Progress in Wear Resistant Materials for Total Hip Arthroplasty
Abstract
:1. Introduction
2. History of Development of Artificial Hip Joints
3. Ultra-High Molecular Weight Polyethylene
4. Metallic Materials
5. Ceramics
5.1. Alumina
5.2. Zirconia
5.3. Silicon Nitride
5.4. Alumina-Zirconia Composites
6. Ultra-Hard Coatings on Metals
7. Hybrid Design of Oxide Ceramic Layer on Metal: Oxinium™
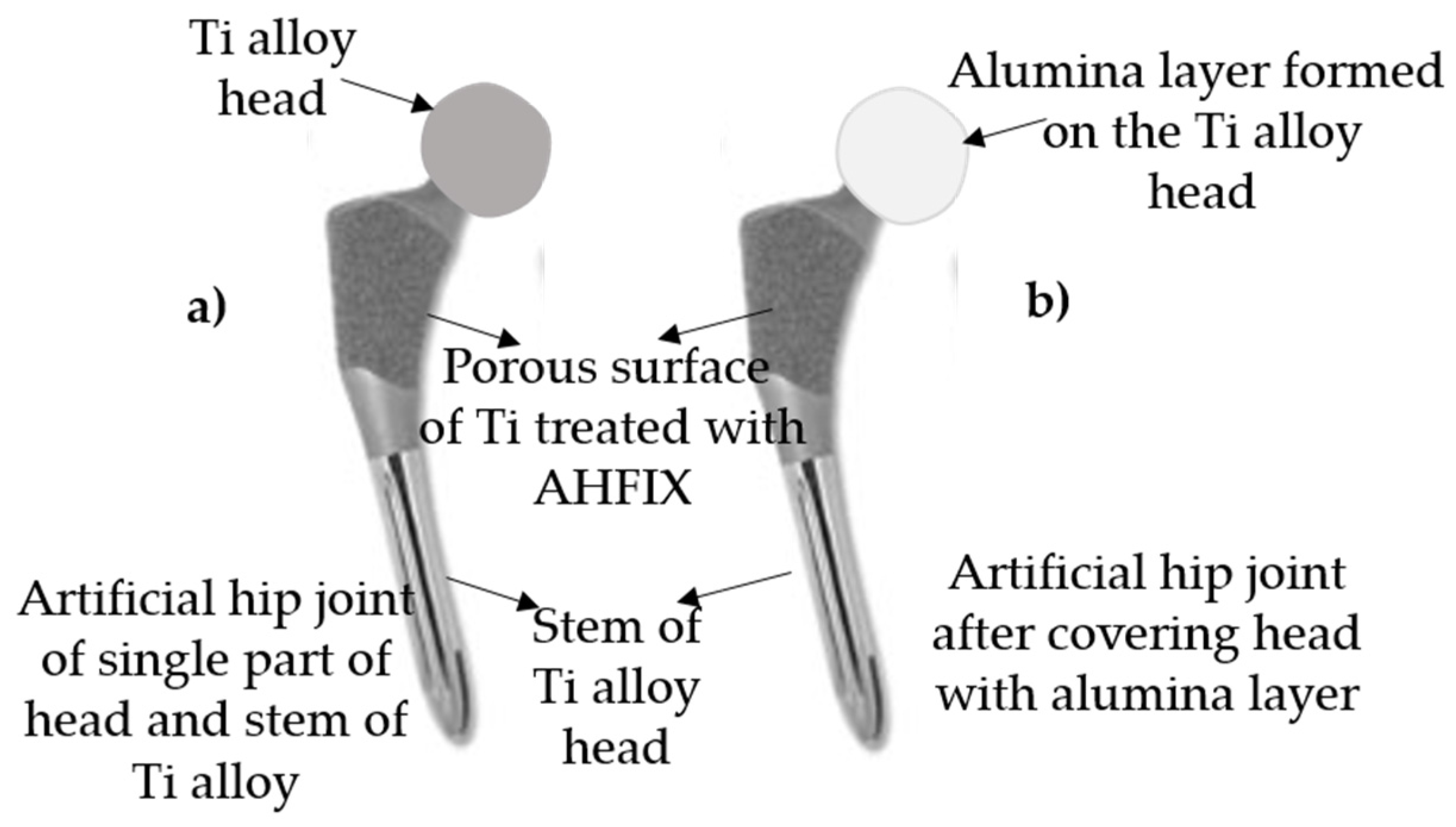
8. Rationale for Design of New Kind of Ceramic/Metal Hybrid Artificial Joint
8.1. Selection and Design of Materials
8.2. Rationale for New Artificial Hip Joint Design
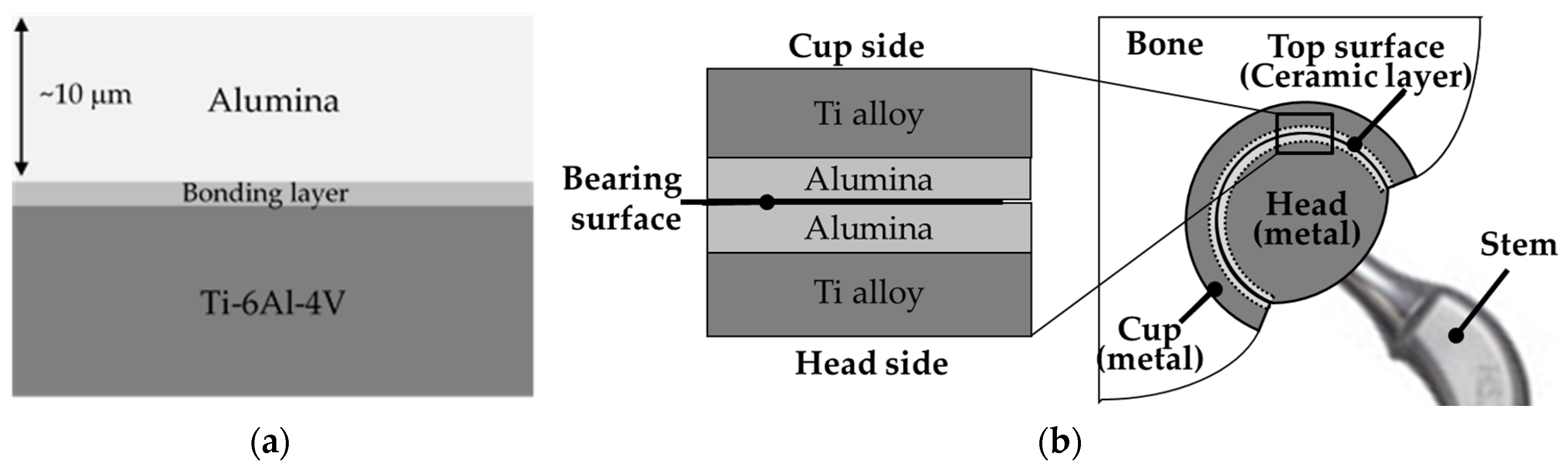
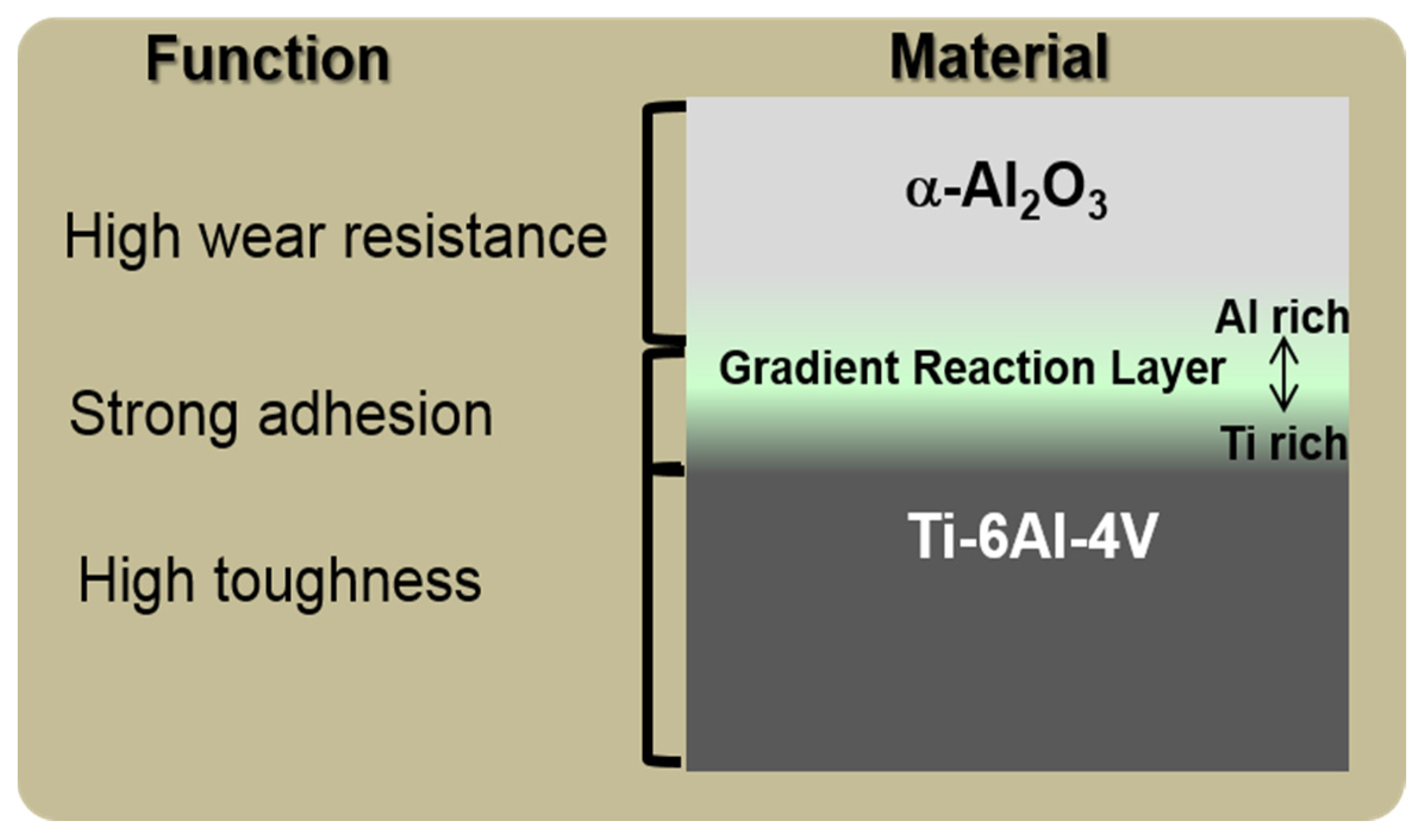
8.3. Science and Technology of Novel Artificial Hip Joint: A Layer of Dense α-Alumina on the Ti Alloy
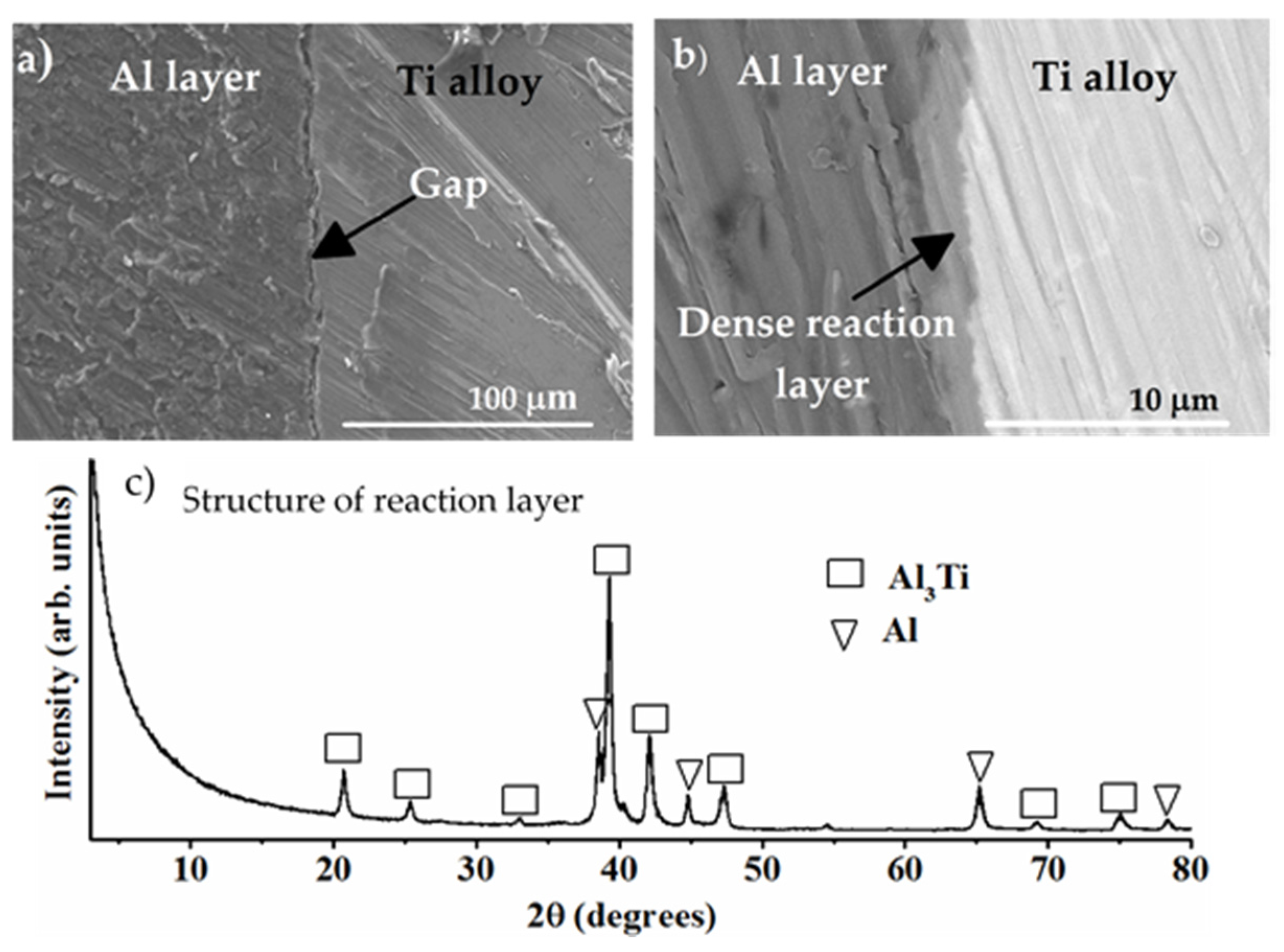
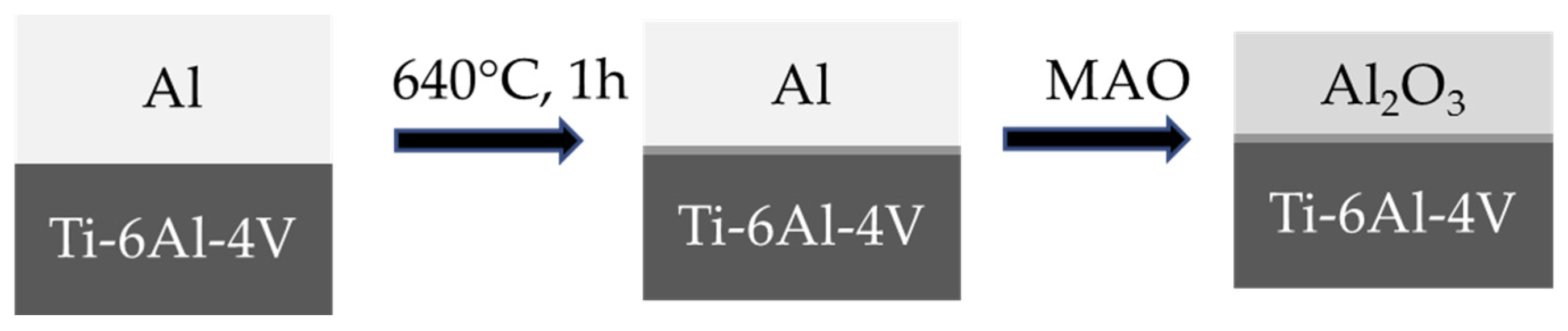
8.3.1. Formation of Al Layer on the Ti Alloy
8.3.2. Formation of Reaction Layer at Alumina-Ti Alloy Interface
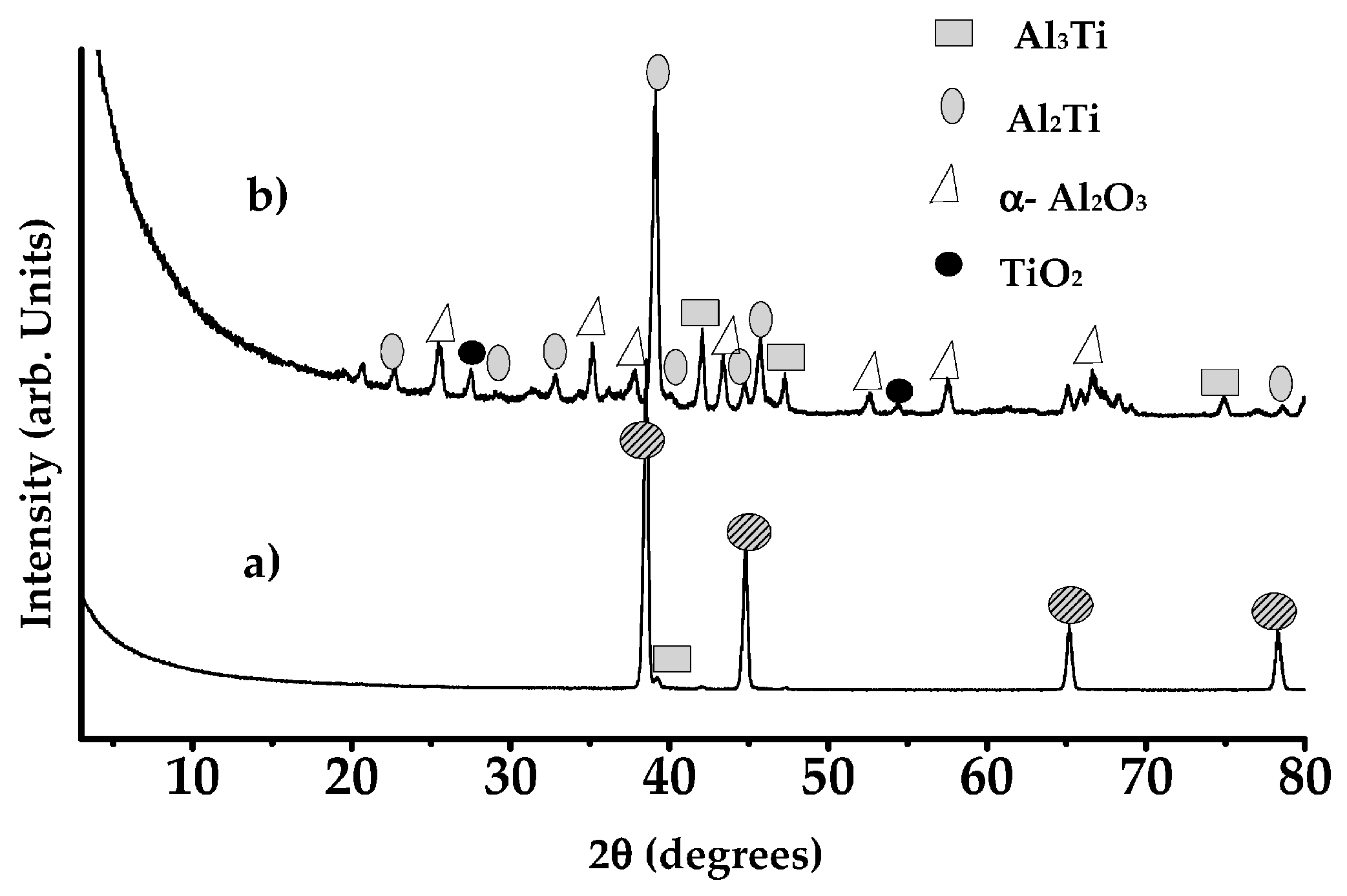
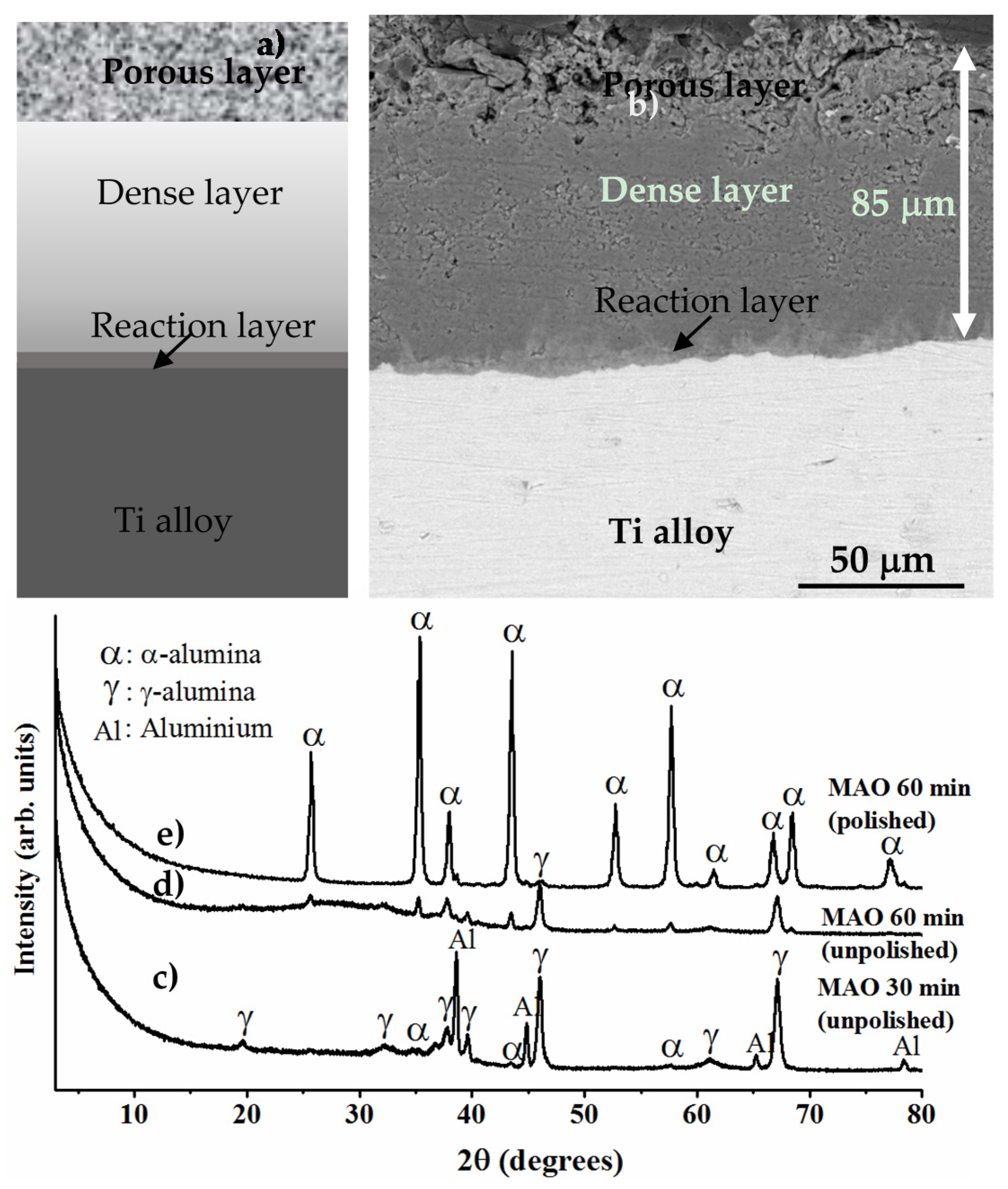
8.3.3. Formation of Alumina Layer on the Ti Alloy
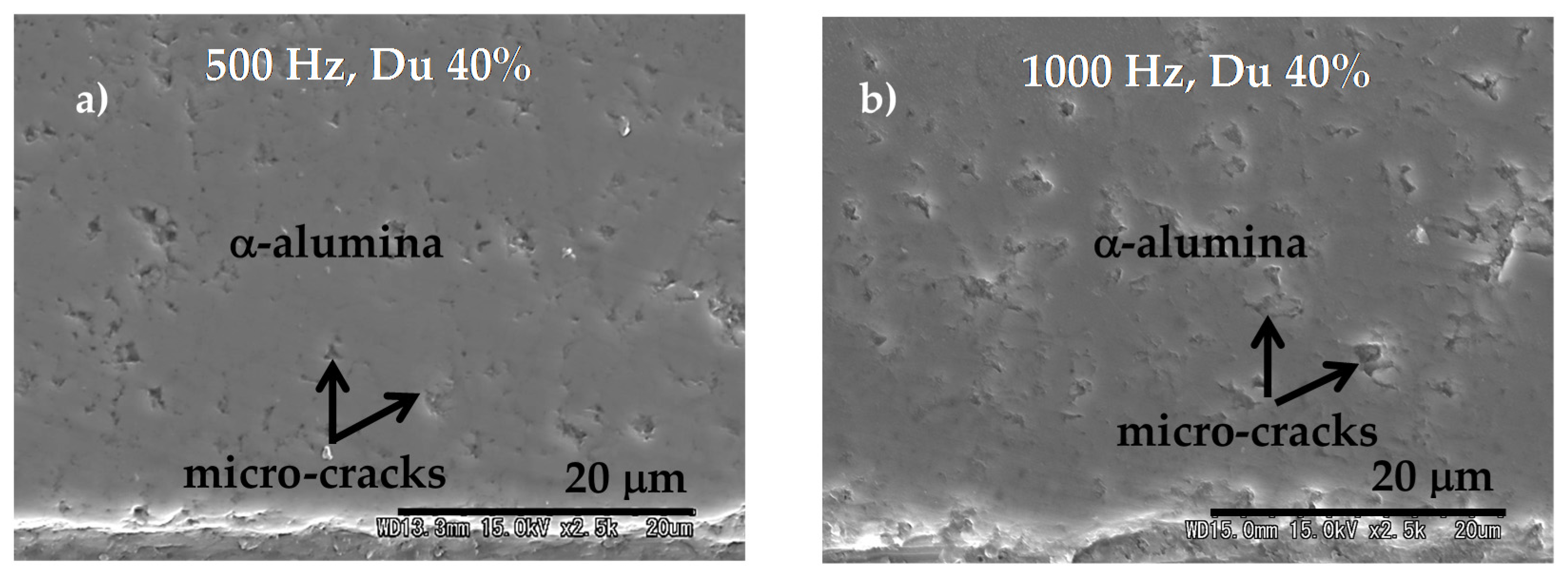
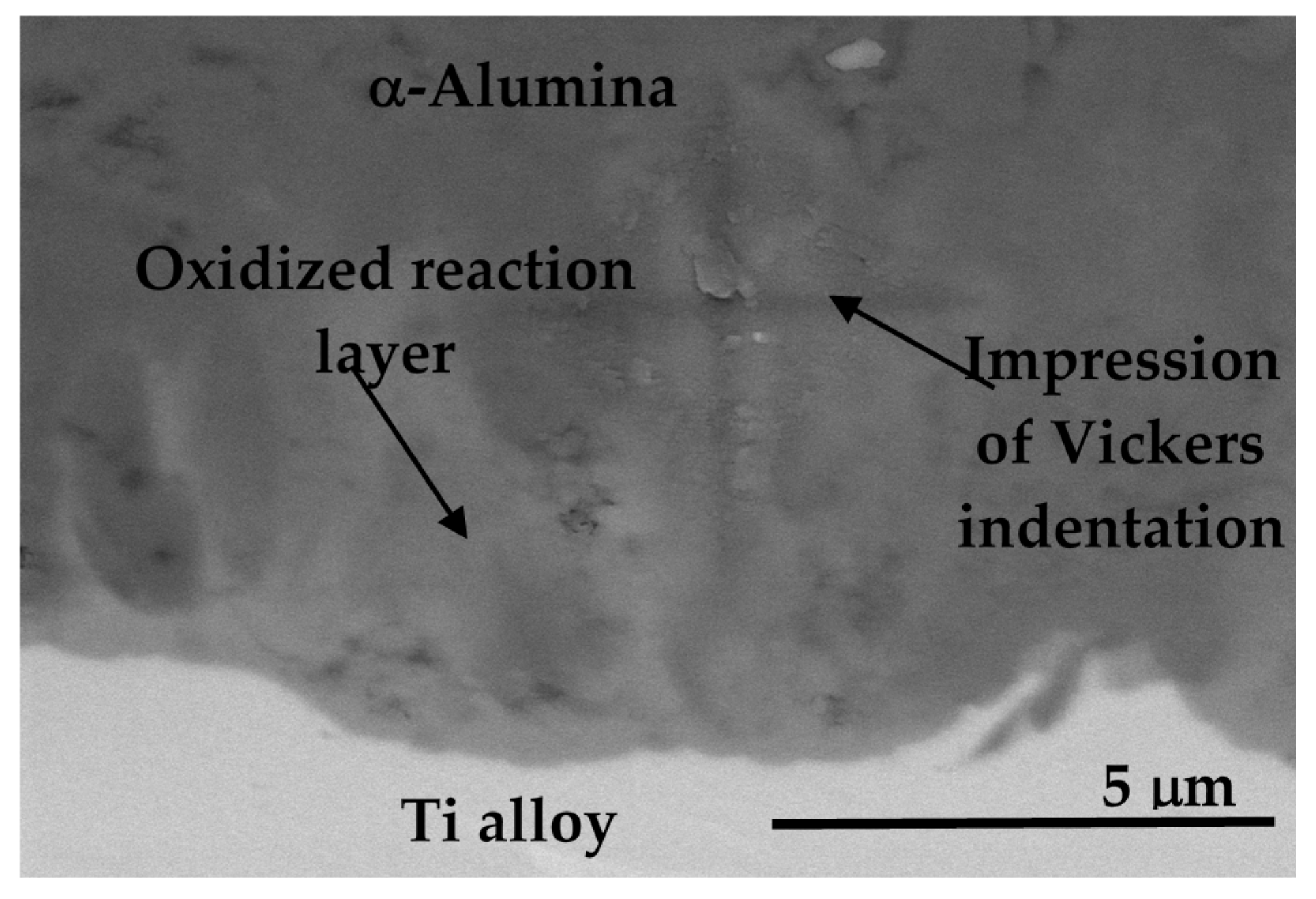
8.3.4. Adhesion of α-Alumina Layer with the Ti Alloy
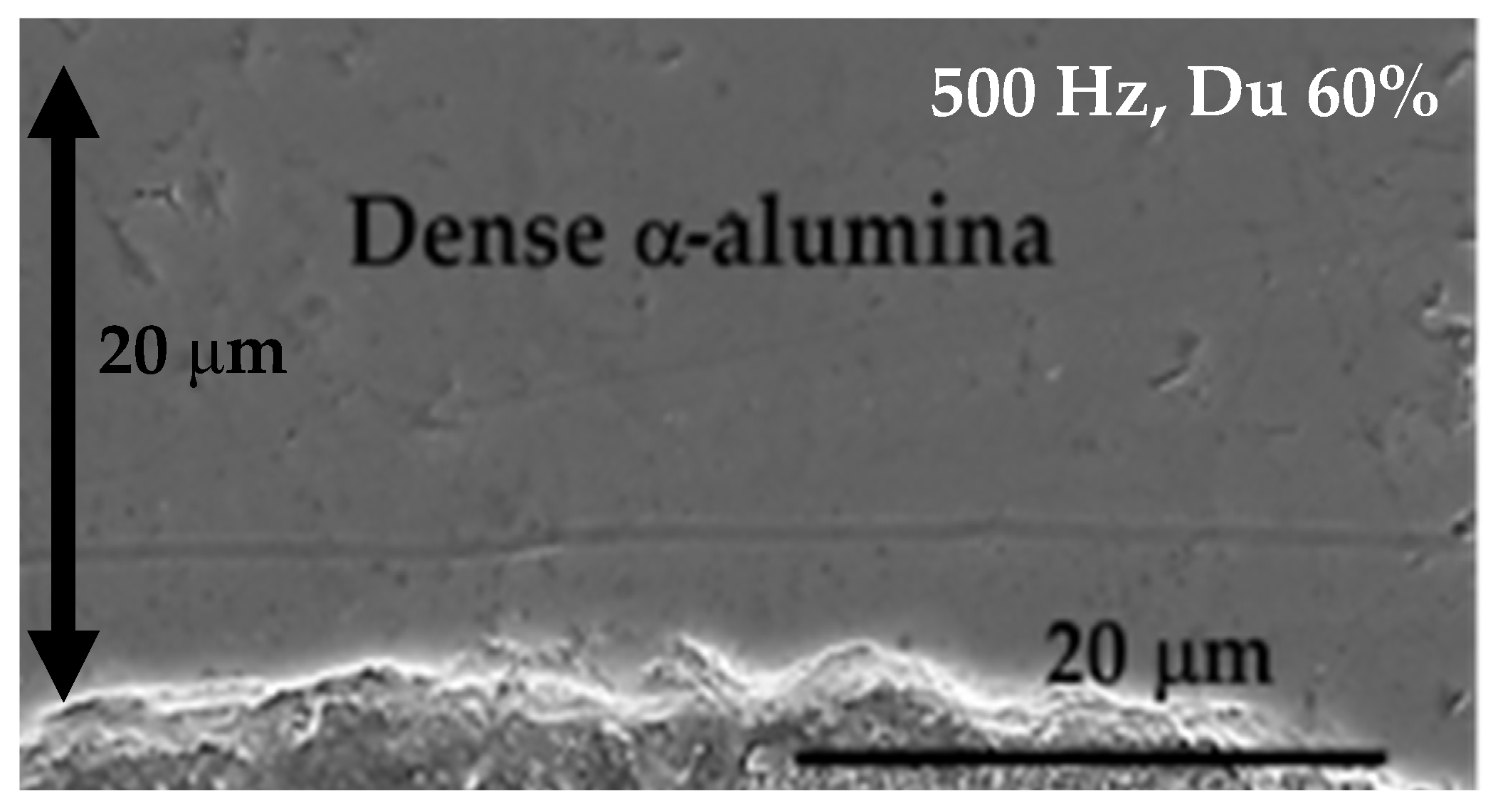
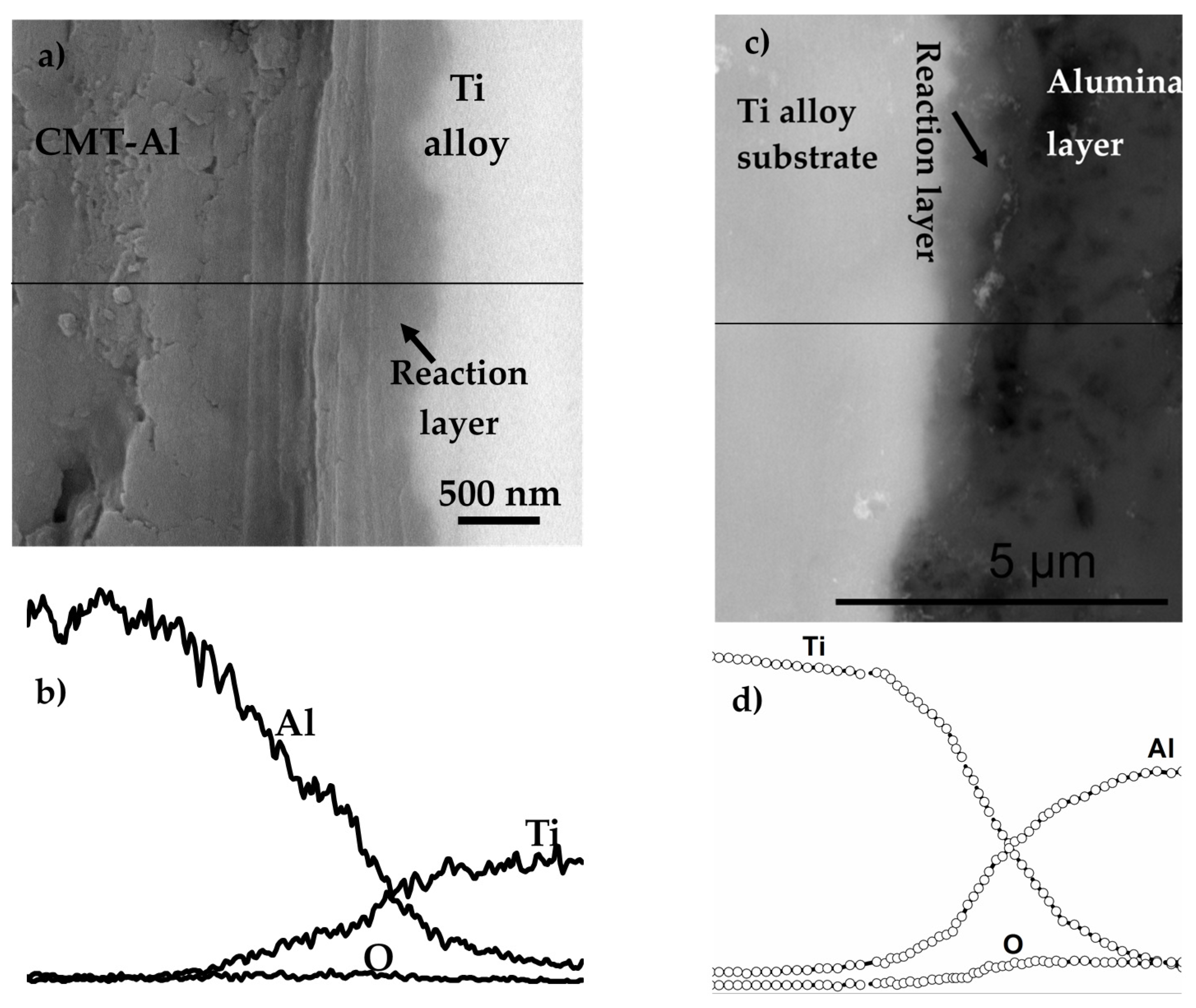
8.3.5. Dense Alumina Layer on Ti Alloy by Cold Metal Transfer and MAO Methods
9. Summary and Conclusions
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Renkawitz, T.; Santori, F.S.; Grifka, J.; Valverde, C.; Morlock, M.M.; Learmonth, I.D. A new short uncemented, proximally fixed anatomic femoral implant with a prominent lateral flare: Design rationals and study design of an international clinical trial. BMC Musculoskelet. Disord. 2008, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Steens, W.; Boettner, F.; Bader, R.; Skripitz, R.; Schneeberger, A. Bone mineral density after implantation of a femoral neck hip prosthesis-a prospective 5 year follow-up. BMC Musculoskelet. Disord. 2015, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yamada, N.; Hanada, S.; Jung, T.K.; Itoi, E. The bone tissue compatibility of a new Ti-Nb-Sn alloy with a low Young’s modulus. Acta Biomater. 2011, 7, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Bao, Z.Z.; Meng, Q.K.; Hu, L.; Zhao, X.Q. A novel metastable Ti-25Nb-2Mo-4Sn alloy with high strength and low Young’s modulus. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 3447–3451. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity beta-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Okazaki, Y. A new Ti-15Zr-4Nb-4Ta alloy for medical applications. Curr. Opin. Solid State Mater. Sci. 2001, 5, 45–53. [Google Scholar] [CrossRef]
- Bai, X.; Sandukas, S.; Appleford, M.R.; Ong, J.L.; Rabiei, A. Deposition and investigation of functionally graded calcium phosphate coatings on titanium. Acta Biomater. 2009, 5, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Sandukas, S.; Appleford, M.; Ong, J.L.; Rabiei, A. Antibacterial effect and cytotoxicity of Ag-doped functionally graded hydroxyapatite coatings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Lucas, L.C.; Lacefield, W.R.; Rigney, E.D. Structure solubility and bond strength of thin calcium-phosphate coatings produced by ion-beam sputter deposition. Biomaterials 1992, 13, 249–254. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Kim, K.H.; Ong, J.L. Review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J. Biomed. Mater. Res. 1996, 32, 409–417. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nishiguchi, S.; Nakamura, T. Graded surface structure of bioactive titanium prepared by chemical treatment. J. Biomed. Mater. Res. 1999, 45, 100–107. [Google Scholar] [CrossRef]
