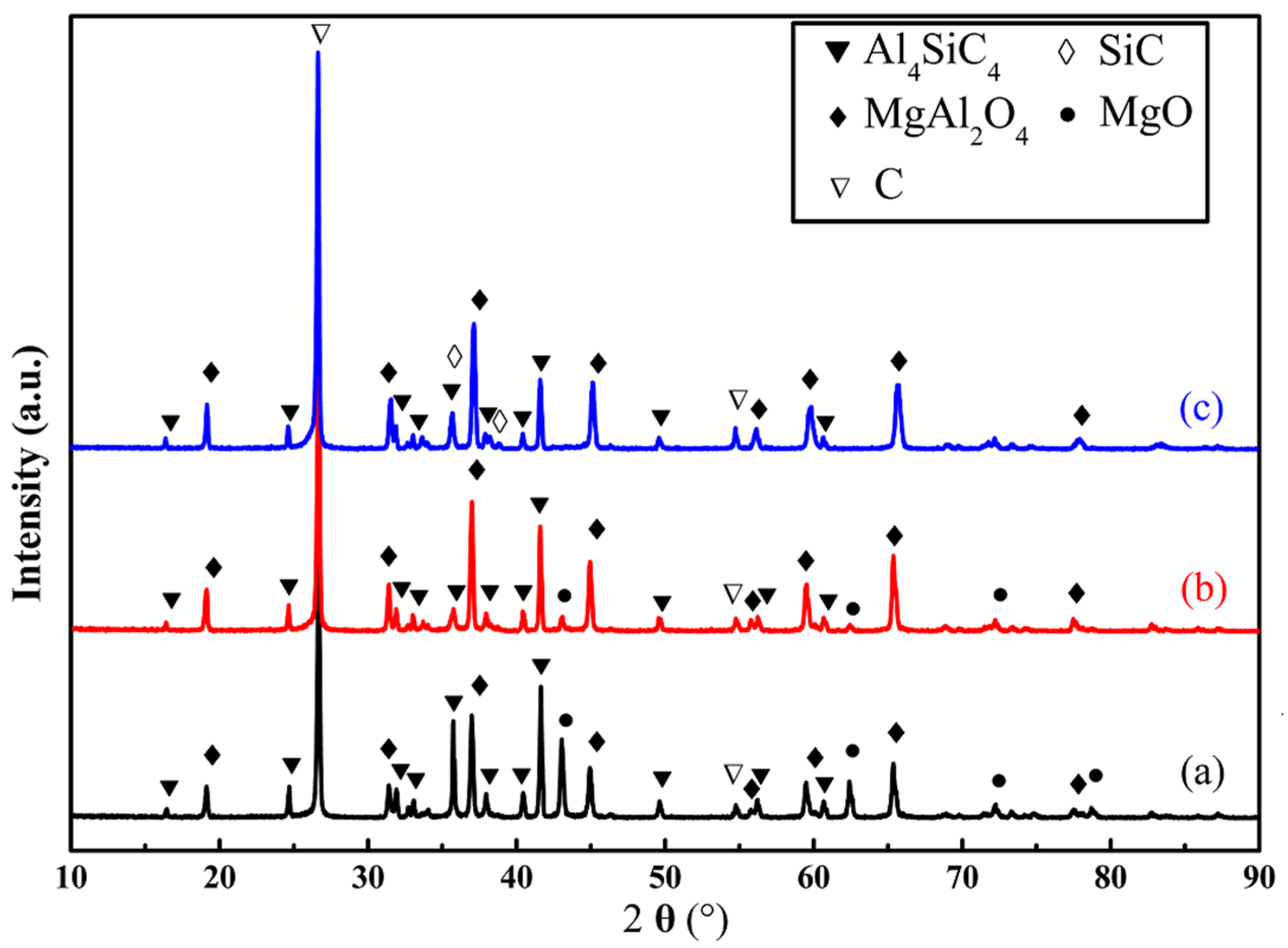
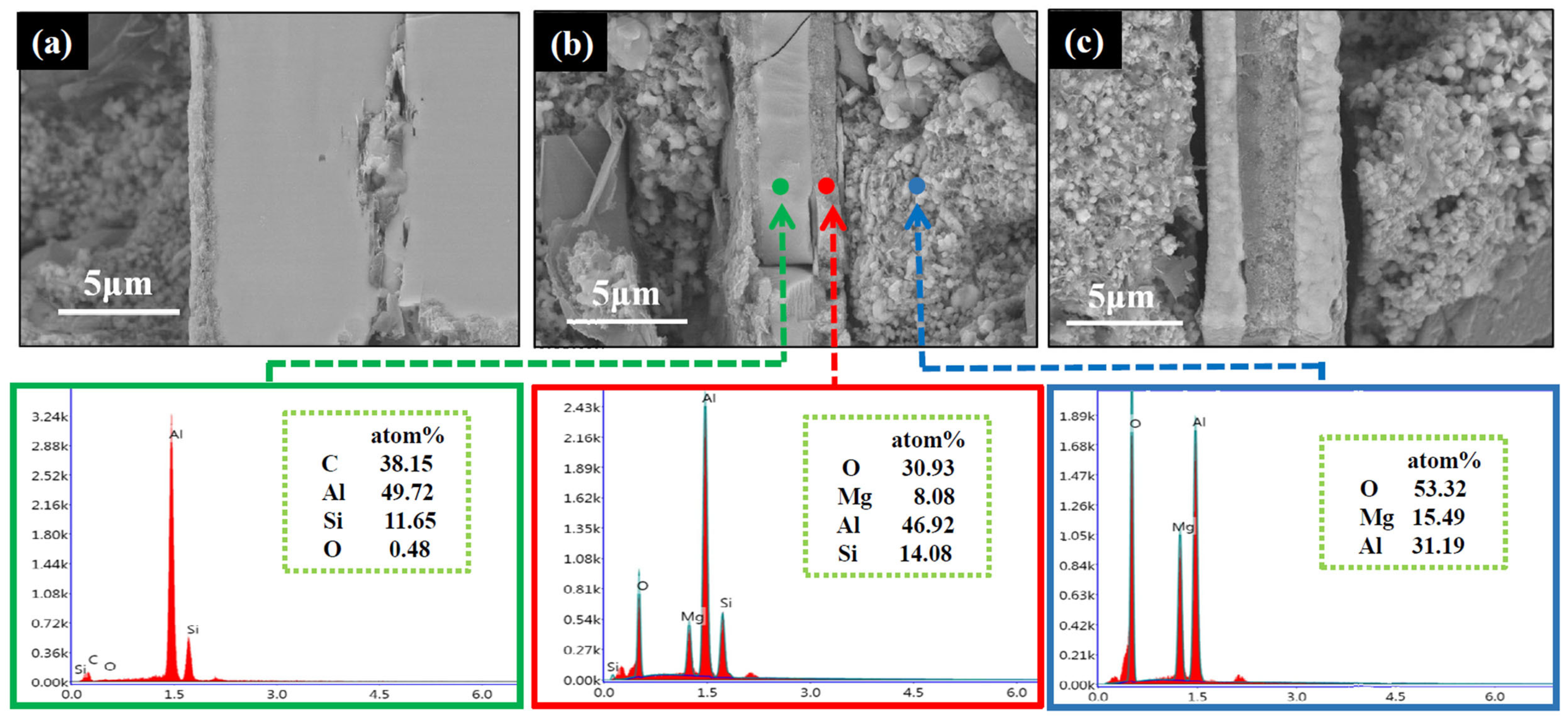
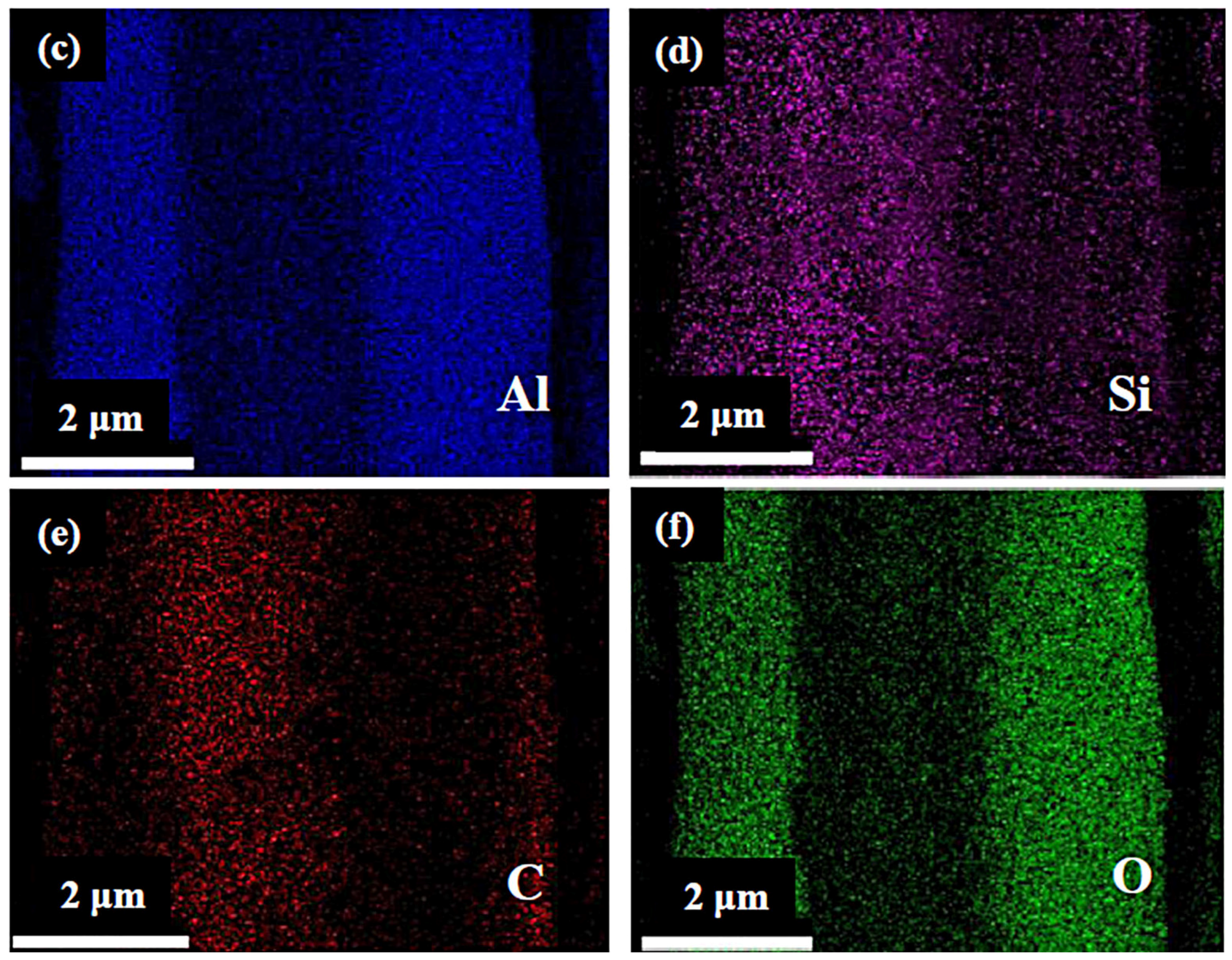
3.2. Evolution of Al4SiC4 in the MgO-C-Al4SiC4 System
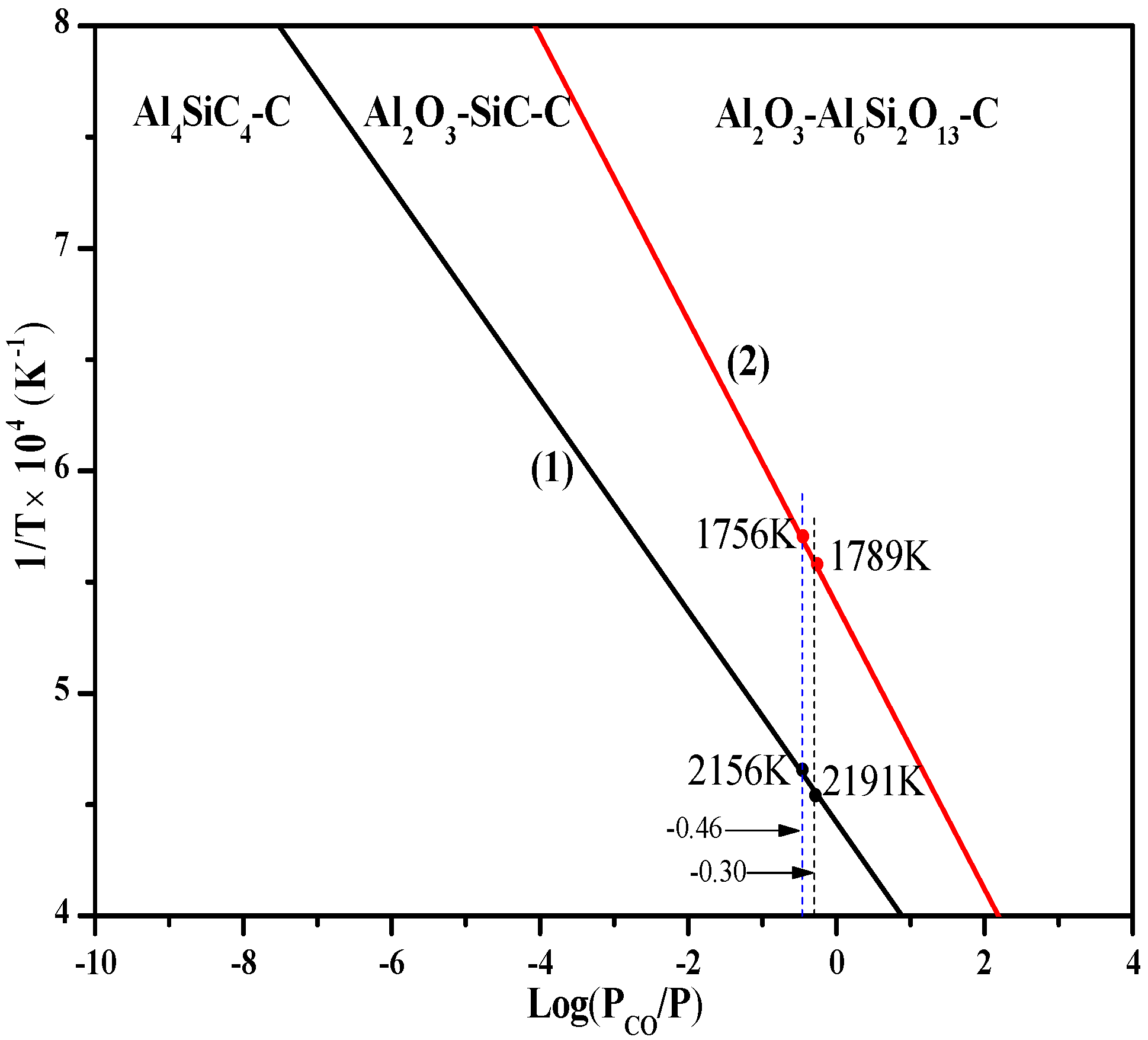
3.3. Thermodynamic Analysis of the Oxidation Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mohoamed, E.; Ewais, M. Carbon based refractories. J. Ceram. Soc. Jpn. 2004, 68, 517–532. [Google Scholar]
- Taffin, C.; Poirier, J. Behaviour of metal additives in MgO-C and Al2O3-C refractories. Interceram 1994, 43, 458–460. [Google Scholar]
- Zhu, T.; Li, Y.; Sang, S.; Xie, Z. Formation of nanocarbon structures in MgO-C refractories matrix: Influence of Al and Si additives. Ceram. Int. 2016, 42, 18833–18843. [Google Scholar] [CrossRef]
- Bavand-Vandchali, M.; Sarpoolaky, H.; Golestani-Fard, F.; Rezaie, H.R. Atmosphere and carbon effects on microstructure and phase analysis of in situ, spinel formation in MgO-C refractories matrix. Ceram. Int. 2009, 35, 861–868. [Google Scholar] [CrossRef]
