Corrosion Resistance and Durability of Superhydrophobic Copper Surface in Corrosive NaCl Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Surface Morphologies and Chemical Compositions
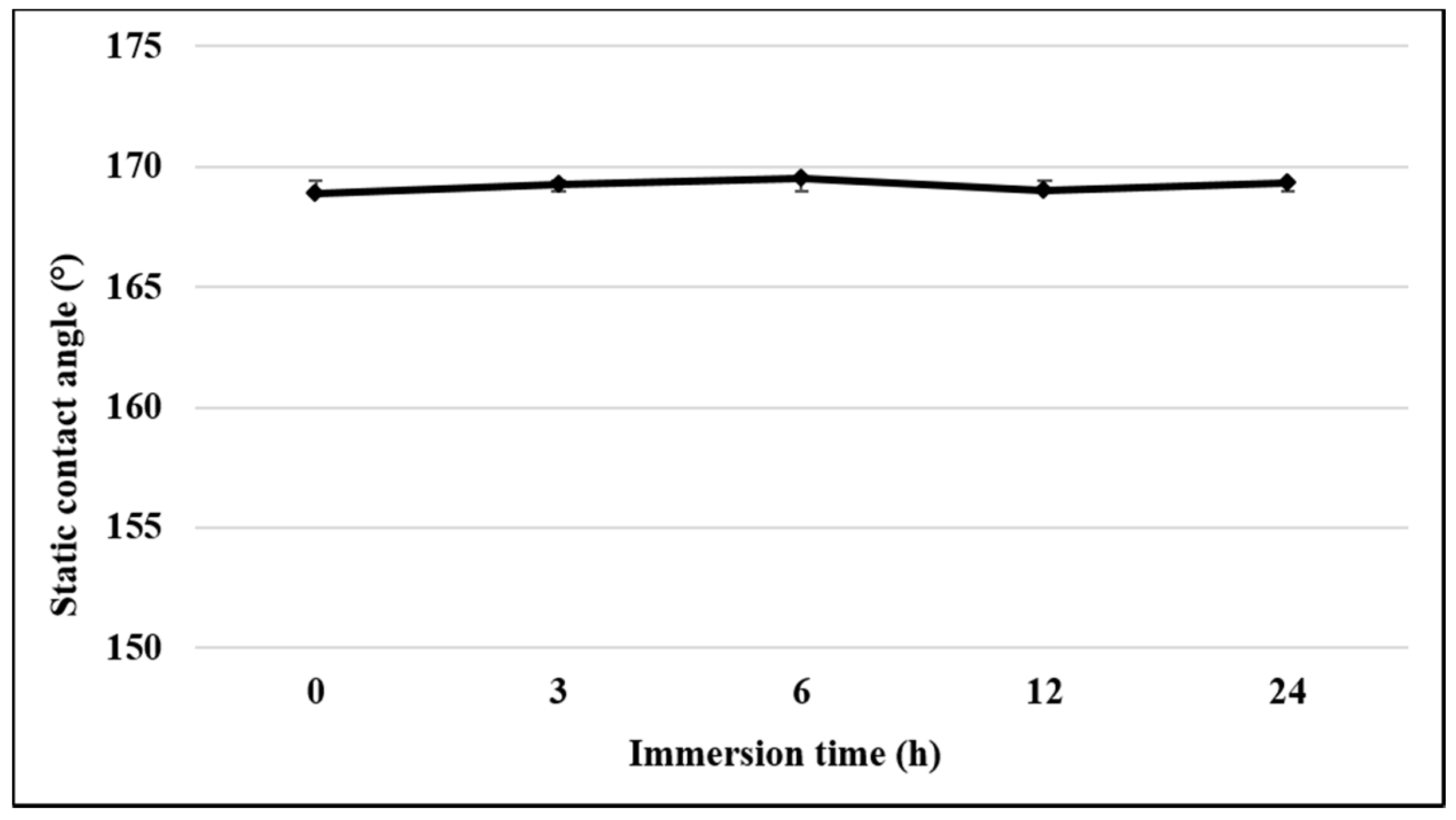
3.2. Chemical Stability and Corrosion Resistance
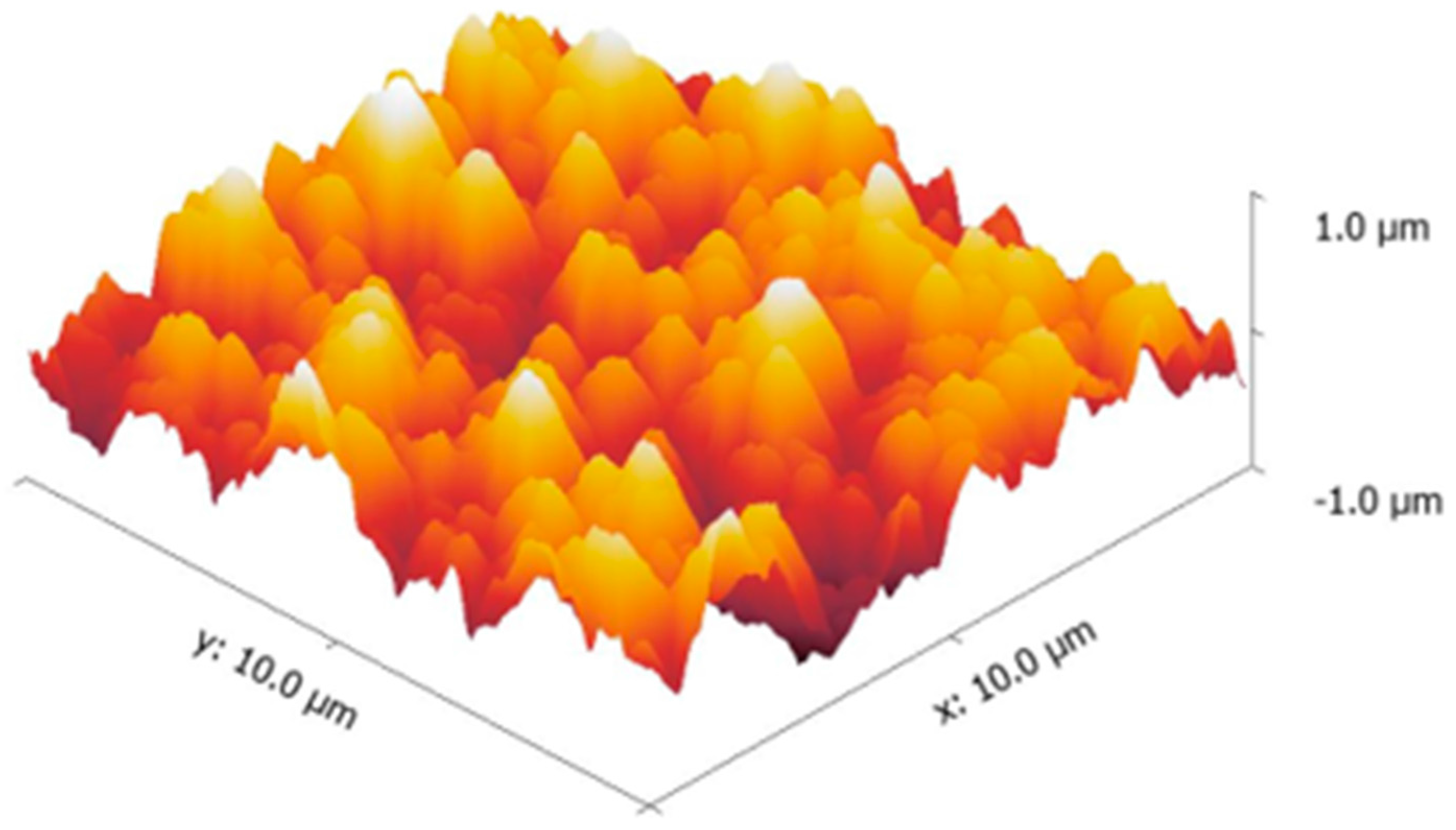
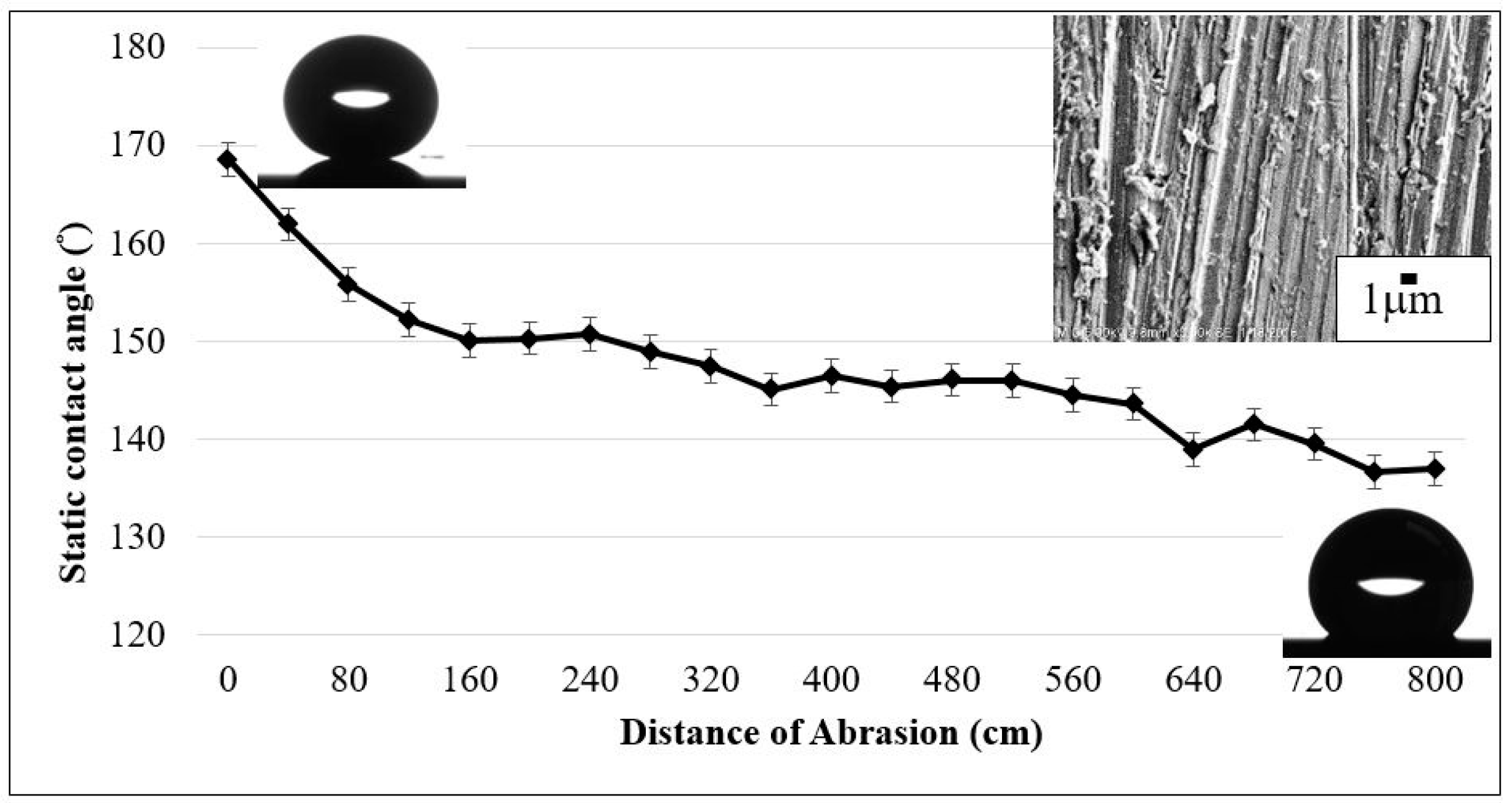
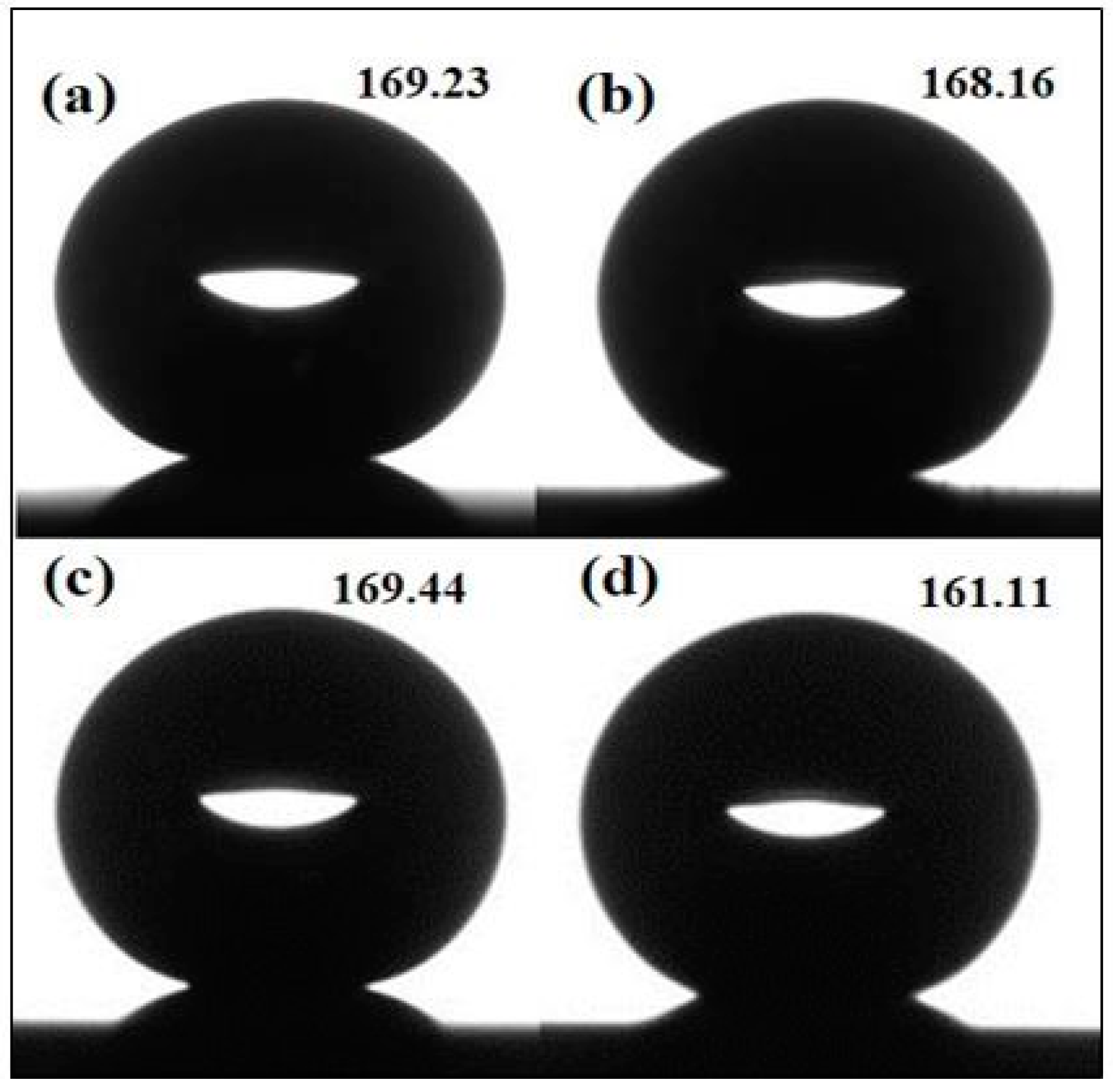
3.3. Mechanical Stability and Property
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Novel method for controllable fabrication of a superhydrophobic CuO surface on AZ91D magnesium alloy. ACS Appl. Mater. Interface 2012, 4, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Xu, X.; Men, X.; Yang, J.; Zhou, X.; Xue, Q. Facile fabrication of a superamphiphobic surface on the copper substrate. J. Colloid Interface Sci. 2012, 367, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hao, L.; Chen, A.; Song, Q.; Chen, C. A rapid one-step process for fabrication of superhydrophobic surface by electrodeposition method. Electrochim. Acta 2012, 59, 168–171. [Google Scholar] [CrossRef]
