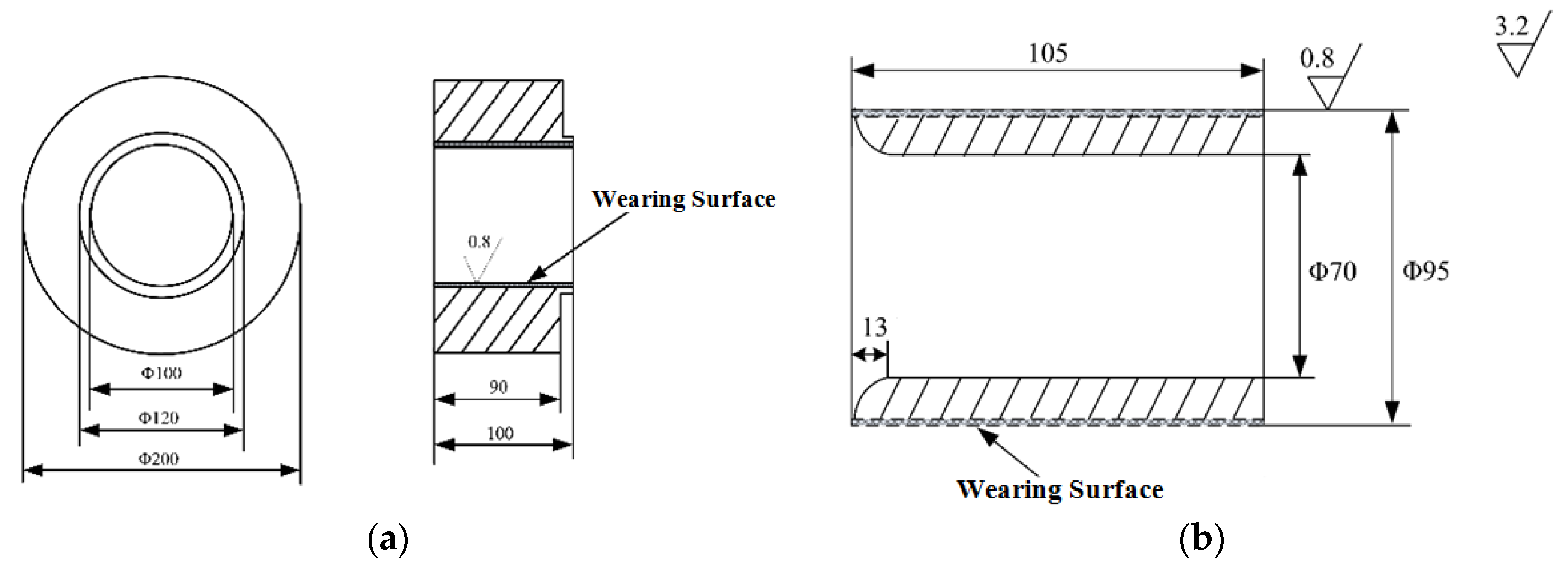
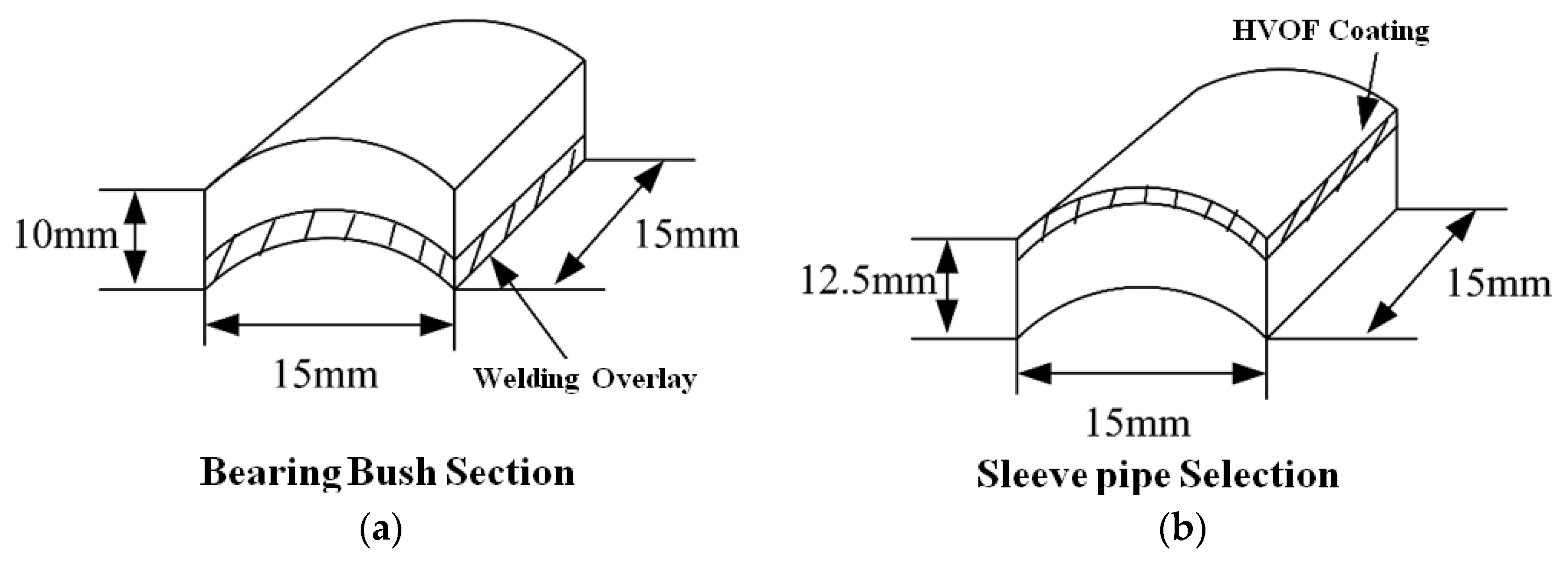
3.1. Coating Morphology, Phase Composition and Hardness
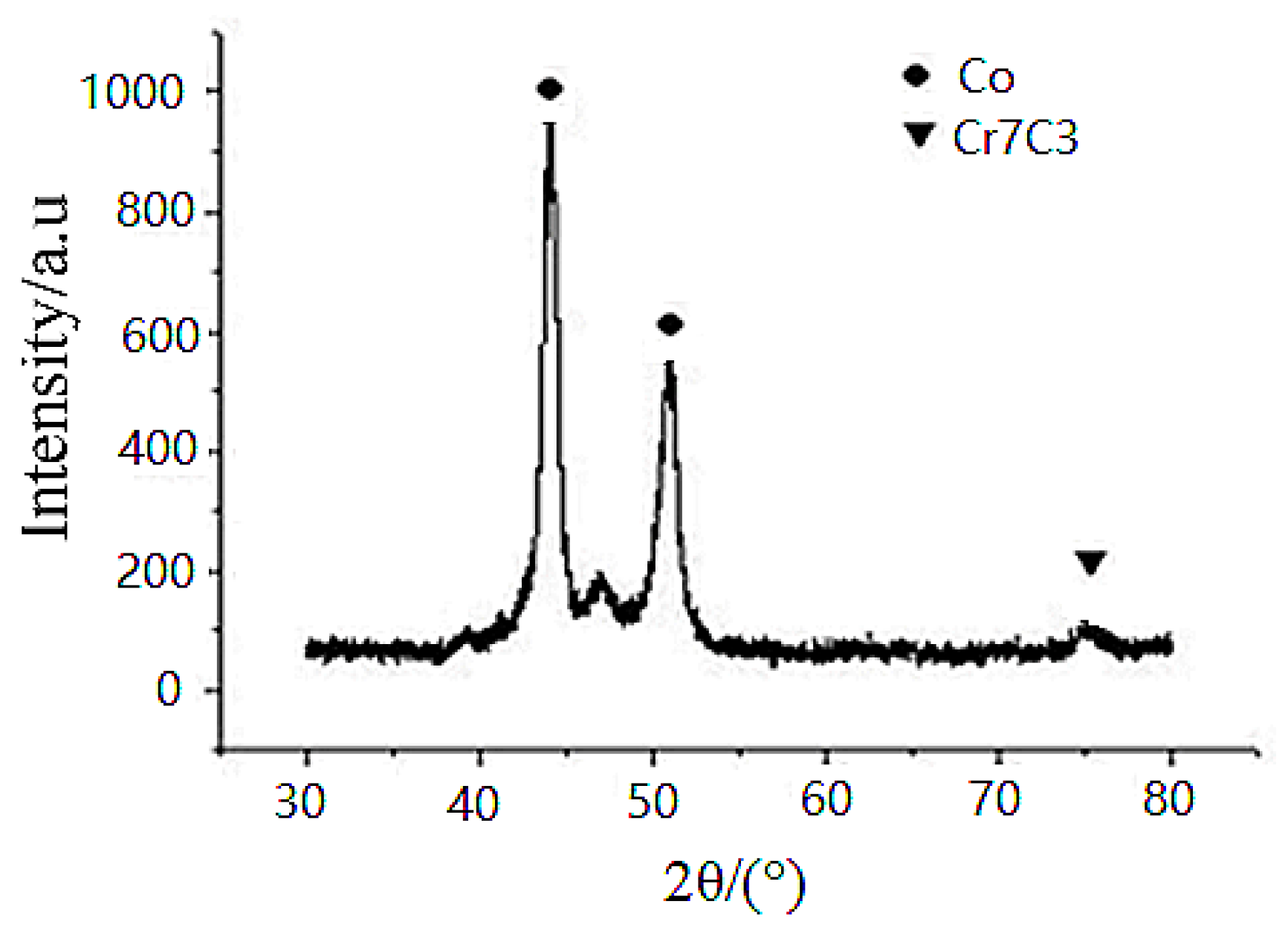
Regarding the bushing surface coating, the XRD spectrum is shown in
Figure 3. Since no Cr and W phases were detected in the XRD spectrum, this probably indicates that Cr and W atoms are in the solid solution of the FCC Co structure, while the Cr-carbide phase is the hard phase dispersed in the Co-Cr matrix phase. The deposited Co-Cr alloy has a microhardness of approximately 530–580HV0.3. A Co-Cr (70:30 atomic ratio) would exhibit a microhardness of approximately 340–350HV0.3. This slight increase in hardness is probably due to the presence of the Cr–carbide phase-dispersion strengthening effect.
