Technological Strategies to Preserve Burrata Cheese Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
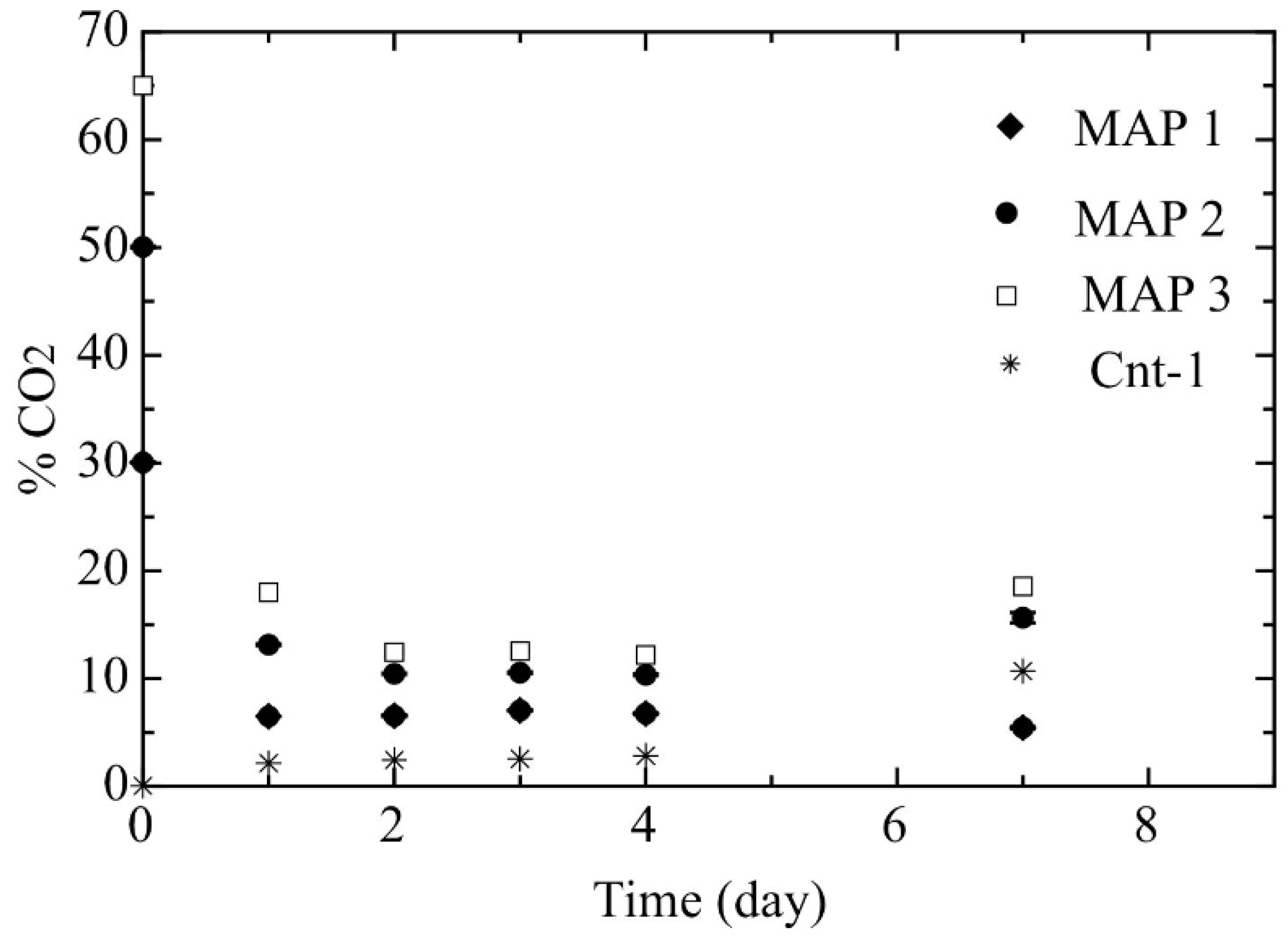
- Step 1: Two burrata samples were placed in a tray containing brine constituted by a NaCl solution (6 g·L−1). Samples were packaged under MAP conditions (MAP-1 30:70 CO2:N2; MAP-2 50:50 CO2:N2 and MAP-3 65:35 CO2:N2) by means of a thermo-sealing machine (Orved, Musile di Piave, Venezia, Italy).