- Kim, H.M.; Takadama, H.; Miyaji, F.; Kokubo, T.; Nishiguchi, S.; Nakamura, T. Formation of bioactive functionally graded structure on Ti-6Al-4V alloy by chemical surface treatment. J. Mater. Sci. Mater. Med. 2000, 11, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kizuki, T.; Takadama, H.; Matsushita, T.; Nakamura, T.; Kokubo, T. Preparation of bioactive Ti metal surface enriched with calcium ions by chemical treatment. Acta Biomater. 2010, 6, 2836–2842. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Pattanayak, D.K.; Yamaguchi, S.; Takadama, H.; Matsushita, T.; Kawai, T.; Takemoto, M.; Fujibayashi, S.; Nakamura, T. Positively charged bioactive Ti metal prepared by simple chemical and heat treatments. J. R. Soc. Interface 2010, 7, S503–S513. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Christensen, S.D.; Malhi, A.S.; Wannomae, K.K.; Muratoglu, O.K. Wear resistance and mechanical properties of highly cross-linked, ultrahigh-molecular weight polyethylene doped with vitamin E. J. Arthroplast. 2006, 21, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Muratoglu, O.K. Vitamin E diffused, highly crosslinked UHMWPE: A review. Int. Orthop. 2011, 35, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Kawaguchi, H.; Ishihara, K.; Kyomoto, M.; Karita, T.; Ito, H.; Nakamura, K.; Takatori, Y. Wear resistance of artificial hip joints with poly(2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: Comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials 2009, 30, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Moro, T.; Iwasaki, Y.; Miyaji, F.; Kawaguchi, H.; Takatori, Y.; Nakamura, K.; Ishihara, K. Superlubricious surface mimicking articular cartilage by grafting poly(2-methacryloyloxyethyl phosphorylcholine) on orthopaedic metal bearings. J. Biomed. Mater. Res. Part A 2009, 91, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.C.; Manaka, M.; Green, D.D.; Williams, P.; Pezzotti, G.; Kim, Y.H.; Ries, M.; Sugano, N.; Sedel, L.; Delauney, C.; et al. Current status of zirconia used in total hip implants. J. Bone Jt. Surg. Am. 2003, 85, 73–84. [Google Scholar] [CrossRef]
- Begand, S.; Oberbach, T.; Glien, W. Investigations of the mechanical properties of an alumina toughened zirconia ceramic for an application in joint prostheses. Key Eng. Mater. 2005, 284, 1019–1022. [Google Scholar] [CrossRef]
- Al-Hajjar, M.; Jennings, L.M.; Begand, S.; Oberbach, T.; Delfosse, D.; Fisher, J. Wear of novel ceramic-on-ceramic bearings under adverse and clinically relevant hip simulator conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, L.W.; Rosen, V.B.; Mangin, S.P.; Treska, M.; Hunter, G. Oxidation microstructures and interfaces in the oxidized zirconium knee. Int. J. Appl. Ceram. Technol. 2005, 2, 221–246. [Google Scholar] [CrossRef]
- Good, V.; Ries, M.; Barrack, R.L.; Widding, K.; Hunter, G.; Heuer, D. Reduced wear with oxidized zirconium femoral heads. J. Bone Jt. Surg. Am. 2003, 85, 105–110. [Google Scholar] [CrossRef]