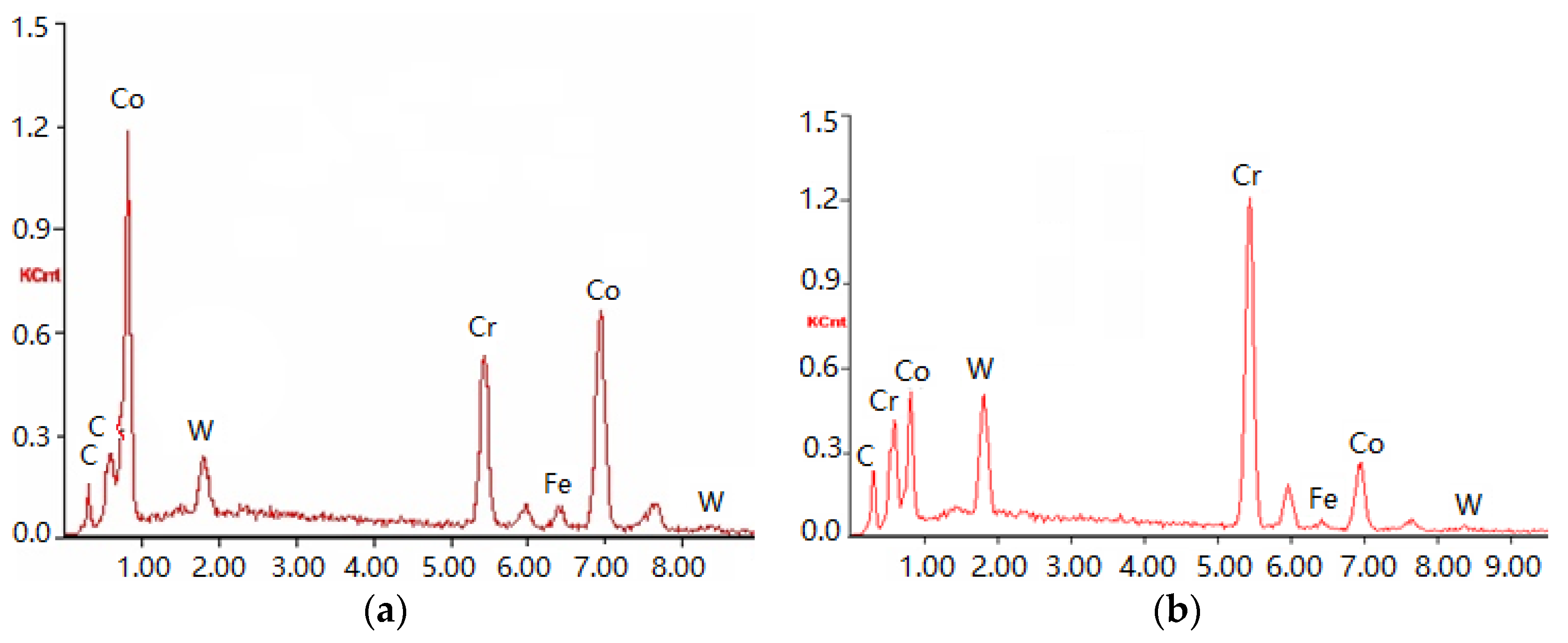
Figure 4 is an image of the welding overlay coating produced on the bushing surface. The two-phase microstructure is clearly shown, including a grey matrix phase and a dark bi-phase. To verify the chemical structure of the two distinct phases, EDS was focused on both the grey matrix Area a and the dark dispersion Area b with their spectra shown in
Figure 5a,b, respectively. The quantitative analysis of the spectrum for these two areas is listed in
Table 5. The hard-facing layer of the sink-roll shaft contains Co, Cr, W, C, Si and iron (Fe) elements, among others. The composition is the same as the powder in
Table 1, but with the Fe element, which could not be found in the powder. This may be produced by a parent metal in the process of welding. The Co content is