- Yao, C.-W.; Alvarado, J.L.; Marsh, C.P.; Jones, B.G.; Collins, M.K. Wetting behavior on hybrid surfaces with hydrophobic and hydrophilic properties. Appl. Surf. Sci. 2014, 290, 59–65. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper, and zinc substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qin, L.; Hao, Y.; Kuang, S.; Bai, X.; Chong, Y.-M.; Zhang, W.; Wang, E. Vertically aligned boron nitride nanosheets: Chemical vapor synthesis, ultraviolet light emission, and superhydrophobicity. ACS Nano 2010, 4, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Barshilia, H.C. Nanometric multiscale rough CuO/Cu(OH)2 superhydrophobic surfaces prepared by a facile one-step solution-immersion process: Transition to superhydrophilicity with oxygen plasma treatment. J. Phys. Chem. C 2011, 115, 18213–18220. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically stable and corrosion resistant superhydrophobic sol–gel coatings on copper substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]
- Dong, C.; Gu, Y.; Zhong, M.; Li, L.; Sezer, K.; Ma, M.; Liu, W. Fabrication of superhydrophobic Cu surfaces with tunable regular micro and random nano-scale structures by hybrid laser texture and chemical etching. J. Mater. Proc. Technol. 2011, 211, 1234–1240. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; She, Z.; Chen, F.; Li, L. Low-cost and large-scale fabrication method for an environmentally-friendly superhydrophobic coating on magnesium alloy. J. Mater. Chem. 2012, 22, 4097–4105. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Birbarah, P.; Foulkes, T.; Yin, S.L.; Rentauskas, M.; Neely, J.; Pilawa-Podgurski, R.C.N.; Miljkovic, N. Jumping-droplet electronics hot-spot cooling. Appl. Phys. Lett. 2017, 110, 123107. [Google Scholar] [CrossRef]
- Wiedenheft, K.F.; Guo, H.A.; Qu, X.; Boreyko, J.B.; Liu, F.; Zhang, K.; Eid, F.; Choudhury, A.; Li, Z.; Chen, C.-H. Hotspot cooling with jumping-drop vapor chambers. Appl. Phys. Lett. 2017, 110, 141601. [Google Scholar] [CrossRef]
- Miljkovic, N.; Enright, R.; Nam, Y.; Lopez, K.; Dou, N.; Sack, J.; Wang, E.N. Jumping-droplet-enhanced condensation on scalable superhydrophobic nanostructured surfaces. Nano Lett. 2013, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Gallant, D.; Chen, X.