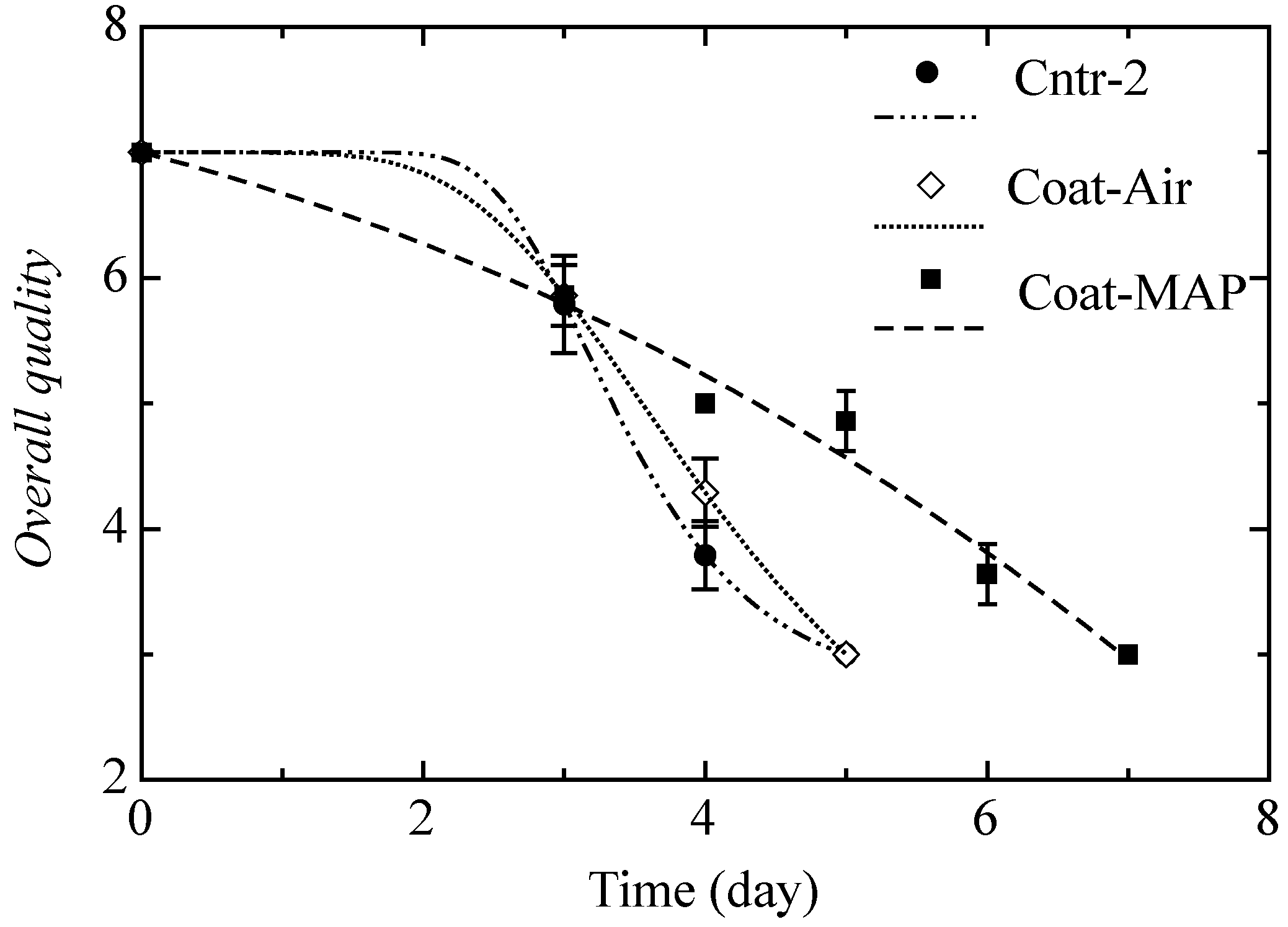
- Step 2: In the second step, the burrata cheese was coated by immersing samples first in a sodium alginic acid solution (2% w/v), then a solution of calcium chloride (5% w/v) was used to promote the alginate-gel-forming process by dipping the product for one min. Two coated samples were packaged with brine in air (Coat-Air) and under MAP (65:35 CO2:N2) (Coat-MAP).