- Burger, W.; Richter, H.G. High strength and toughness alumina matrix composites by transformation toughening and ‘in situ’ platelet reinforcement (ZPTA)—The new generation of bioceramics. Key Eng. Mater. 2000, 192–195, 545–548. [Google Scholar] [CrossRef]
- Brown, A.S. Hip New World. ASME Mech. Eng. 2006, 128, 28–33. [Google Scholar]
- Charnley, J.; Kamangar, A.; Longfield, M.D. The optimum size of prosthetic heads in relation to wear of plastic sockets in total replacement of hip. Med. Biol. Eng. Comput. 1969, 7, 31–39. [Google Scholar] [CrossRef]
- Amstutz, H.C.; Campbell, P.; Kossovsky, N.; Clarke, I.C. Mechanism and clinical significance of wear debris-induced osteolysis. Clin. Orthop. Relat. Res. 1992, 276, 7–18. [Google Scholar] [CrossRef]
- Boutin, P. Total arthroplasty of the hip by fritted alumina prosthesis. Experimental study and 1st clinical applications. Orthop. Traumatol. Surg. Res. 2014, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. The prolem is osteolysis. Clin. Orthop. Relat. Res. 1995, 311, 46–53. [Google Scholar]
- Kim, Y.H.; Kim, J.S.; Park, J.W.; Joo, J.H. Periacetabular osteolysis is the problem in contemporary total hip arthroplasty in young patients. J. Arthroplast. 2012, 27, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S. Compendium of highly crosslinked UHMWPEs. In UHMWPE Biomaterials Handbook; Kurtz, S., Ed.; Elsevier: New York, NY, USA, 2009; pp. 291–308. [Google Scholar]
- Oral, E.; Malhi, A.S.; Muratoglu, O.K. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials 2006, 27, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Reinitz, S.D.; Currier, B.H.; Van Citters, D.W.; Levine, R.A.; Collier, J.P. Oxidation and other property changes of retrieved sequentially annealed UHMWPE acetabular and tibial bearings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Neils, A.L.; Doshi, B.N.; Fu, J.; Muratoglu, O.K. Effects of simulated oxidation on the in vitro wear and mechanical properties of irradiated and melted highly crosslinked UHMWPE. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Bhateja, S.K.; Duerst, R.W.; Aus, E.B.; Andrews, E.H. Free radicals trapped in polyethylene crystals. J. Macromol. Sci. Part B Phys. 1995, 34, 263–272. [Google Scholar] [CrossRef]
- Jahan, M.S.; King, M.C.; Haggard, W.O.; Sevo, K.L.; Parr, J.E. A study of long-lived free radicals in gamma-irradiated medical grade polyethylene. Radiat. Phys. Chem. 2001, 62, 141–144. [Google Scholar] [CrossRef]
- Lewis, G. Properties of crosslinked ultra-high-molecular-weight polyethylene. Biomaterials 2001, 22, 371–401. [Google Scholar] [CrossRef]
- Dumbleton, J.H.; D’Antonio, J.A.; Manley, M.T.; Capello, W.N.; Wang, A. The basis for a second-generation highly cross-linked UHMWPE. Clin. Orthop. Relat. Res. 2006, 453, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.H.; Van Citters, D.W.; Currier, J.H.; Carlson, E.M.; Tibbo, M.E.; Collier, J.P. In vivo oxidation in retrieved highly crosslinked tibial inserts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 441–448. [Google Scholar] [PubMed]
- Kurtz, S.M.; Gawel, H.A.; Patel, J.D. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin. Orthop. Relat. Res. 2011, 469, 2262–2277. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Neils, A.L.; Wannomae, K.K.; Muratoglu, O.K. Novel active stabilization technology in highly crosslinked UHMWPEs for superior stability. Radiat. Phys. Chem. 2014, 105, 6–11. [Google Scholar] [CrossRef]