higher in the primary phase a, while the C is very low. This can determine that it is a γ solid solution phase (γ-Co), which forms some solid solution with a certain amount of alloy elements, such as Cr and W. In the primary phase at the grain boundary eutectic b, the C content is higher due to being concentrated with Cr and W, which shows that the phase is Cr
7C
3-type carbides with a γ-Co eutectic structure.
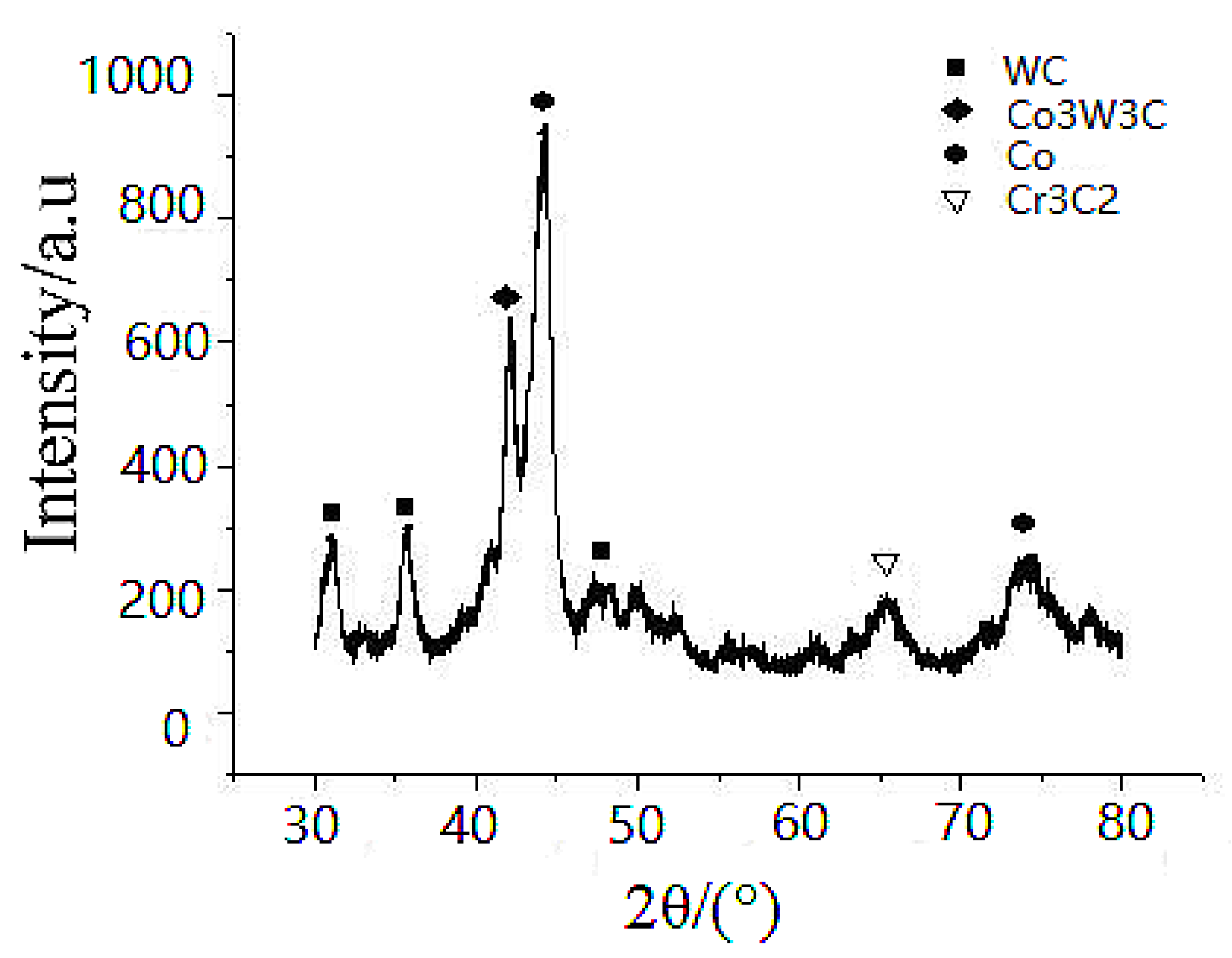
Regarding the sleeve-pipe surface coating, the XRD spectrum is shown in
Figure 6 and the XRD spectra is quite complex. It indicated that the XRD spectra of the coating shows diffraction patterns of the following: (a) major FCC-Co phase with peaks of (111), (200) and (220) at 2θ angle of ~44°, ~51° and ~72°, respectively; (b) hexagonal W carbide (WC phase) peaks of (001), (100), and (101) at 2θ angles of ~31.5°, 35.6° and 48.3°, respectively; (c) η-carbide phase Co
3W
3C at 2θ angles of ~42°; (d) a minor Cr-carbide Cr
3C
2 phase identified at 2θ angles of ~65°.
The measured Vickers microhardness is approximately 900–1200HV0.3, indicating that the deposited sleeve surface is a complex Co-Cr-Ni alloy that has a co-existence of multi-phasic carbide phases.
Figure 7 is an image of the post-laser treatment of the HVOF-sprayed coating produced on the sleeve-pipe surface. It is clearly a multi-phasic microstructure, which includes a grey facet large particulate phase (Area a), a grey contiguous smaller precipitate phase (Area b), a large irregularly shaped crystal phase (Area c) and a dark matrix phase (Area d). The EDS spectrum for all areas is listed in
Table 6.
The above results show that the coating composition and microstructure are relatively complicated. The alloy system is a Co-Cr-Ni-W series, which contains a higher proportion of Cr and Co solid solutions as well as a certain proportion of W. There are three types of precipitated phase distribution. The zone in Area a is a white WC phase, which should belong to the mix with the WC powder. The Area b zone is a grey compound carbide phase including Cr, Co, Ni and W, which should belong to the Co3W3C phase. Furthermore, this compound contains some W elements in the γ-Co solid solution. The Area c zone has some small circular particles, which are distributed like “eggs”. Its composition is mainly C and W, meaning that it should belong to the WC or W2C compounds.