- Step 3: In this step, the antimicrobial compounds were added to the burrata filling during the production process. In particular, lysozyme (500 mg·kg−1) and Na2-EDTA (50 mM) were dissolved in the cream and then mixed with the fiordilatte pieces. Samples of burrata with the antimicrobial compounds were packaged in air (LysEDTA-Air) and under MAP (65:35 CO2:N2) (LysEDTA-MAP). Moreover, burrata with the antimicrobial compounds was coated as described in Step 2 and packaged under MAP (65:35 CO2:N2) [Coat-LysEDTA].
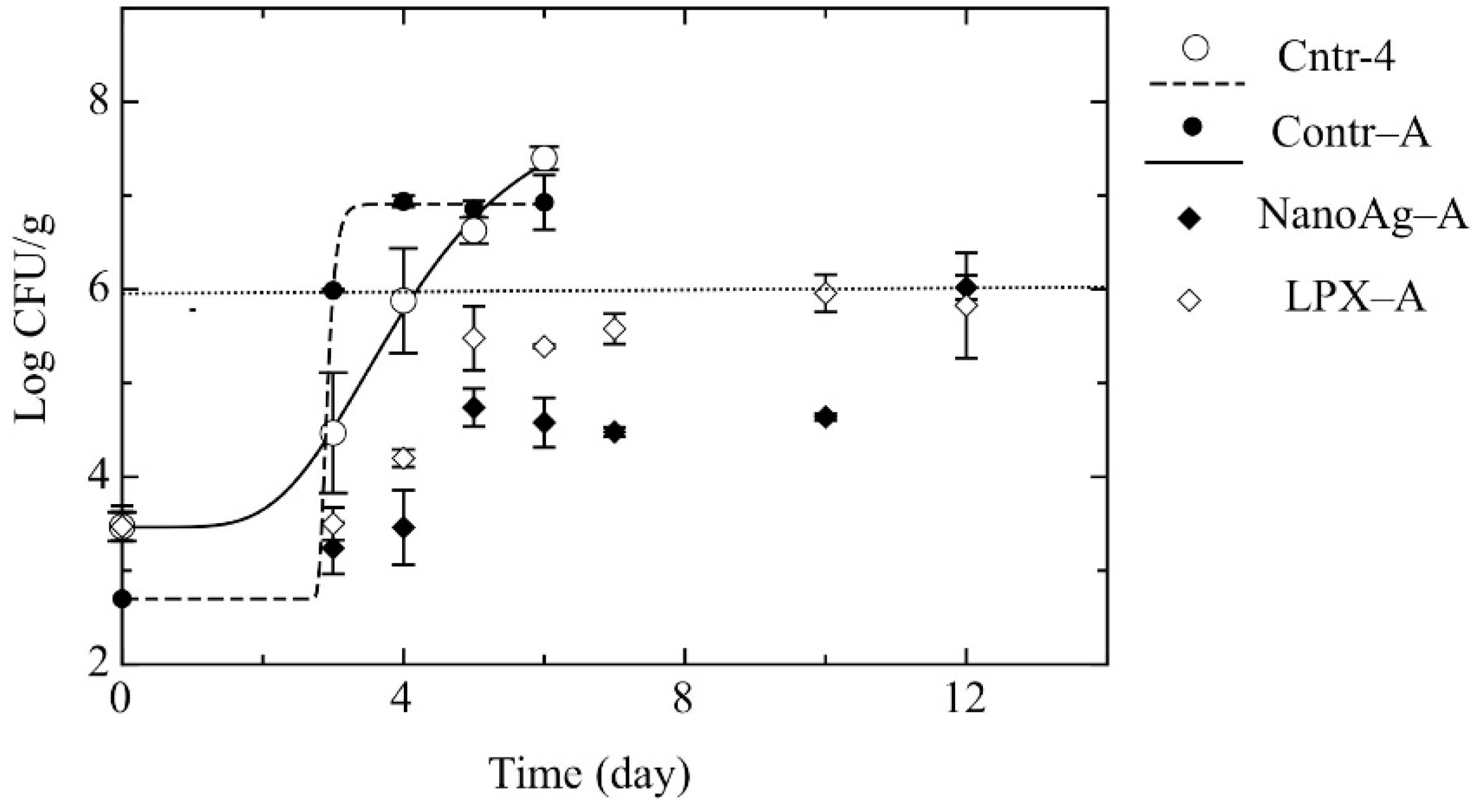
- Step 4: In this step, burrata cheese with the antimicrobial compounds (lysozyme and Na2-EDTA) was produced as described in Step 3. Subsequently, the samples were coated with alginic acid loaded with silver nanoparticles (250 mg·kg−1) [NanoAg-A] or the lactoperoxidase system (10,000 mg·kg−1) [LPX-A] and packaged under MAP (65:35 CO2:N2).
2.3. Microbiological Analyses and Determination of pH
2.4. Headspace Gas Composition
2.5. Sensory Analysis
2.6. Shelf Life Calculation
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costa, C.; Conte, A.; Del Nobile, M.A. Use of Metal Nanoparticles for Active Packaging Applications. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 399–404. [Google Scholar]
- Gammariello, D.; Conte, A.; Di Giulio, S.; Attanasio, M.; Del Nobile, M.A. Shelf life of stracciatella cheese under modified atmosphere packaging. J. Dairy Sci. 2009, 92, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.A.; Conte, A.; Incoronato, A.L.; Panza, O. Modified atmosphere packaging to improve the microbial stability of Ricotta. Afr. J. Microbiol. Res. 2009, 3, 37–142. [Google Scholar]
- Selim, S. Antimicrobial activity of essential oils against Vancomycin-resistant enterococchi (VRE) and EscherichiaColi O157, H7 in feta soft cheese and minced beef meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Chouliara, I.; Karatapanis, A.E.; Kontominas, M.G.; Savvaidis, I.N. Shelf-life of a Greek whey cheese under modified atmosphere packaging. Int. Dairy J. 2007, 17, 358–364. [Google Scholar] [CrossRef]
- Dermiki, M.; Ntzimani, A.; Badeka, A.; Savvaidis, I.N.; Kontominas, N.G. Shelf-life extension and quality attributes of the whey cheese ‘‘Myzithra Kalathaki’’ using modified atmosphere packaging. LWT 2008, 41, 284–294. [Google Scholar] [CrossRef]
