Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors
Abstract
:1. Introduction
2. Experimental
2.1. Sol-Gel Formulation
2.2. Coatings Preparation
2.3. Coating Characterization
3. Results and Discussion
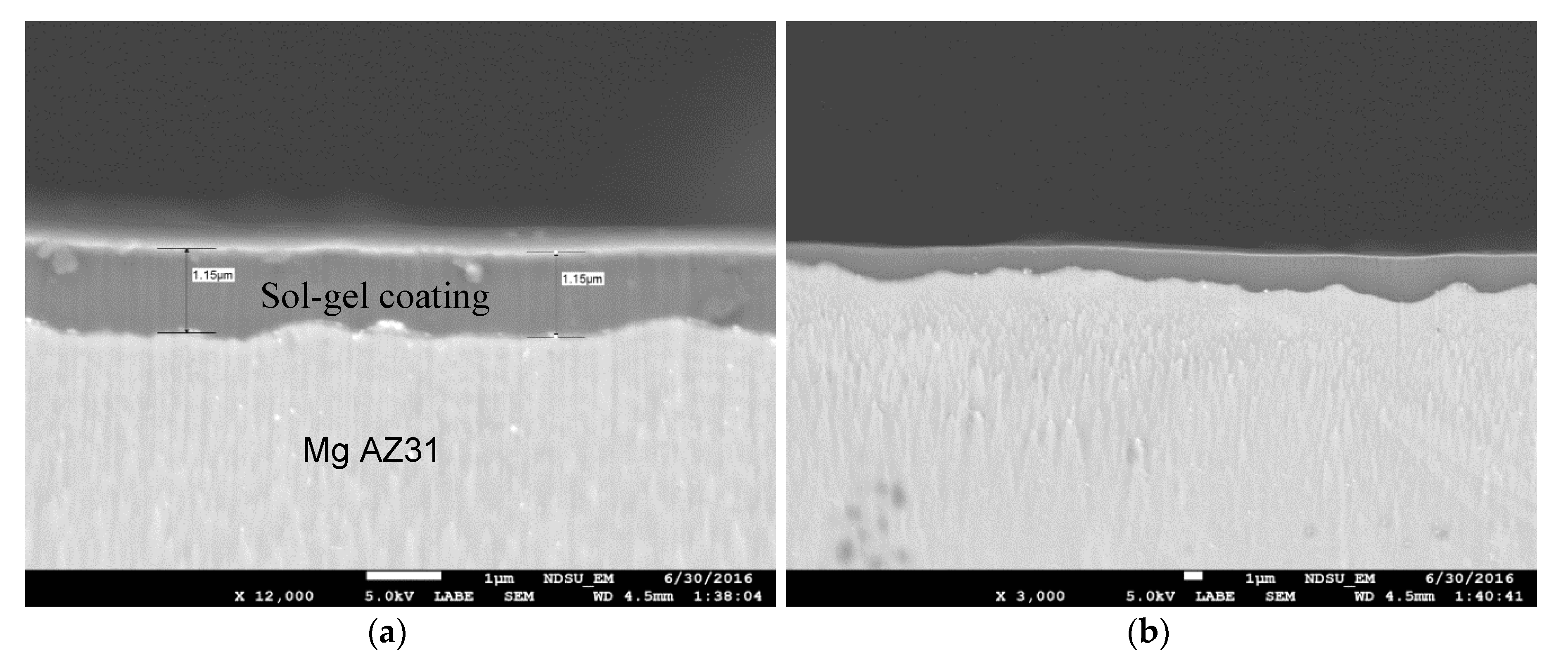
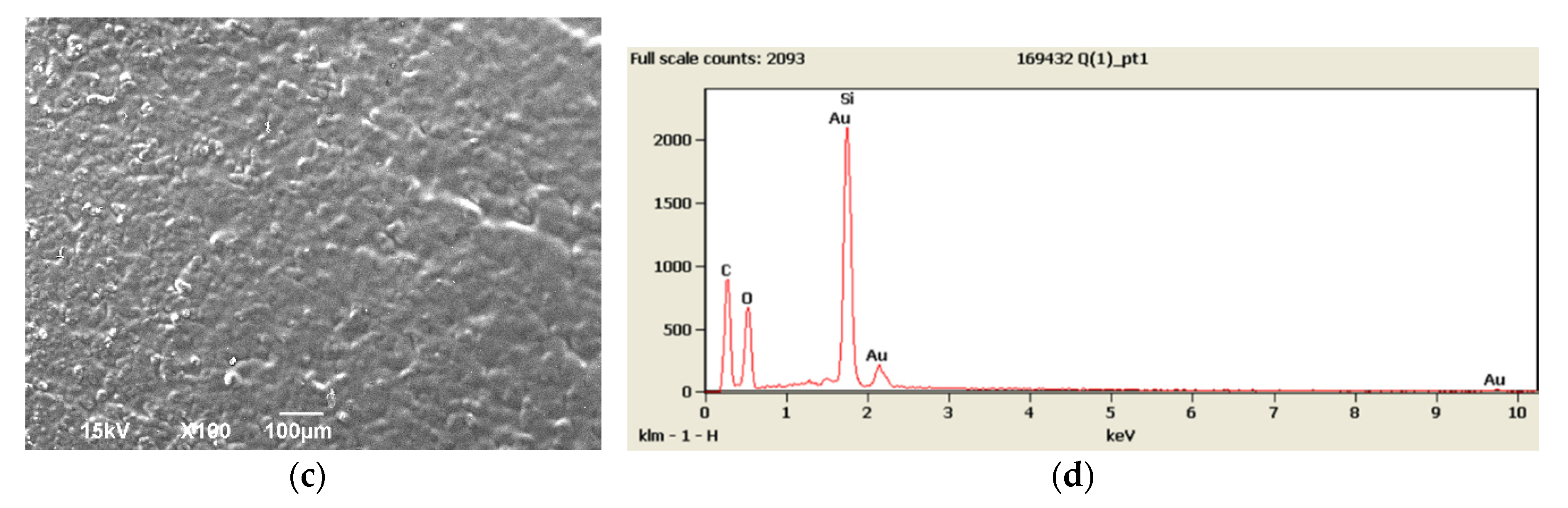
3.1. SEM and EDX Results
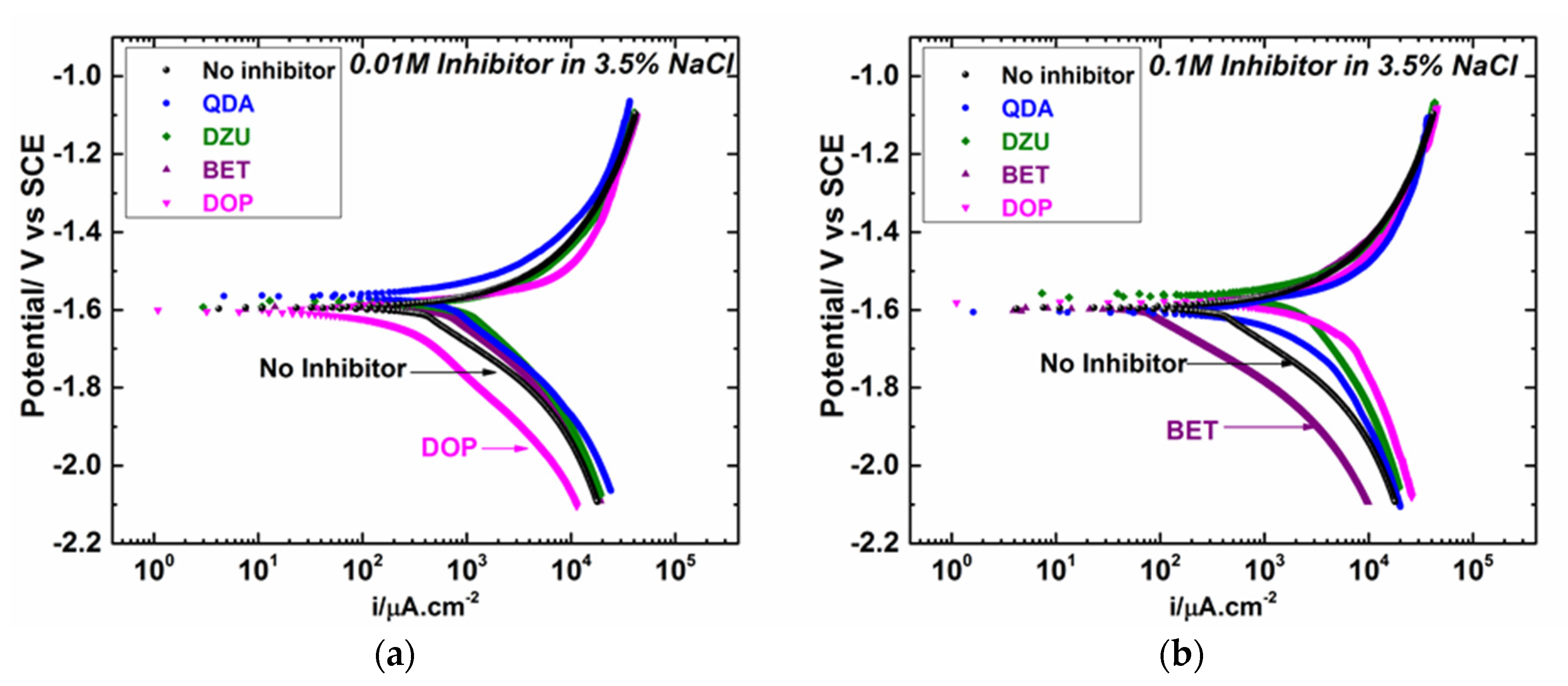
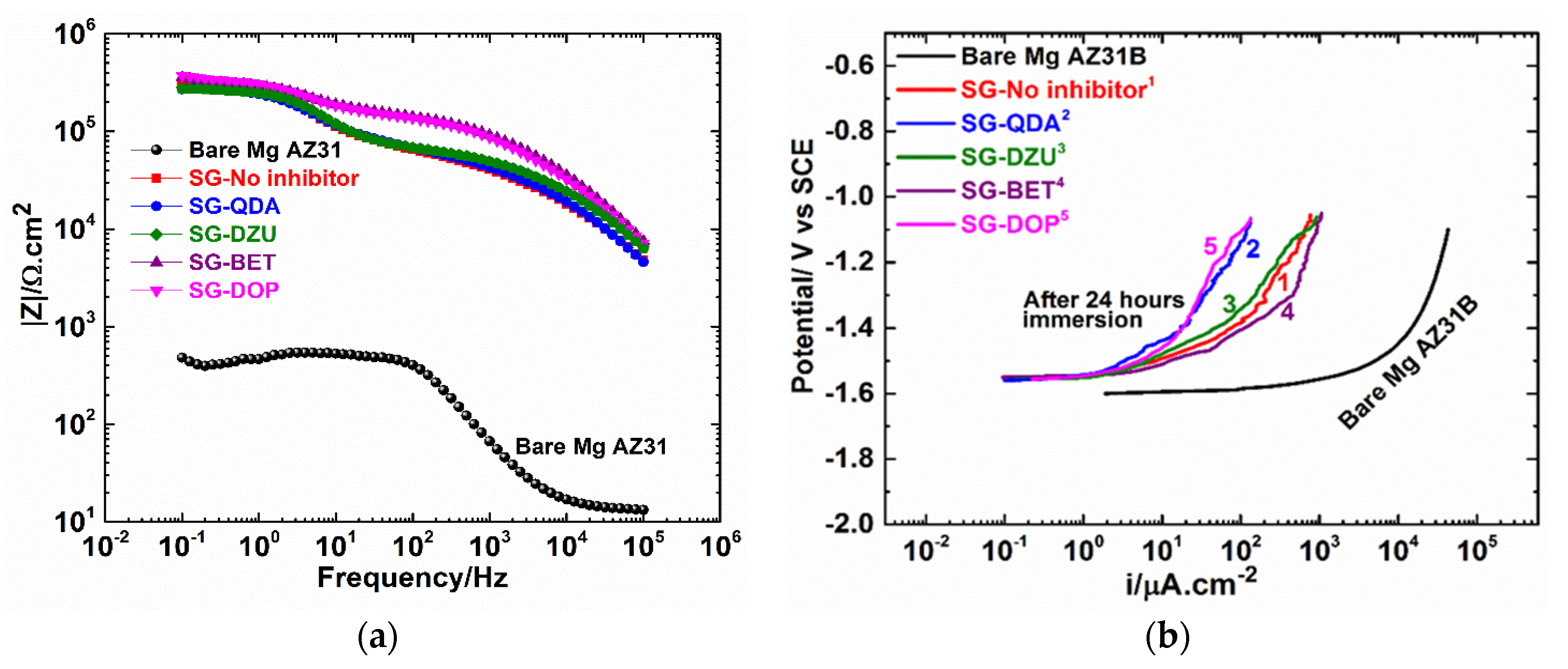
3.2. Electrochemical Polarization and EIS Results
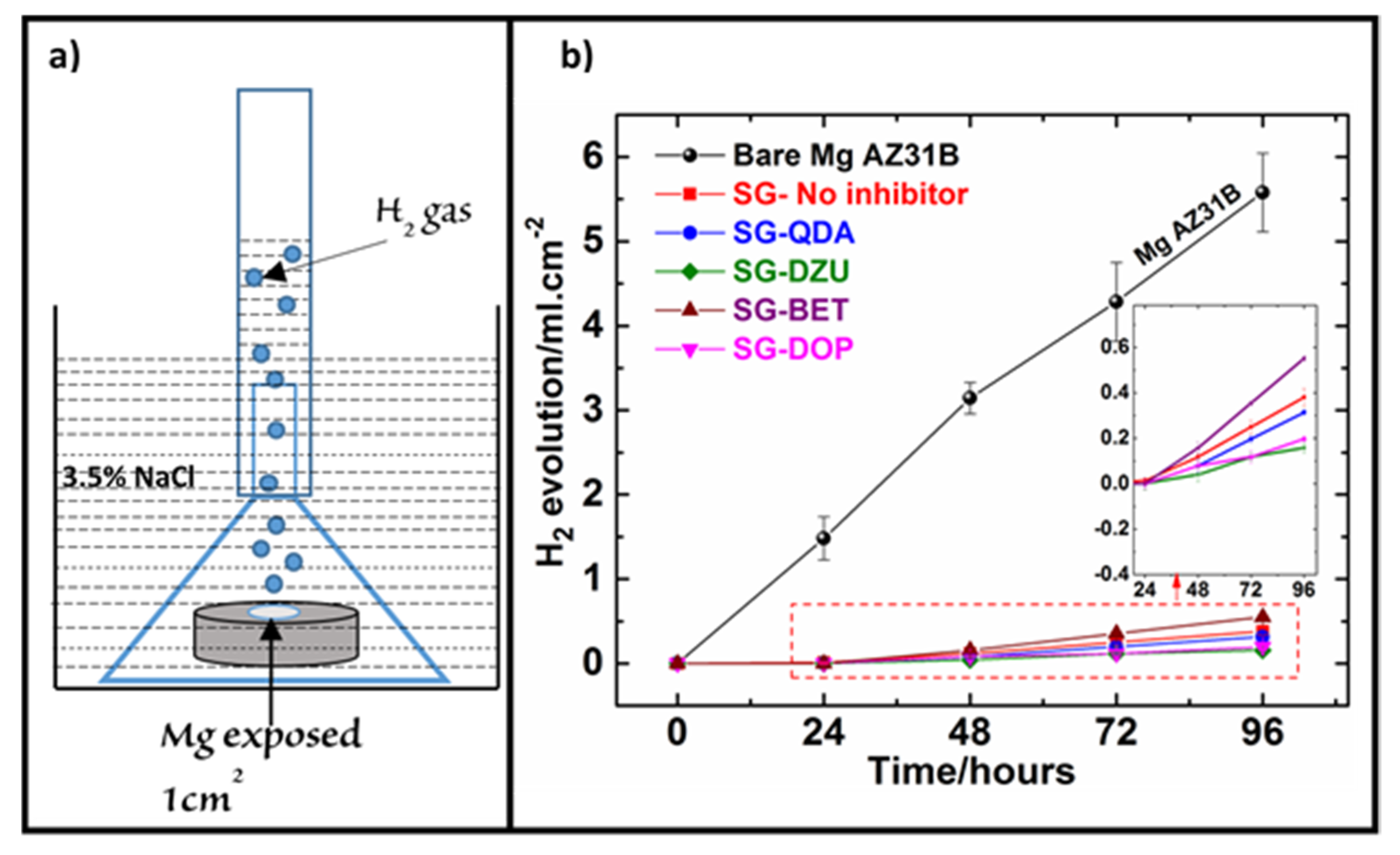
3.3. Hydrogen Evolution Measurement (HEM)
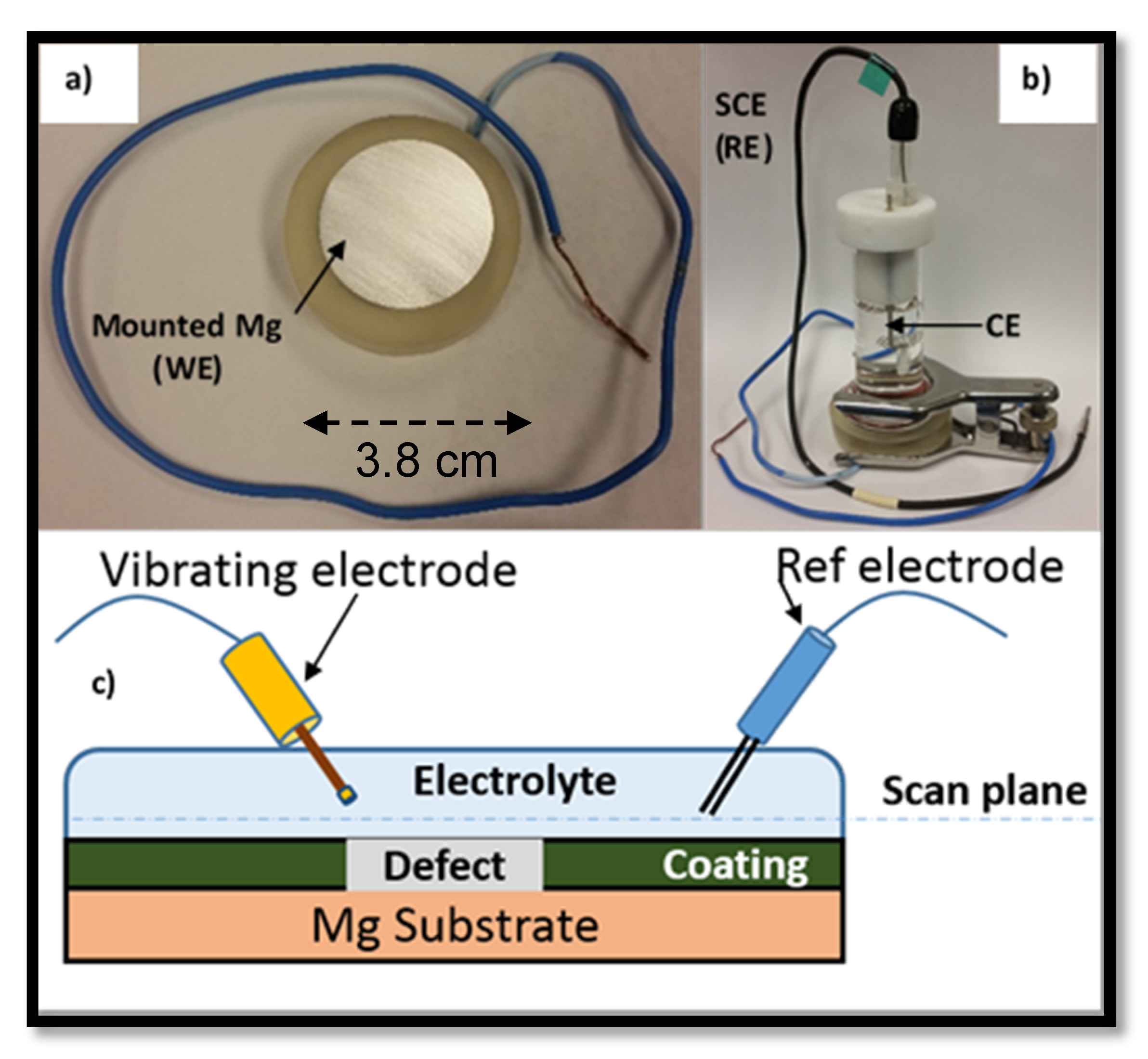
3.4. Scanning Vibrating Electrode Technique (SVET) Measurements
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Dislich, H. Sol-gel: Science, processes and products. J. Non. Cryst. Solids 1986, 80, 115–121. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Almeida, R.; Soutar, A.; Tadanaga, K.; Yang, H.; Watanabe, T. Coatings made by sol-gel and chemical nanotechnology. J. Sol.-Gel Sci. Technol. 2008, 47, 203–236. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Boissière, C.; Innocenzi, P.; Sanchez, C.; Grosso, D. Hydrophobic, antireflective, self-cleaning, and antifogging sol-gel coatings: An example of multifunctional nanostructured materials for photovoltaic cells. Chem. Mater. 2010, 22, 4406–4413. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pedraza, F.; Vhatkar, R.S. Silica based superhydrophobic coating for long-term industrial and domestic applications. J. Alloys Compd. 2016, 663, 487–493. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Latthe, S.S.; Gao, L.; An, S.; Yoon, S.S.; Liu, B.; Xing, R. Self-cleaning transparent superhydrophobic coatings through simple sol-gel processing of fluoroalkylsilane. Appl. Surf. Sci. 2015, 351, 897–903. [Google Scholar] [CrossRef]
- Dislich, H.; Non, J. Sol-Gel 1984–2004 (?). Cryst. Solids 1985, 73, 599–612. [Google Scholar] [CrossRef]
- Damborenea, J.; Pellegri, N.; Sanctis, O.; Durán, A. Electrochemical behaviour of SiO2 sol-gel coatings on stainless steel. J. Sol-Gel Sci. Technol. 1995, 4, 239–244. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M.; Annergren, I.F.; Liu, Y.N. Multilayer sol-gel coatings for corrosion protection of magnesium. Surf. Coat. Technol. 2005, 198, 478–482. [Google Scholar] [CrossRef]
- Mekeridis, E.D.; Kartsonakis, I.A.; Kordas, G.C. Multilayer organic-inorganic coating incorporating TiO2 nanocontainers loaded with inhibitors for corrosion protection of AA2024-T3. Prog. Org. Coat. 2012, 73, 142–148. [Google Scholar] [CrossRef]