3.2. Corrosion of Bushing Surface Coatings
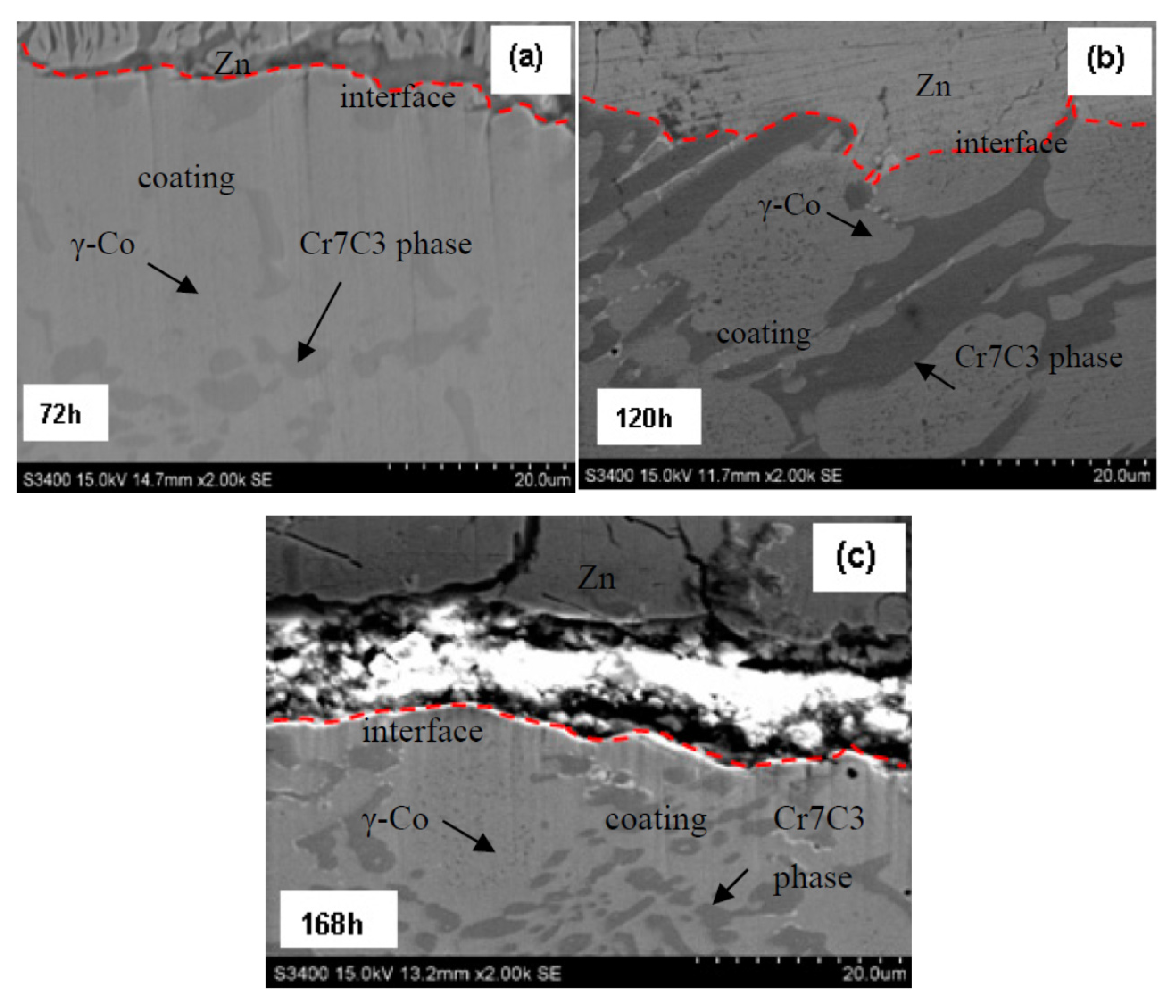
The coating has an original thickness of 5 mm. Their after-corrosion thicknesses are 4.475 mm for 72 h, 4.15 mm for 120 h and 3.65 mm for 168 h, which leads to a coating corrosion rate of 0.008 mm/h. The after-corrosion samples at the sampled times for the bushing coating for 72 h, 120 h and 168 h are shown in
Figure 8a–c, respectively.
Figure 8a shows two regions after the 72 h corrosion, including: (a) the top region with the molten Zn adhered onto the coating interface and (b) the bottom region with the coating. During the Zn corrosion, the coating morphology does not change, a new phase is not formed, while corrosion channels or cracks are not formed in the coating. In addition, the fact that there is no observed corrosion rate difference between the light-coloured alloy phase and the dark-coloured particulate phase (or eutectic phase) is likely to be the primary reason there is a uniform corrosion mechanism without the formation of cracks or interconnected porosity channels for the welding-overlaid bushing surface coating in the corrosion experiments.