- Conte, A.; Brescia, I.; Del Nobile, M.A. Lysozyme/EDTA disodium salt and modified-atmosphere packaging to prolong the shelf life of burrata cheese. J. Dairy Sci. 2011, 94, 5289–5297. [Google Scholar] [CrossRef] [PubMed]
- Belewu, M.A.; Ahmed El-Imam, A.M.; Adeyemi, K.D.; Oladunjoye, S.A. Eucalyptus oil and lemon grass oil: Effect on chemical composition and shelf-life of soft cheese. Environ. Nat. Resour. Res. 2012, 2, 114–118. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Souza, B.W.S.; Avides, M.D.C.; Vicente, A.A. Shelf life extension of ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against Listeria monocytogenes. Agric. Food Chem. 2010, 58, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, A.L.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Agar hydrogel with silver nanoparticles to prolong the self life of Fiord di Latte cheese. J. Dairy Sci. 2011, 94, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Lucera, A.; Lacivita, V.; Saccotelli, M.A.; Conte, A.; Del Nobile, M.A. Packaging optimisation for portioned Canestrato di Moliterno cheese. Int. J. Dairy Technol. 2016, 69, 401–409. [Google Scholar] [CrossRef]
- Fernandez, A.; Soriano, E.; Lopez Carballo, G.; Picouet, P.; Lloret, E.; Gavara, R.; Hernandez-Mùnoz, P. Preservation of aseptic conditions in absorbent pads by using silver nanotechnology. Food Res. Int. 2009, 42, 1105–1112. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Conte, A.; Longano, D.; Costa, C.; Ditaranto, N.; Ancona, A.; Cioffi, N.; Scrocco, C.; Sabbatini, L.; Contò, F.; Del Nobile, M.A. A novel preservation technique applied to fiordilatte cheese. Innov. Food Sci. Emerg. Technol. 2013, 19, 158–165. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Lucera, A.; Esposto, D.; Conte, A.; Faccia, M.; Zambrini, A.V.; Del Nobile, M.A. Packaging optimisation to prolong the shelf life of fiordilatte cheese. J. Dairy Res. 2015, 1, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Conte, A.; Buonocore, G.G.; Lavorgna, M.; Del Nobile, M.A. Calcium-alginate coating loaded with silver-montmorillonite nanoparticles to prolong the shelf-life of fresh-cut carrots. Food Res. Int. 2012, 48, 164–169. [Google Scholar] [CrossRef]