- López, D.A.; Rosero-Navarro, N.C.; Ballarre, J.; Durán, A.; Aparicio, M.; Ceré, S. Multilayer silica-methacrylate hybrid coatings prepared by sol-gel on stainless steel 316L: Electrochemical evaluation. Surf. Coat. Technol. 2008, 202, 2194–2201. [Google Scholar] [CrossRef]
- López, A.J.; Rams, J.; Ureña, A. Sol-gel coatings of low sintering temperature for corrosion protection of ZE41 magnesium alloy. Surf. Coat. Technol. 2011, 205, 4183–4191. [Google Scholar] [CrossRef]
- Wittmar, A.; Wittmar, M.; Caparrotti, H.; Veith, M. The influence of the inhibitor particle sizes to the corrosion properties of hybrid sol-gel coatings. J. Sol-Gel Sci. Technol. 2011, 59, 621–628. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of active corrosion protection via combination of inhibitor-loaded nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Mordike, B.L.; Ebert, T. Magnesium properties—application—potential. Mater. Sci. Eng. 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium’’ in the automotive industry. J. Mater. Chem. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B. Magnesium alloys development towards the 21st century. Mater. Sci. Forum 2000, 350, 19–30. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Karavai, O.V.; Bastos, A.C.; Zheludkevich, M.L.; Taryba, M.G.; Lamaka, S.V.; Ferreira, M.G.S. Localized electrochemical study of corrosion inhibition in microdefects on coated AZ31 magnesium alloy. Electrochim. Acta 2010, 55, 5401–5406. [Google Scholar] [CrossRef]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol-gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Xue, H.B.; Meis, N.N.A.H.; Esteves, A.C.C.; Ferreira, M.G.S. Fault-tolerant hybrid epoxy-silane coating for corrosion protection of magnesium alloy AZ31. Prog. Org. Coat. 2015, 80, 98–105. [Google Scholar] [CrossRef]
- Ivanou, D.K.; Yasakau, K.A.; Kallip, S.; Lisenkov, A.D.; Starykevich, M.; Lamaka, S.V.; Ferreira, M.G.S.; Zheludkevich, M.L. Active corrosion protection coating for a ZE41 magnesium alloy created by combining PEO and sol-gel techniques. RSC Adv. 2016, 6, 12553–12560. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Balaskas, A.C.; Koumoulos, E.P.; Charitidis, C.A.; Kordas, G. ORMOSIL-epoxy coatings with ceramic containers for corrosion protection of magnesium alloys ZK10. Prog. Org. Coat. 2013, 76, 459–470. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Koumoulos, E.P.; Charitidis, C.A.; Kordas, G. Hybrid organic-inorganic coatings including nanocontainers for corrosion protection of magnesium alloy ZK30. J. Nanopart. Res. 2013, 15, 1871. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C. Influence of TiO2 nanocontainers on hybrid organic-inorganic coatings for corrosion protection of magnesium alloy. Int. J. Struct. Integr. 2013, 4, 127–142. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R.; Gobara, M. Scratch-resistant anticorrosion sol-gel coating for the protection of AZ31 magnesium alloy via a low temperature sol-gel route. Corros. Sci. 2010, 52, 2565–2570. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Obot, I.B.; Murulana, L.C. Quinoline and its derivatives as effective corrosion inhibitors for mild steel in acidic medium. Int. J. Electrochem. Sci. 2010, 5, 1574–1586. [Google Scholar]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.F.P.; Ferreira, M.G.S. Novel inorganic host layered double hydroxides intercalated with guest organic inhibitors for anticorrosion applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Ferreira, M.G.S. High effective organic corrosion inhibitors for 2024 aluminium alloy. Electrochim. Acta 2007, 52, 7231–7247. [Google Scholar] [CrossRef]
- Scharnagl, N.; Blawert, C.; Dietzel, W. Corrosion protection of magnesium alloy AZ31 by coating with poly(ether imides) (PEI). Surf. Coat. Technol. 2009, 203, 1423–1428. [Google Scholar] [CrossRef]
- Zheng, W.; Fan, H.; Wang, L.; Jin, Z. Oxidative self-polymerization of dopamine in an acidic environment. Langmuir 2015, 31, 11671–11677. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-ispired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Howson, M.R. Removal of Sulfides using Chlorite and an Amphoteric Ammonium Betaine. U.S. Patent 5,082,576, 21 January 1992. [Google Scholar]
- Isaacs, H.S.; Kendig, M.W. Determination of surface inhomogeneties using a scanning probe impedance technique. Corrosion 1980, 36, 269–274. [Google Scholar] [CrossRef]
- Jadhav, N.; Vetter, C.A.; Gelling, V.J. Characterization and electrochemical investigations of polypyrrole/aluminum flake composite pigments on AA 2024-T3 substrate. ECS Trans. 2012, 41, 75–89. [Google Scholar]
- Jadhav, N.; Vetter, C.A.; Gelling, V.J. The effect of polymer morphology on the performance of a corrosion inhibiting polypyrrole/aluminum flake composite pigment. Electrochim. Acta 2013, 102, 28–43. [Google Scholar] [CrossRef]
- Upadhyay, V.; Qi, X.; Wilson, N.; Battocchi, D.; Bierwagen, G.; Forsmark, J. Electrochemical characterization of coated self-piercing rivets for magnesium applications. SAE Int. J. Mater. Manuf. 2016, 9, 187–199. [Google Scholar] [CrossRef]