- Hayashi, S.; Takanaga, S.; Takahashi, H.; Watanabe, A. Behavior of boric compounds added in MgO-C bricks. Taikabutsu Overseas 1991, 11, 12–19. [Google Scholar]
- Li, R.; Xiao, G.Q.; Wang, H. Effect of carbon black and ZrB2 on the oxidation resistance and microstructure of low-carbon MgO-C refractories. Bull. Chin. Ceram. Soc. 2013, 32, 738–742. [Google Scholar]
- Luz, A.P.; Souza, T.M.; Pagliosa, C.; Brito, M.A.M.; Pandolfelli, V.C. In-situ hot elastic modulus evolution of MgO-C refractories containing Al, Si or Al-Mg antioxidants. Ceram. Int. 2016, 42, 9836–9843. [Google Scholar] [CrossRef]
- Tada, H.; Nomura, O.; Nishio, H.; Ichikawa, K. Effect of aluminum on densification of MgO-C bricks. Refractories 1995, 47, 60–65. [Google Scholar]
- Yu, J.; Yamaguchi, A. Hydration of synthesized Al4C3 and its prevention effect by Si addition. J. Ceram. Soc. Jpn. 1995, 103, 475–478. [Google Scholar] [CrossRef]
- Peng, X.; Li, L.; Peng, D. The progress of low-carbon MgO-C composite study. Refractories 2003, 37, 355–357. [Google Scholar]