-G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process. Appl. Surf. Sci. 2013, 282, 689–694. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wan, Y.; Wu, J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coating on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- Enright, R.; Miljkovic, N.; Dou, N.; Nam, Y.; Wang, E.N. Condensation on superhydrophobic copper oxide nanostructures. J. Heat Transf. 2013, 135, 091304. [Google Scholar] [CrossRef]
- Nam, Y.; Ju, Y.S. A comparative study of the morphology and wetting characteristics of micro/nanostructured Cu surfaces for phase change heat transfer applications. J. Adhes. Sci. Technol. 2013, 27, 2163–2176. [Google Scholar] [CrossRef]
- Chavan, S.; Cha, H.; Orejon, D.; Nawaz, K.; Singla, N.; Yeung, Y.F.; Park, D.; Kang, D.H.; Chang, Y.; Takata, Y.; et al. Heat transfer through a condensate droplet on hydrophobic and nanostructured superhydrophobic surfaces. Langmuir 2016, 32, 7774–7787. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.Y.; McCarthy, T.J. Self-Assembly is not the only reaction possible between alkyltrichlorosilanes and surfaces: Monomolecular and oligomeric covalently attached layers of dichloro- and trichloroalkylsilanes on silicon. Langmuir 2000, 16, 7268–7274. [Google Scholar] [CrossRef]
- Chen, H.-H.; Anbarasan, R.; Kuo, L.-S.; Hsu, C.-C.; Chen, P.-H.; Chiang, K.-F. Fabrication of hierarchical structured superhydrophobic copper surface by in-situ method with micro/nano scaled particles. Mater. Lett. 2012, 66, 299–301. [Google Scholar] [CrossRef]
- Yang, H.; Pi, P.; Cai, Z.-Q.; Wen, X.; Wang, X.; Cheng, J.; Yang, Z.-R. Facile preparation of super-hydrophobic and super-oleophilic silica film on stainless steel mesh via sol–gel process. Appl. Surf. Sci. 2010, 256, 4095–4102. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.; Lu, S.; Xi, J.; Wang, Y. Fabrication of superhydrophobic surfaces with hierarchical structure through a solution-immersion process on copper and galvanized iron substrates. Langmuir 2008, 24, 10895–10900. [Google Scholar] [CrossRef] [PubMed]
- Fransolet, A.M.; Tarte, P. Infrared spectra of analyzed samples of the amblygonite-montrebasite series: A new rapid semi-quantitative determination of fluorine. Am. Mineral. 1977, 62, 559–564. [Google Scholar]