- Green, J.M.; Hallab, N.J.; Liao, Y.S.; Narayan, V.; Schwarz, E.M.; Xie, C. Anti-oxidation treatment of ultra high molecular weight polyethylene components to decrease periprosthetic osteolysis: Evaluation of osteolytic and osteogenic properties of wear debris particles in a murine calvaria model. Curr. Rheumatol. Rep. 2013, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, N.H.; Greene, M.E.; Nebergall, A.K.; Nielsen, P.T.; Laursen, M.B.; Troelsen, A.; Malchau, H. Three year RSA evaluation of vitamin E diffused highly cross-linked polyethylene liners and cup stability. J. Arthroplast. 2015, 30, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K. Highly lubricated polymer interfaces for advanced artificial hip joints through biomimetic design. Polym. J. 2015, 47, 585–597. [Google Scholar] [CrossRef]
- Bragdon, C.R.; Doerner, M.; Martell, J.; Jarrett, B.; Palm, H.; Malchau, H. The 2012 John Charnley Award: Clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin. Orthop. Relat. Res. 2013, 471, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Skipor, A.K.; Campbell, P.A.; Patterson, L.M.; Anstutz, H.C.; Schmalzried, T.P.; Jacobs, J.J. Serum and urine metal levels in patients with metal-on-metal surface arthroplasty. J. Mater. Sci. Mater. Med. 2002, 13, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Wimmer, M.A. New insights into wear and biological effects of metal-on-metal bearings. J. Bone Jt. Surg. Am. 2011, 93, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Briaticovangosa, G.; Kaestner, W.; Lally, C.; Bontinck, W.J. Nickel, cobalt and chromium in consumer products: A role in allergic contact-dermatitis? Contact Dermat. 1993, 28, 15–25. [Google Scholar] [CrossRef]
- Head, W.C.; Bauk, D.J.; Emerson, R.H. Titanium as the material of choice for cementless femoral components in total hip arthroplasty. Clin. Orthop. Relat. Res. 1995, 311, 85–90. [Google Scholar]
- Walker, P.R.; Leblanc, J.; Sikorska, M. Effects of aluminum and other cations on the structure of brain and liver chromatin. Biochemistry 1989, 28, 3911–3915. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Ushida, T.; Tateishi, T.; Okazaki, Y.; Asao, S. Effect of Ti, Al, and V ions on the relative growth rate of fibroblasts (L929) and osteoblasts (MC3T3-E1) cells. Bio-Med. Mater. Eng. 1996, 6, 79–86. [Google Scholar]
- Long, M.; Crooks, R.; Rack, H.J. High-cycle fatigue performance of solution-treated metastable-beta titanium alloys. Acta Mater. 1999, 47, 661–669. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Surface modification of a Ti-6Al-4V alloy by thermal oxidation. Surf. Coat. Technol. 2005, 192, 164–170. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Effect of thermal oxidation on corrosion and corrosion-wear behaviour of a Ti–6Al–4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kurella, A.; Dahotre, N.B. Laser surface modification of Ti–6Al–4V: Wear and corrosion characterization in simulated biofluid. J. Biomater Appl. 2006, 21, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, Y.; Hu, W.; Luo, L. Surface modification of Ti-6Al-4V alloy via friction-stir processing: Microstructure evolution and dry sliding wear performance. Surf. Coat. Technol. 2014, 239, 160–170. [Google Scholar] [CrossRef]