Figure 8b shows the SEM image of the sample after 120 h of corrosion. There is some evidence that the matrix phase revealed a slightly higher corrosion rate than the dark-carbide particulate phase due to the presence of carbide particles protruding at the coating/molten Zn interface. However, the preferred corrosive resistance of the carbide particles did not result in a dramatic change in coating corrosion rate or the formation of cracks or corrosion channels in the coating. This is probably because the volume fraction of the carbide phase is too small to cause a dramatic corrosion rate difference in the material. This preferred corrosion difference is also evident in the 168 h corrosion sample, which is shown in
Figure 8c for the protruding dark-carbide phase at the coating/molten Zn interface. Therefore, the fabricated bushing surface coating has a uniform corrosion mechanism and its corrosion rate is predictable for the sink-roller assembly in continuous galvanised Zn-coating production applications.
3.3. Corrosion of Sleeve-Pipe Surface Coatings
The HVOF sleeve-pipe surface coating after laser treatment has a thickness of 1 mm. Their thicknesses after corrosion are 0.8 mm for 72 h, 0.6 mm for 120 h and 0.3 mm for 168 h, which leads to a coating corrosion rate of 0.0042 mm/h. This can be divided into a corrosion rate of 0.0033 mm/h for the first 120 h and 0.0063 mm/h for the last 48 h from 120 h to 168 h in experiments. The corrosion rate of the HVOF sleeve-pipe surface coating without laser treatment is 0.104 mm/h for the first 72 h, with the failure of the coatings occurring after 120 h from the previous experiment.
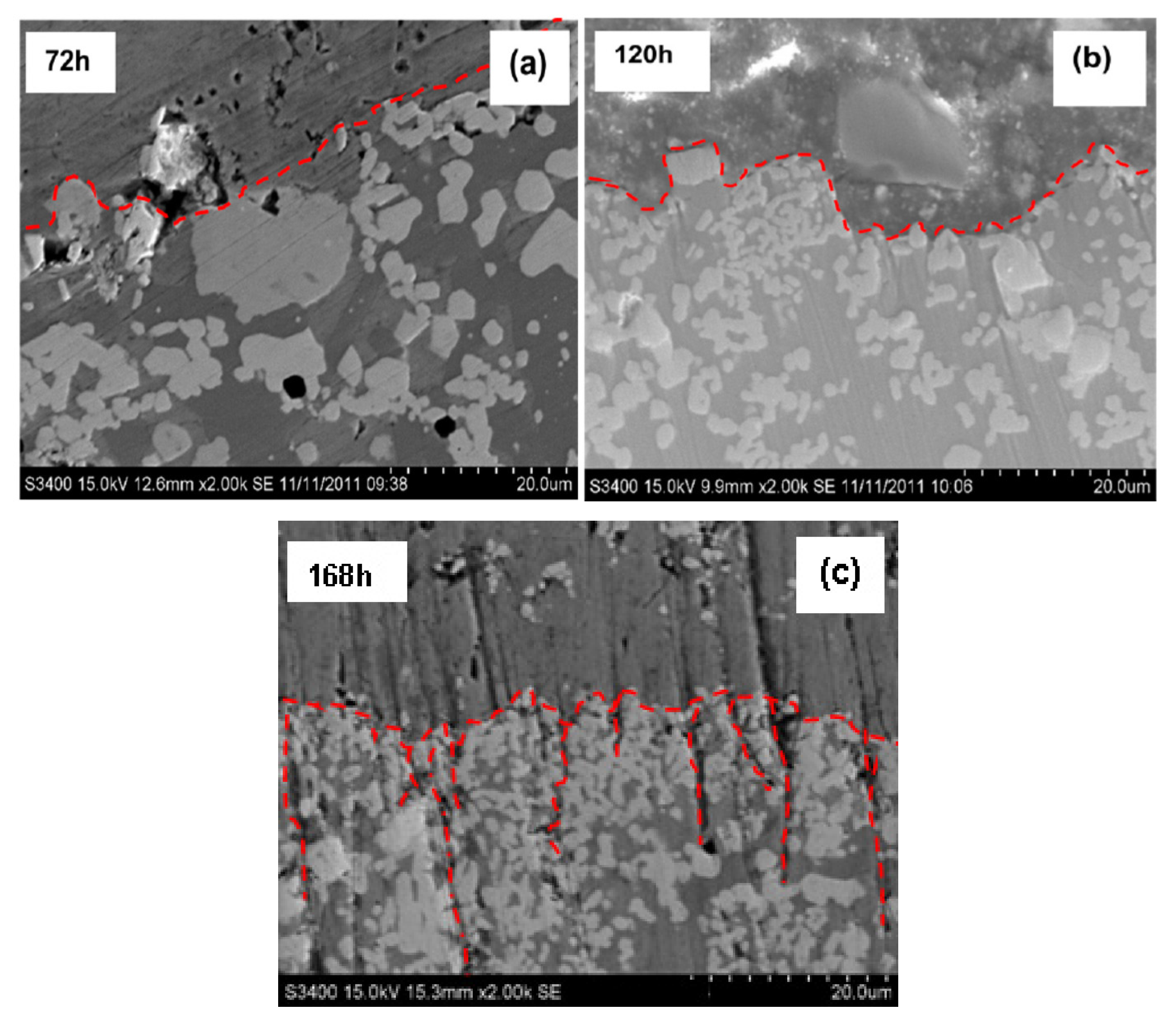
The samples after corrosion at various times for the sleeve-pipe coatings at 72 h, 120 h and 168 h are shown in
Figure 9a–c, respectively.
Similar to the bushing coatings, the 72 h corrosion experiment (
Figure 9a) also shows two regions: (a) the top left region with the presence of molten Zn on the coating surface and (b) the right bottom region with the coating. At this stage of the experiment, the coating morphology does not change, no new phase is formed and no corrosion channels or cracks are formed in the coating. It appears that the large carbide particles may be more corrosive-resistant to the molten Zn due to the particulates protruding more at the coating/molten Zn interface. However, no obvious corrosion rate difference exists between the matrix alloy phase (slightly dark-coloured) and the white-coloured particulate phases. That can still classify it as a selective phase corrosion mechanism at this stage of corrosion for the HVOF-coated sleeve surface.