- Salh, R. Defect related luminescence in silicon dioxide network: A review. In Crystalline Silicon-Properties and Uses; Basu, S., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Radev, L.; Mostafa, N.Y.; Michailova, I.; Salvado, I.M.M.; Fernandes, M.H.V. In vitro bioactivity of collagen/calcium phosphate silicate composites, cross-linked with chondroitin sulfate. Int. J. Mater. Chem. 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Nimittrakoolchai, O.U.; Supothina, S. Preparation of stable ultrahydrophobic and superoleophobic silica-based coating. J. Nanosci. Nanotechnol. 2012, 12, 4962–4968. [Google Scholar] [CrossRef] [PubMed]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interface 2011, 3, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Anbarasan, R.; Kuo, L.-S.; Tsai, M.-Y.; Chen, P.-H.; Chiang, K.-F. Synthesis, characterizations and hydrophobicity of micro/nano scaled heptadecafluorononanoic acid decorated copper nanoparticle. Nano Micro Lett. 2010, 2, 101–105. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Morton, E. Castable cements to prevent corrosion of metals in molten salts. Sol. Energy Mater. Sol. Cells 2016, 153, 44–51. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interface 2014, 6, 8762–8770. [Google Scholar] [CrossRef] [PubMed]
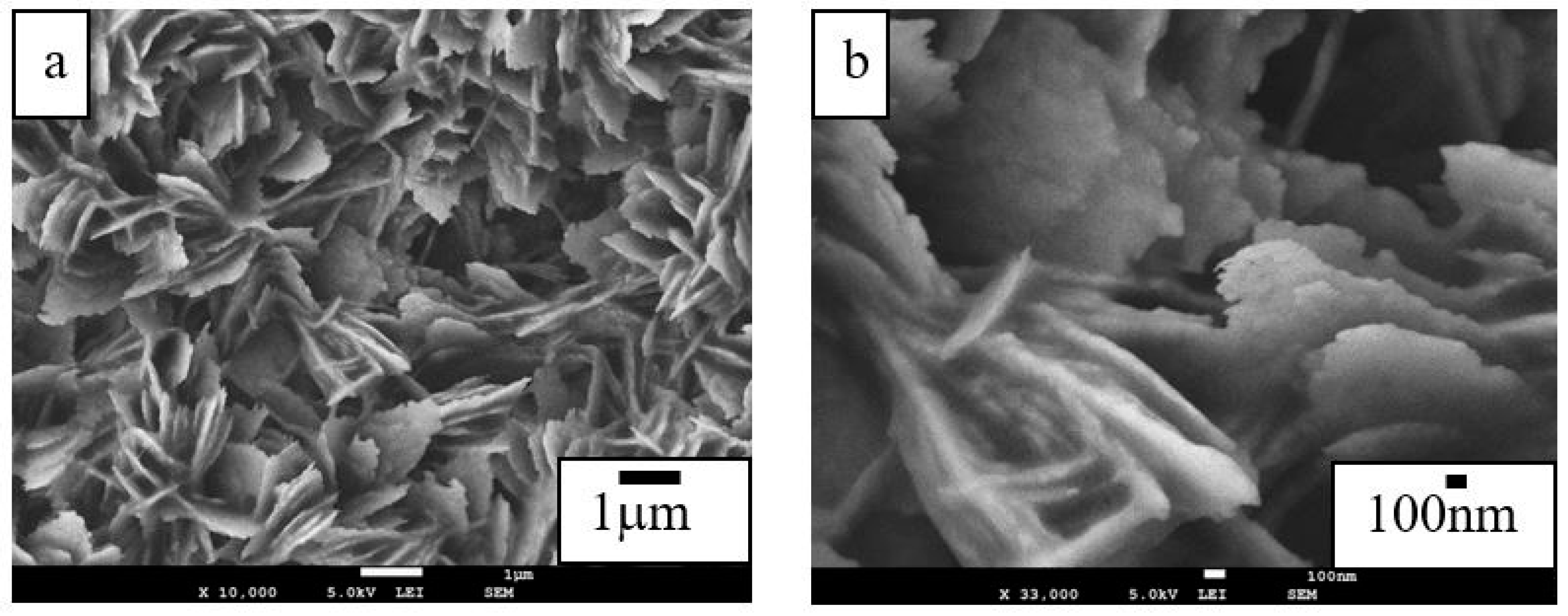
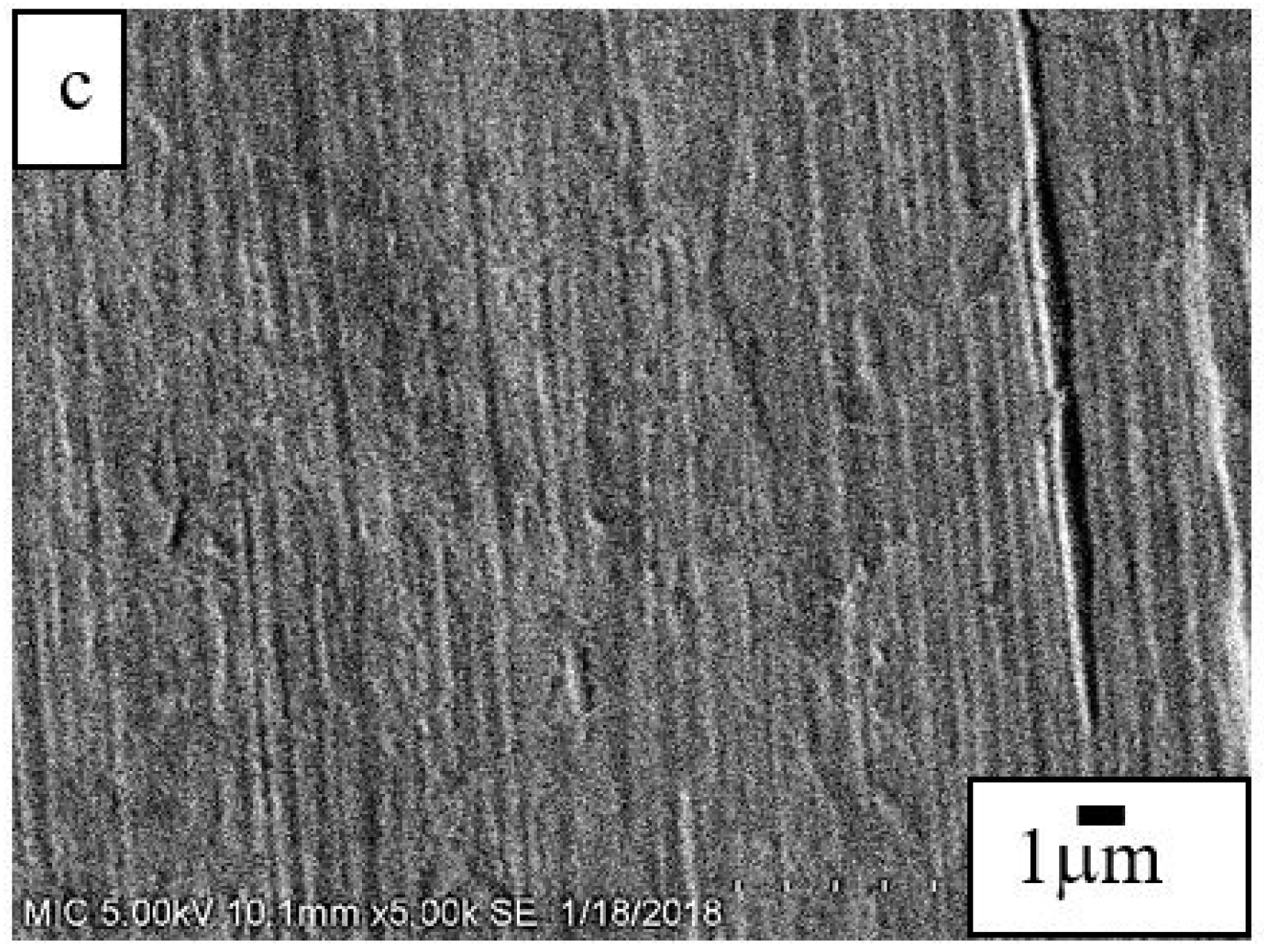

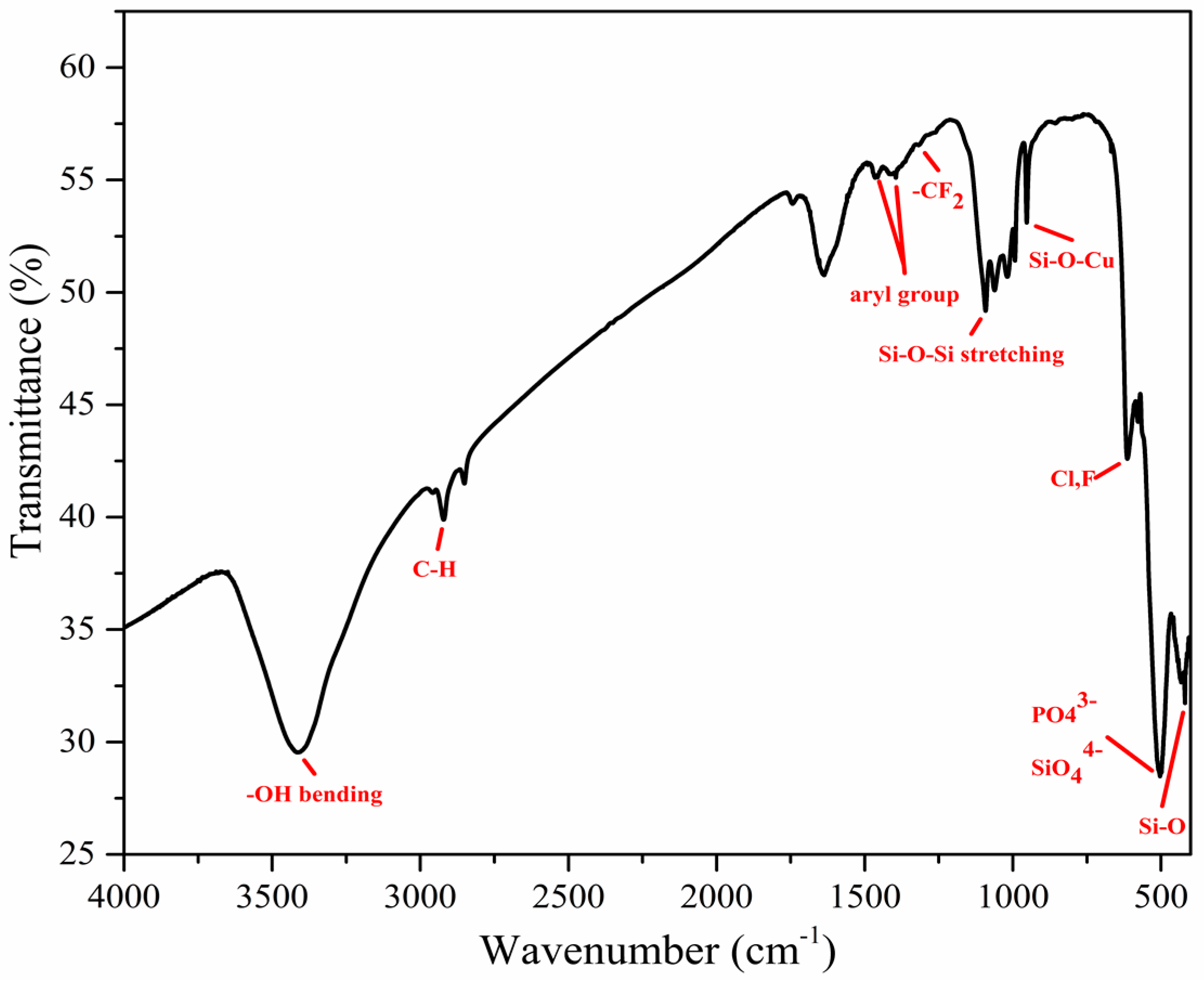
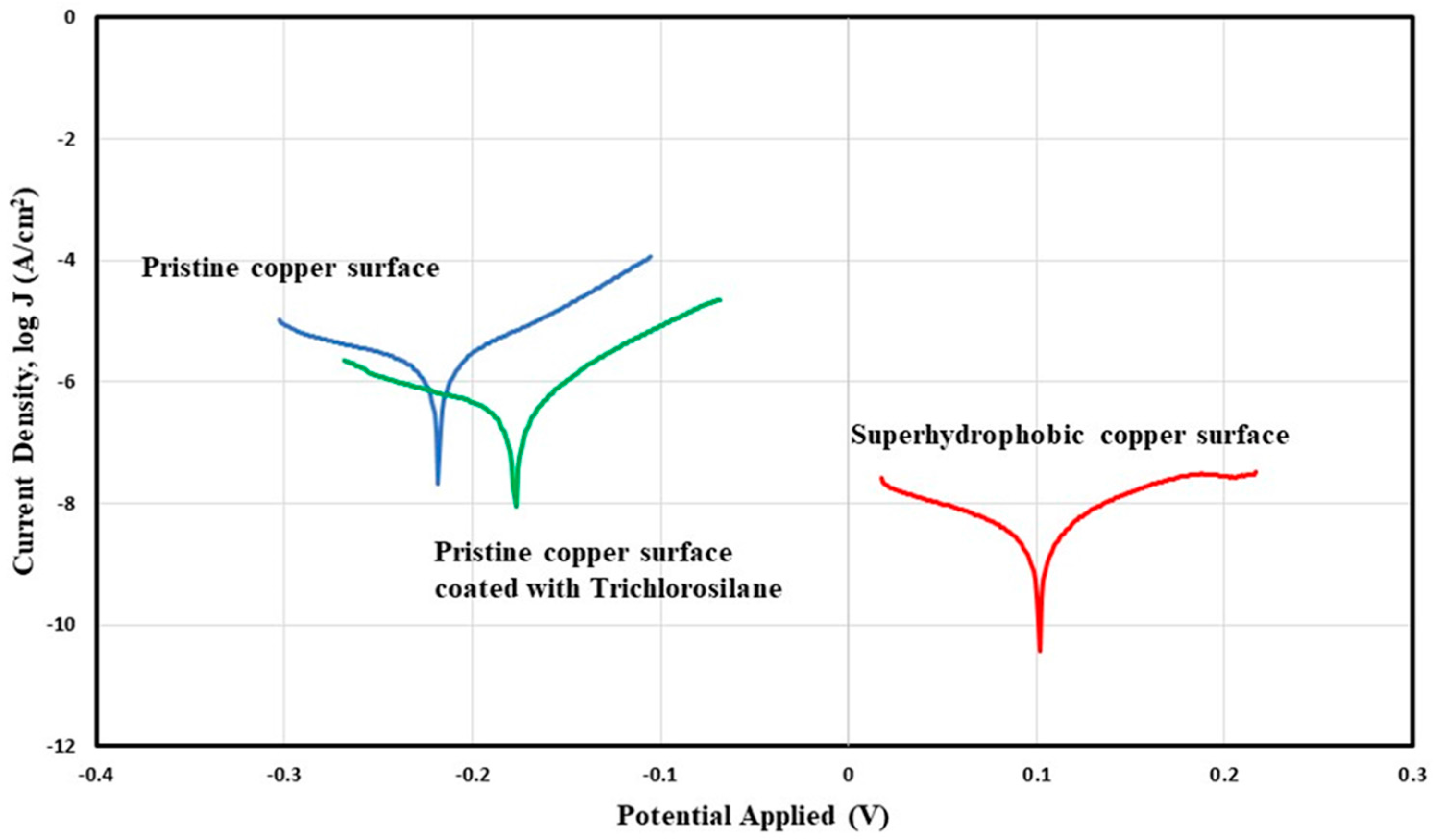
| Sample | Ecorr (V) | Jcorr (A/cm2) | Corrosion Rate (mm/year) |
|---|---|---|---|
| Pristine copper surface | −2.20 × 10−1 ± 1.00 × 10−3 | 2.12 × 10−6 ± 4.02 × 10−7 | 4.89 × 10−2 ± 9.63 × 10−3 |
| Copper surface coated with Trichlorosilane | −1.7 × 10−1 ± 8.91 × 10−3 | 6.6 × 10−7 ± 5.83 × 10−7 | 1.53 × 10−2 ± 1.35 × 10−2 |
| Superhydrophobic copper | 10.6 × 10−2 ± 3.61 × 10−2 | 4.62 × 10−9 ± 2.99 × 10−9 | 1.07 × 10−4 ± 6.95 × 10−5 |
| Property | Surface | ||
|---|---|---|---|
| Superhydrophobic Copper Surface | Superhydrophoic Copper Surface after Corrosion Test | Superhydrophobic Copper Surface after Immersion in 3.5 wt % NaCl for 24 h | |
| 3-Dimensional image | 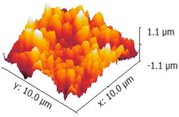 | 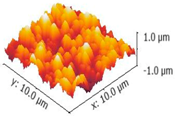 |  |