- Oonishi, H.; Clarke, I.C.; Good, V.; Amino, H.; Ueno, M.; Masuda, S.; Oomamiuda, K.; Ishimaru, H.; Yamamoto, M.; Tsuji, E. Needs of bioceramics to longevity of total joint arthroplasty. Key Eng. Mater. 2003, 240–242, 735–754. [Google Scholar] [CrossRef]
- Sedel, L. Evolution of alumina-on-alumina implants—A review. Clin. Orthop. Relat. Res. 2000, 379, 48–54. [Google Scholar] [CrossRef]
- Sedel, L. Clinical applications of ceramic-ceramic combinations in joint replacement. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woohhead: Cambridge, UK; CRC: Boca Raton, FL, USA, 2008; pp. 688–698. [Google Scholar]
- Jarrett, C.A.; Ranawat, A.S.; Bruzzone, M.; Blum, Y.C.; Rodriguez, J.A.; Ranawat, C.S. The squeaking hip: a phenomenon of ceramic-on-ceramic total hip arthroplasty. J. Bone Jt. Surg. Am. 2009, 91, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Hannouche, D.; Zaoui, A.; Zadegan, F.; Sedel, L.; Nizard, R. Thirty years of experience with alumina-on-alumina bearings in total hip arthroplasty. Int. Orthop. 2011, 35, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.C.; Manley, M.T. How do alternative bearing surfaces influence wear behavior? J. Am. Acad. Orthop. Surg. 2008, 16, S86–S93. [Google Scholar] [CrossRef] [PubMed]
- Tipper, J.L.; Hatton, A.; Nevelos, J.E.; Ingham, E.; Doyle, C.; Streicher, R.; Nevelos, A.B.; Fisher, J. Alumina-alumina artificial hip joints. Part II: Characterisation of the wear debris from in vitro hip joint simulations. Biomaterials 2002, 23, 3441–3448. [Google Scholar] [CrossRef]
- Bizot, P.; Banallec, L.; Sedel, L.; Nizard, R. Alumina-on-alumina total hip prostheses in patients 40 years of age or younger. Clin. Orthop. Relat. Res. 2000, 379, 68–76. [Google Scholar] [CrossRef]
- Bizot, P.; Larrouy, M.; Witvoet, J.; Sedel, L.; Nizard, R. Press-fit metal-backed alumina sockets: A minimum 5-year followup study. Clin. Orthop. Relat. Res. 2000, 379, 134–142. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Cho, S.H. A comparison of polyethylene wear in hips with cobalt-chrome or zirconia heads. A prospective, randomised study. J. Bone Jt. Surg. Br. 2001, 83, 742–750. [Google Scholar] [CrossRef]
- Wroblewski, M.; Siney, P.D.; Nagai, H.; Fleming, P.A. Wear of ultra-high-molecular-weight polyethylene cup articulating with 22.225 mm zirconia diameter head in cemented total hip arthroplasty. J. Orthop. Sci. 2004, 9, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Skyrme, A.D.; Richards, S.; John, A.; Chia, M.; Walter, W.K.; Walter, W.L.; Zicat, B. Polyethylene wear rates with Zirconia and cobalt chrome heads in the ABG hip. Hip Int. 2005, 15, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G.; Pilloni, L.; Burger, W.; Muratori, F.; Richter, H.G. On the fracture of a zirconia ball head. J. Mater. Sci. Mater. Med. 2006, 17, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Kocagoz, S.; Arnholt, C.; Huet, R.; Ueno, M.; Walter, W.L. Advances in zirconia toughened alumina biomaterials for total joint replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Pezzotti, G.; Sakakura, S.; Ries, M.; Clarke, I. Zirconia ceramic femoral heads in the USA-retrieved zirconia heads-2 to 10 years out. In Proceedings of the 49th Annual Meeting of the Orthopaedic Research Society, New Orleans, LA, USA, 2–5 February 2003. [Google Scholar]
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Chevalier, J.; Pezzotti, G. Ceramics and ceramic coatings in orthopaedics. J. Eur. Ceram. Soc. 2015, 35, 4327–4369. [Google Scholar] [CrossRef]