- Gammariello, D.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Bio-based nanocomposite coating to preserve quality of Fior di latte cheese. J. Dairy Sci. 2011, 94, 5298–5304. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, M.; Bevilacqua, A.; Corbo, M.R.; Pati, S.; Del Nobile, M.A. Use of active compounds for prolonging the shelf life of mozzarella cheese. Int. Dairy J. 2008, 18, 624–630. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Lucera, A.; Sinigaglia, M.; Corbo, M.R. Synergic antimicrobial activity of lysozyme, nisin, and EDTA against Listeria monocytogenes in ostrich meat patties. J. Food Sci. 2010, 75, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; O’Rourke, A.L.; McLay, J.; Simmonds, R. Use of a ground beef model to assess the effect of the lactoperoxidase system on the growth of Escherichia coli O157:H7, Listeria monocytogenes and Staphylococcus aureus in red meat. Int. J. Food Microbiol. 2000, 57, 147–158. [Google Scholar] [CrossRef]
- Vannini, L.; Lanciotti, R.; Baldi, D.; Guerzoni, M.E. Interactions between high pressure homogenization and antimicrobial activity of lysozyme and lactoperoxidase. Int. J. Food Microbiol. 2004, 94, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Seifu, E.; Buys, E.M.; Donkin, E.F. Significance of the lactoperoxidase system in the dairy industry and its potential applications: A review. Trends Food Sci. Technol. 2005, 16, 137–154. [Google Scholar] [CrossRef]
- Seifu, E.; Buys, E.M.; Donkin, E.F.; Petzer, I.M. Antibacterial activity of the lactoperoxidase system against food-borne pathogens in Saanen and South African Indigenous goat milk. Food Contr. 2004, 15, 447–452. [Google Scholar] [CrossRef]
- Conte, A.; Gammariello, D.; Di Giulio, S.; Attanasio, M.; Del Nobile, M.A. Active coating and modified-atmosphere packaging to extend the shelf life of Fior di Latte cheese. J. Dairy Sci. 2009, 92, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Angiolillo, L.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. The effect of incorporating calcium lactate in the saline solution on improving the shelf life of Fiordilatte cheese. Int. J. Dairy Technol. 2013, 66, 373–381. [Google Scholar] [CrossRef]