Figure 9b shows the SEM image of the sample after 120 h of corrosion. There is clear evidence that the coating exhibited severe molten Zn corrosion at this stage of the experiment. Both the top matrix phase and large carbide particulates were corroded away from the coating into the molten Zn. This resulted in smaller carbide particle particulates, which were not subjected to laser treatment, and the matrix coatings further towards the substrate being left behind. In addition, a noticeable higher corrosion rate of the matrix alloy phase was found when compared to the white carbide particulate phase. However, the preferred corrosion resistance of the carbide particles did not result in dramatic changes in the coating corrosion rate or the formation of cracks or corrosion channels in the coating. This preferred corrosion difference is obvious in the 168 h corrosion sample. This is shown in
Figure 9c, where white carbide particulates protrude with severe cracks and channels having opened in the coating to expose the severe corrosion of the matrix phase. Following this, the corrosion rates are obviously higher for the matrix phase when compared to the white carbide particulates. In Co-Cr-Ni alloy coatings, the corrosion begins from the anodic microstructural phase. If the form of the anodic phase is continuous, such as a grain-boundary coating structure, a rapid penetration of the component could occur.
It should be emphasised here that the current report only involved the report of bushing and sleeve coating performance in molten Zn without subjecting an applied force. During the actual sink-roller operation in a production environment, conditions are much more aggressive and complicated, as the bushing and sleeve pipe will be subjected to molten Zn corrosion and surface wear. The coating wear mechanisms are quite complex. At the initial stage of coating wear, there will be two sliding surfaces with the liquid Zn acting as a lubricant and corrosive agent. As time goes on, corrosion, such as Co-Zn, Cr-Zn and surface wear debris (such as metals or alloys of Cr, Co and hard-phase carbides, including WC, Cr-C and η-Co-W-C), will be added to the surface, which further complicates the wear-and-tear process of the surface coating. We are now trying to improve the coating qualities and to plan the wear performance of the coating in both simulated and actual production environments. The results will be subsequently published.