- Chen, F.C.; Ardell, A.J. Fracture toughness of ceramics and semi-brittle alloys using a miniaturized disk-bend test. Mater. Res. Innov. 2000, 3, 250–262. [Google Scholar] [CrossRef]
- Roebben, G.; Sarbu, C.; Lube, T.; Van der Biest, O. Quantitative determination of the volume fraction of intergranular amorphous phase in sintered silicon nitride. Mater. Sci. Eng. A 2004, 370, 453–458. [Google Scholar] [CrossRef]
- Olofsson, J.; Pettersson, M.; Teuscher, N.; Heilmann, A.; Larsson, K.; Grandfield, K.; Persson, C.; Jacobson, S.; Engqvist, H. Fabrication and evaluation of SixNy coatings for total joint replacements. J. Mater. Sci. Mater. Med. 2012, 23, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Berlind, T.; Schmidt, S.; Jacobson, S.; Hultman, L.; Persson, C.; Engqvist, H. Structure and composition of silicon nitride and silicon carbon nitride coatings for joint replacements. Surf. Coat. Technol. 2013, 235, 827–834. [Google Scholar] [CrossRef]
- Bal, B.S.; Khandkar, A.; Lakshminarayanan, R.; Clarke, I.; Hoffman, A.A.; Rahaman, M.N. Fabrication and testing of silicon nitride bearings in total hip arthroplasty winner of the 2007 “HAP” PAUL Award. J. Arthroplast. 2009, 24, 110–116. [Google Scholar] [CrossRef] [PubMed]
- McEntire, B.J.; Lakshminarayanan, R.; Ray, D.A.; Clarke, I.C.; Puppulin, L.; Pezzotti, G. Silicon nitride bearings for total joint arthroplasty. Lubricants 2016, 4, 35. [Google Scholar] [CrossRef]
- Green, D.; Donaldson, T.; Williams, P.; Pezzotti, G.; Clarke, I. Long term strip wear rates of 3rd and 4th generation ceramic-on-ceramic under microseparation. In Proceedings of the 53rd Annual Meeting of the Orthopaedic Research Society, San Diego, CA, USA, 11–14 February 2007; p. 1776. [Google Scholar]
- Clarke, I.; Gustafson, A. The design of ceramics for joint replacement. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead: Cambridge, UK, 2008; pp. 106–132. [Google Scholar]
- Begand, S.; Oberbach, T.; Glien, W. ATZ—A new material with a high potential in joint replacement. Key Eng. Mater. 2005, 284–286, 983–986. [Google Scholar] [CrossRef]
- Kremers, H.M.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of total hip and knee replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.J. Nanostructured diamondlike carbon thin films for medical applications. Mater. Sci. Eng. C 2005, 25, 405–416. [Google Scholar] [CrossRef]
- Pappas, M.J.; Makris, G.; Buechel, F.F. Titanium nitride ceramic film against polyethylene: A 48-million cycle wear test. Clin. Orthop. Relat. Res. 1995, 317, 64–70. [Google Scholar]
- Hauert, R.; Falub, C.V.; Thorwarth, G.; Thorwarth, K.; Affolter, C.; Stiefel, M.; Podleska, L.E.; Taeger, G. Retrospective lifetime estimation of failed and explanted diamond-like carbon coated hip joint balls. Acta Biomater. 2012, 8, 3170–3176. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Lackner, J.M.; Major, L.; Morita, T.; Sawae, Y.; Bin Mamat, A.; Stavness, I.; Roy, C.K.; Krupka, I. Improved wear resistance of functional diamond like carbon coated Ti-6Al-4V alloys in an edge loading conditions. J. Mech. Behav. Biomed. Mater. 2016, 59, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Catledge, S.A.; Vohra, Y.K. Effect of nitrogen addition on the microstructure and mechanical properties of diamond films grown using high-methane concentrations. J. Appl. Phys. 1999, 86, 698–700. [Google Scholar] [CrossRef]