- Zhu, B.Q.; Zhang, W.J.; Yao, Y.S. Current situation and development of low-carbon magnesia-carbon materials research. Refractories 2006, 40, 90–95. [Google Scholar]
- Tamura, S.; Ochiai, T.; Takanaga, S.; Kanai, T.; Nakamura, H. The development of nano structured matrix. In Proceedings of the 8th Unified International Technical Conference on Refractories (UNITECR), Osaka, Japan, 19–22 October 2003. [Google Scholar]
- Bo, L.; Sun, J.L.; Tang, G.S.; Liu, K.Q.; Liu, L.I.; Liu, Y.F. Effects of nanometer carbon black on performance of low-carbon MgO-C composites. J. Iron Steel Res. Int. 2010, 17, 75–78. [Google Scholar]
- Takanaga, S.; Fujiwara, Y.; Hatta, M. Development of MgO-rimmed MgO-C brick. In Proceedings of the 9th Unified International Technical Conference on Refractories (UNITECR), Orlando, FL, USA, 8–11 November 2005. [Google Scholar]
- Li, L.; Tang, G.S.; He, Z.Y.; Li, K.Q.; Pan, X.Y. Effects of dispersion and content of nanometer carbon on mechanical performance of low carbon MgO-C materials. In Proceedings of the 11th Unified International Technical Conference on Refractories (UNITECR), Salvador, Brazil, 13–16 October 2009. [Google Scholar]