- Frankel, G.S.; Samaniego, A.; Birbilis, N. Evolution of hydrogen at dissolving magnesium surfaces. Corros. Sci. 2013, 70, 104–111. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; StJohn, D. An hydrogen evolution method for the estimation of the corrosion rate of magnesium alloys. Magnes. Technol. 2001, 2001, 254–262. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Birbilis, N.; Williams, G.; Gusieva, K.; Samaniego, A.; Gibson, M.A.; McMurray, H.N. Poisoning the corrosion of magnesium. Electrochem. Commun. 2013, 34, 295–298. [Google Scholar] [CrossRef]
- Lin, J.; Upadhyay, V.; Qi, X.; Battocchi, D.; Bierwagen, G.P. Hydrogen evolution and early blistering from magnesium-rich primers on AA2024-T3. Corrosion 2016, 72, 377–383. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, V.; Bergseth, Z.; Kelly, B.; Battocchi, D. Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors. Coatings 2017, 7, 86. https://doi.org/10.3390/coatings7070086
Upadhyay V, Bergseth Z, Kelly B, Battocchi D. Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors. Coatings. 2017; 7(7):86. https://doi.org/10.3390/coatings7070086
Chicago/Turabian StyleUpadhyay, Vinod, Zachary Bergseth, Brett Kelly, and Dante Battocchi. 2017. "Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors" Coatings 7, no. 7: 86. https://doi.org/10.3390/coatings7070086