- Chaix, E.; Guillaume, C.; Guillard, V. Oxygen and Carbon Dioxide Solubility and Diffusivity in Solid Food Matrices: A Review of Past and Current Knowledge. Compr. Rev. Food Sci. Food Saf. 2014, 13, 261–286. [Google Scholar] [CrossRef]
- Cruz, R.S.; Soares, N.D.F.F.; de Andrade, N.J. Evaluation of oxygen absorber on antimicrobial preservation of lasagna—Type fresh pasta under vacuum packed. Ciênc. Agroretec. 2006, 30, 1135–1138. [Google Scholar] [CrossRef]
- Kampf, N.; Nussinovitch, A. Hydrocolloid coating of cheeses. Food Hydrocoll. 2000, 14, 531–537. [Google Scholar] [CrossRef]
- Laurienzo, P.; Malinconico, M.; Pizzano, R.; Manzo, C.; Piciocchi, N.; Sorrentino, A.; Volpe, M.G. Natural polysaccharide-based gels for dairy food preservation. J. Dairy Sci. 2006, 89, 2856–2864. [Google Scholar] [CrossRef]

| Experimental Steps | Antimicrobial Compounds in the Burrata Filling | Coating with Antimicrobial Compound | Headspace Gas Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Antimicrobial | Lysozyme/Na2-EDTA | Absent | No. Antimicrobial | Silver Nanoparticles | Lactoperoxi_dase system | Air | MAP-1 | MAP-2 | MAP-3 | ||
| Step-1 | Cntr-1 | √ | √ | √ | |||||||
| MAP-1 | √ | √ | √ | ||||||||
| MAP-2 | √ | √ | √ | ||||||||
| MAP-3 | √ | √ | √ | ||||||||
| Step-2 | Cntr-2 | √ | √ | √ | |||||||
| Coat-Air | √ | √ | √ | ||||||||
| Coat-MAP | √ | √ | √ | ||||||||
| Step-3 | Cntr-3 | √ | √ | √ | |||||||
| LysEDTA-Air | √ | √ | √ | ||||||||
| LysEDTA-MAP | √ | √ | √ | ||||||||
| Coat-Lys EDTA | √ | √ | √ | ||||||||
| Step-4 | Cntr-4 | √ | √ | √ | |||||||
| Cntr-A | √ | √ | √ | ||||||||
| NanoAg-A | √ | √ | √ | ||||||||
| LPX-A | √ | √ | √ | ||||||||
| Experimental Steps | MAL (Day) | SAL (Day) | Shelf Life (Day) | Shelf Life Increase (%) | |
|---|---|---|---|---|---|
| Step-1 | Cntr-1 | 1.87 ± 0.11 a | 2.11 ± 0.04 a | 1.87 ± 0.11 a | – |
| MAP-1 | 1.98 ± 0.05 a | 3.81 ± 0.66 b | 1.98 ± 0.05 a | 5.88 | |
| MAP-2 | 2.34 ± 0.04 b | 3.84 ± 0.01 b | 2.34 ± 0.04 b | 25.13 | |
| MAP-3 | 2.53 ± 0.09 c | 5.04 ± 0.51 c | 2.53 ± 0.09 c | 35.29 | |
| Step-2 | Cntr-2 | 1.68 ± 0.02 a | 3.69 ± 0.01 a | 1.68 ± 0.02 a | – |
| Coat-Air | 1.80 ± 0.18 a | 4.19 ± 0.01 b | 1.80 ± 0.18 a | 7.15 | |