- Viala, C.; Fortier, P.; Bouix, J. Stable and metastable phase equilibria in the chemical interaction between aluminium and silicon carbide. J. Mater. Sci. 1990, 25, 1842–1850. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Zhang, S.W. Synthesis and some properties of Al4SiC4. J. Am. Ceram. Soc. 1995, 103, 20–24. [Google Scholar] [CrossRef]
- Huang, X.X.; Wen, G.W.; Cheng, X.M.; Zhang, B.Y. Oxidation behavior of Al4SiC4 ceramic up to 1700 °C. Corros. Sci. 2007, 49, 2059–2570. [Google Scholar] [CrossRef]
- Itatani, K.; Takahashi, F.; Aizawa, M.; Okada, I.; Davies, I.J.; Suemasu, H.; Nozue, A. Densification and microstructural developments during the sintering of aluminum silicon carbide. J. Mater. Sci. 2002, 37, 335–342. [Google Scholar] [CrossRef]
- Zhang, S.; Yamaguchi, A. Effect of Al4SiC4 Addition to Carbon-Containing Refractories. J. Ceram. Soc. Jpn. 1995, 103, 235–239. [Google Scholar] [CrossRef]
- Zhang, S.; Yamaguchi, A. A comparison of Al, Si and Al4SiC4 added to Al2O3 single bond refractories. In Proceedings of the Unified International Technical Conference on Refractories (UNITECR), New Orleans, LA, USA, 4–7 November 1997. [Google Scholar]
- Li, L.; Wang, S.F.; Chen, S.B. The improvement in oxidation resistance of magnesia-carbon refractory bricks by Al4SiC4. Mater. Sci. Forum 2011, 686, 671–677. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Wang, L. Self-propagating high-temperature synthesis of Al4SiC4 and B4C and their application in carbon-containing refractories. Refractories 2011, 45, 401–407. [Google Scholar]
- Chen, J.H.; Zhang, Z.H.; Mi, W.J.; Wang, E.H.; Li, B.; Chou, K.C.; Hou, X.M. Fabrication and oxidation behavior of Al4SiC4 powders. J. Am. Ceram. Soc. 2017. [Google Scholar] [CrossRef]
- Yokokawa, H.; Fujishige, M.; Ujiie, S.; Dokiya, M. Phase relations associated with the aluminum blast furnace: Aluminum oxycarbide melts and Al–C–X (X = Fe, Si) liquid alloys. Metall. Mater. Trans. B 1987, 18, 433–444. [Google Scholar] [CrossRef]