- Catledge, S.A.; Vaid, R.; Diggins, P.; Weimer, J.J.; Koopman, M.; Vohra, Y.K. Improved adhesion of ultra-hard carbon films on cobalt-chromium orthopaedic implant alloy. J. Mater. Sci. Mater. Med. 2011, 22, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Papo, M.J.; Catledge, S.A.; Vohra, Y.K. Mechanical wear behavior of nanocrystalline and multilayer diamond coatings on temporomandibular joint implants. J. Mater. Sci. Mater. Med. 2004, 15, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Abreu, C.S.; Oliveira, F.J.; Gomes, J.R.; Silva, R.F. Tribological characterization of NCD in physiological fluids. Diam. Relat. Mater. 2008, 17, 848–852. [Google Scholar] [CrossRef]
- Vila, M.; Amaral, M.; Oliveira, F.J.; Silva, R.F.; Fernandes, A.J.S.; Soares, M.R. Residual stress minimum in nanocrystalline diamond films. Appl. Phys. Lett. 2006, 89, 093109. [Google Scholar] [CrossRef]
- Ries, M.D.; Salehi, A.; Widding, K.; Hunter, G. Polyethylene wear performance of oxidized zirconium and cobalt-chromium knee components under abrasive conditions. J. Bone Jt. Surg. Am. 2002, 84, 129–135. [Google Scholar] [CrossRef]
- Evangelista, G.T.; Fulkerson, E.; Kummer, E.; Di Cesare, P.E. Surface damage to an Oxinium femoral head prosthesis after dislocation. J. Bone Jt. Surg. Br. 2007, 89, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, W.L.; Strauss, E.J.; Cardinale, M.; Herrera, L.; Kummer, F.J. Surface oxidized zirconium total hip arthroplasty head damage due to closed reduction. J. Arthroplast. 2009, 24, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Matsushita, T.; Kokubo, T.; Takadama, H. Formation of alumina layer on Ti alloy for artificial hip joint. Key Eng. Mater. 2014, 614, 200–205. [Google Scholar] [CrossRef]
- Khanna, R.; Kokubo, T.; Matsushita, T.; Nomura, Y.; Nose, N.; Oomori, Y.; Yoshida, T.; Wakita, K.; Takadama, H. Novel artificial hip joint: A layer of alumina on Ti–6Al–4V alloy formed by micro-arc oxidation. Mater. Sci. Eng. C 2015, 55, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Kokubo, T.; Matsushita, T.; Takadama, H. Fabrication of dense α-alumina layer on Ti–6Al–4V alloy hybrid for bearing surfaces of artificial hip joint. Mater. Sci. Eng. C 2016, 69, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Rajeev, G.; Takadama, H.; Rao Bakshi, S. Fabrication of dense alumina layer on Ti alloy hybrid by cold metal transfer and micro-arc oxidation methods. J. Mater. Res. 2017. [Google Scholar] [CrossRef]
- Angadji, A.; Royle, M.; Collins, S.N.; Shelton, J.C. Influence of cup orientation on the wear performance of metal-on-metal hip replacements. Proc. Inst. Mech. Eng. H 2009, 223, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.M.; O’Brien, M.K.; Stroud, N.J.; Pedersen, D.R.; Callaghan, J.J.; Brown, T.D. Hard-on-hard total hip impingement causes extreme contact stress concentrations. Clin. Orthop. Relat. Res. 2011, 469, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Barrack, R.L.; Burak, C.; Skinner, H.B. Concerns about ceramics in THA. Clin. Orthop. Relat. Res. 2004, 429, 73–79. [Google Scholar] [CrossRef]