| Roughness Rq (nm) | 333.86 ± 5 | 322.54 ± 16 | 326.82 ± 5 |
| Stiffness value (N/m) | 4.25 ± 0.05 | 4.24 ± 0.26 | 4.23 ± 0.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.-W.; Sebastian, D.; Lian, I.; Günaydın-Şen, Ö.; Clarke, R.; Clayton, K.; Chen, C.-Y.; Kharel, K.; Chen, Y.; Li, Q. Corrosion Resistance and Durability of Superhydrophobic Copper Surface in Corrosive NaCl Aqueous Solution. Coatings 2018, 8, 70. https://doi.org/10.3390/coatings8020070
Yao C-W, Sebastian D, Lian I, Günaydın-Şen Ö, Clarke R, Clayton K, Chen C-Y, Kharel K, Chen Y, Li Q. Corrosion Resistance and Durability of Superhydrophobic Copper Surface in Corrosive NaCl Aqueous Solution. Coatings. 2018; 8(2):70. https://doi.org/10.3390/coatings8020070
Chicago/Turabian StyleYao, Chun-Wei, Divine Sebastian, Ian Lian, Özge Günaydın-Şen, Robbie Clarke, Kirby Clayton, Chiou-Yun Chen, Krishna Kharel, Yanyu Chen, and Qibo Li. 2018. "Corrosion Resistance and Durability of Superhydrophobic Copper Surface in Corrosive NaCl Aqueous Solution" Coatings 8, no. 2: 70. https://doi.org/10.3390/coatings8020070