- JANAF Thermochemical Data, 3rd ed.; National Standard Reference Data System; US National Bureau of Standards: Gaithersburg, MD, USA, 1971.

| Temperature | Phase Content (wt %) | ||||
|---|---|---|---|---|---|
| MgO | C | Al4SiC4 | MgAl2O4 | SiC | |
| 1400 °C | 25.78 | 10.44 | 8.23 | 55.55 | – |
| 1500 °C | 4.21 | 11.26 | 6.94 | 77.59 | – |
| 1600 °C | – | 11.75 | 4.95 | 77.72 | 5.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Xing, X.; Wang, E.; Li, B.; Chen, J.; Sun, J.; Hou, X. Oxidation Behavior and Mechanism of Al4SiC4 in MgO-C-Al4SiC4 System. Coatings 2017, 7, 85. https://doi.org/10.3390/coatings7070085
Yao H, Xing X, Wang E, Li B, Chen J, Sun J, Hou X. Oxidation Behavior and Mechanism of Al4SiC4 in MgO-C-Al4SiC4 System. Coatings. 2017; 7(7):85. https://doi.org/10.3390/coatings7070085
Chicago/Turabian StyleYao, Huabai, Xinming Xing, Enhui Wang, Bin Li, Junhong Chen, Jialin Sun, and Xinmei Hou. 2017. "Oxidation Behavior and Mechanism of Al4SiC4 in MgO-C-Al4SiC4 System" Coatings 7, no. 7: 85. https://doi.org/10.3390/coatings7070085