- Langton, D.J.; Jameson, S.S.; Joyce, T.J.; Gandhi, J.N.; Sidaginamale, R.; Mereddy, P.; Lord, J.; Nargol, A.V. Accelerating failure rate of the ASR total hip replacement. J. Bone Jt. Surg. Br. 2011, 93, 1011–1106. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Tay, G.H.; Godbolt, D.B.; Crawford, R.W. Pseudotumor in a well-fixed metal-on-polyethylene uncemented hip arthroplasty. J. Arthroplast. 2012, 27, 493.e13–493.e17. [Google Scholar] [CrossRef] [PubMed]
- So, K.; Kaneuji, A.; Matsumoto, T.; Matsuda, S.; Akiyama, H. Is the bone-bonding ability of a cementless total hip prosthesis enhanced by alkaline and heat treatments? Clin. Orthop. Relat. Res. 2013, 471, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Briggs, E.P.; Walpole, A.R.; Wilshaw, P.R.; Karlsson, M.; Palsgard, E. Formation of highly adherent nano-porous alumina on Ti-based substrates: A novel bone implant coating. J. Mater. Sci. Mater. Med. 2004, 15, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Varlese, F.A.; Tului, M.; Sabbadini, S.; Pellissero, F.; Sebastiani, M.; Bemporad, E. Optimized coating procedure for the protection of TiAl intermetallic alloy against high temperature oxidation. Intermetallics 2013, 37, 76–82. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Q.M.; Sun, C.; Wang, F.H. Preparation and oxidation resistance of a crack-free Al diffusion coating on Ti22Al26Nb. Corros. Sci. 2007, 49, 3598–3609. [Google Scholar] [CrossRef]
- Chu, M.S.; Wu, S.K. The improvement of high temperature oxidation of Ti-50Al by sputtering Al film and subsequent interdiffusion treatment. Acta Mater. 2003, 51, 3109–3120. [Google Scholar] [CrossRef]
- Novoselova, T.; Celotto, S.; Morgan, R.; Fox, P.; O’Neill, W. Formation of TiAl intermetallics by heat treatment of cold-sprayed precursor deposits. J. Alloy. Compd. 2007, 436, 69–77. [Google Scholar] [CrossRef]
- Balani, K.; Laha, T.; Agarwal, A.; Karthikeyan, J.; Munroe, N. Effect of carrier gases on microstructural and electrochemical behavior of cold-sprayed 1100 aluminum coating. Surf. Coat. Technol. 2005, 195, 272–279. [Google Scholar] [CrossRef]
- Kang, K.; Won, J.; Bae, G.; Ha, S.; Lee, C. Interfacial bonding and microstructural evolution of Al in kinetic spraying. J. Mater. Sci. 2012, 47, 4649–4659. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Song, H.W.; Yerokhin, A.L.; Dowey, S.J.; Matthews, A. Thickness effects on the mechanical properties of micro-arc discharge oxide coatings on aluminium alloys. Surf. Coat. Technol. 1999, 116, 1055–1060. [Google Scholar] [CrossRef]
- Sundararajan, G.; Krishna, L.R. Mechanisms underlying the formation of thick alumina coatings through the MAO coating technology. Surf. Coat. Technol. 2003, 167, 269–277. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Pilkington, A.; Leyland, A.; Matthews, A. Oxide ceramic coatings on aluminium alloys produced by a pulsed bipolar plasma electrolytic oxidation process. Surf. Coat. Technol. 2005, 199, 150–157. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, R.; Ong, J.L.; Oral, E.; Narayan, R.J. Progress in Wear Resistant Materials for Total Hip Arthroplasty. Coatings 2017, 7, 99. https://doi.org/10.3390/coatings7070099
Khanna R, Ong JL, Oral E, Narayan RJ. Progress in Wear Resistant Materials for Total Hip Arthroplasty. Coatings. 2017; 7(7):99. https://doi.org/10.3390/coatings7070099
Chicago/Turabian StyleKhanna, Rohit, Joo L. Ong, Ebru Oral, and Roger J. Narayan. 2017. "Progress in Wear Resistant Materials for Total Hip Arthroplasty" Coatings 7, no. 7: 99. https://doi.org/10.3390/coatings7070099