| Coat-MAP | 2.61 ± 0.06 b | 5.76 ± 0.27 c | 2.61 ± 0.06 b | 55.35 | |
| Step-3 | Cntr-3 | 1.67 ± 0.10 a | 3.81 ± 0.02 a | 1.67 ± 0.10 a | – |
| LysEDTA-Air | 2.10 ± 0.16 a | 3.39 ± 0.18 b | 2.10 ± 0.16 a | 25.74 | |
| LysEDTA-MAP | 3.85 ± 0.05 b | 4.36 ± 0.01 c | 3.85 ± 0.05 b | 130.53 | |
| Coat-Lys EDTA | 4.55 ± 0.76 b | 5.34 ± 0.02 d | 4.55 ± 0.76 b | 172.45 | |
| Step-4 | Cntr-4 | 3.00 ± 1.00 a | 6.47 ± 0.14 a | 3.00 ± 1.00 a | – |
| Cntr-A | 4.19 ± 0.13 a | 5.62 ± 0.52 a | 4.19 ± 0.13 a | 39.66 | |
| NanoAg-A | 10 < day < 12 | 10.01 ± 0.64 b | 10.01 ± 0.64 b | 233.66 | |
| LPX-A | 9 < day < 10 | 8.21 ± 0.63 c | 8.21 ± 0.63 c | 173.66 | |
| Microorganisms | Sampling Time | Cntr-2 | Coat-Air | Coat-MAP |
|---|---|---|---|---|
| (day) | (log CFU/g) | (log CFU/g) | (log CFU/g) | |
| Enterobacteriaceae | 0 | 1.78 ± 0.24 | 1.78 ± 0.24 | 1.78 ± 0.25 |
| 3 | 5.55 ± 0.06 | 5.56 ± 0.08 | 5.84 ± 0.34 | |
| 4 | 7.44 ± 0.19 | 7.29 ± 0.30 | 6.62 ± 0.73 | |
| 6 | – | – | 7.43 ± 0.80 | |
| Pseudomonas spp. | 0 | 3.41 ± 0.10 | 3.41 ± 0.10 | 3.41 ± 0.10 |
| 3 | 6.54 ± 0.11 | 6.60 ± 0.01 | 6.29 ± 0.29 | |
| 4 | 6.57 ± 0.30 | 6.32 ± 0.16 | 5.93 ± 0.21 | |
| 6 | – | – | 6.73 ± 0.21 |
| Samples | Lactic Acid Bacteria | Mesophylic Lactococci | ||
|---|---|---|---|---|
| log(i) CFU/g | log(f) CFU/g | log(i) CFU/g | log(f) CFU/g | |
| Cntr-4 | 5.11 ± 0.37 | 7.36 ± 0.35 | 6.08 ± 0.07 | 7.42 ± 0.11 |
| Cntr-A | 4.88 ± 0.03 | 6.99 ± 0.04 | 6.15 ± 0.09 | 7.34 ± 0.02 |
| NanoAg-A | 4.88 ± 0.03 | 7.12 ± 0.02 | 6.05 ± 0.03 | 7.49 ± 0.13 |
| LPX-A | 4.71 ± 0.05 | 7.27 ± 0.21 | 6.14 ± 0.17 | 7.62 ± 0.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.; Lucera, A.; Conte, A.; Zambrini, A.V.; Del Nobile, M.A. Technological Strategies to Preserve Burrata Cheese Quality. Coatings 2017, 7, 97. https://doi.org/10.3390/coatings7070097
Costa C, Lucera A, Conte A, Zambrini AV, Del Nobile MA. Technological Strategies to Preserve Burrata Cheese Quality. Coatings. 2017; 7(7):97. https://doi.org/10.3390/coatings7070097
Chicago/Turabian StyleCosta, Cristina, Annalisa Lucera, Amalia Conte, Angelo Vittorio Zambrini, and Matteo Alessandro Del Nobile. 2017. "Technological Strategies to Preserve Burrata Cheese Quality" Coatings 7, no. 7: 97. https://doi.org/10.3390/coatings7070097





