A Note on the Dyeing of Wool Fabrics Using Natural Dyes Extracted from Rotten Wood-Inhabiting Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Fungi
2.2. Dyes Production
2.3. Fabric Dyeing
3. Results
3.1. Isolation and Identification of Fungi
3.2. Production of Fungal Dyes
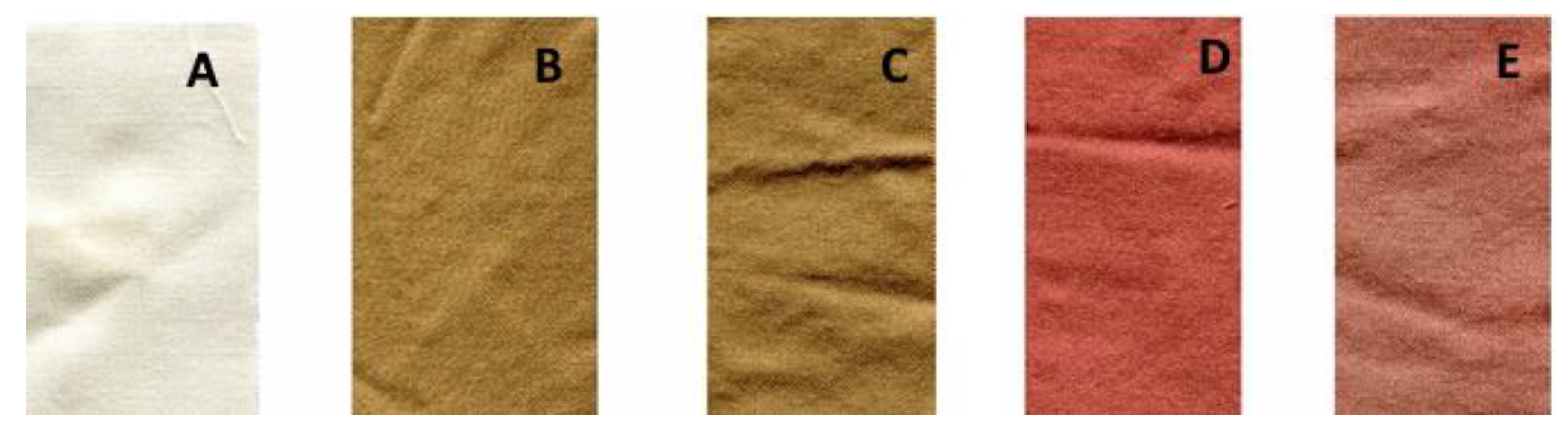
3.3. Dye of Wool Samples
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Venil, C.; Wahidin, M.; Ahmad, W. Production of bacterial pigments in low cost medium and formulation of biodegradable ink. Indian J. Exp. Biol. 2017, 55, 441–447. [Google Scholar]
- Panesar, R.; Kaur, S.; Panesar, P. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Srivastava, S. Food adulteration affecting the nutrition and health of human beings. Biol. Sci. Med. 2015, 1, 65–70. [Google Scholar]
- Manikprabhu, D.; Lingappa, K. γ Actinorhodin a natural and attorney source for synthetic dye to detect acid production of fungi. Saudi J. Biol. Sci. 2013, 20, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.P.N.; Xiao, M.; Li, W.-J. Fungal and Bacterial Pigments: Secondary Metabolites with Wide Applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar]
- Chan-Bacab, M.J.; Sanmartín, P.; Camacho-Chab, J.C.; Palomo-Ascanio, K.B.; Huitz-Quimé, H.E.; Ortega-Morales, B.O. Characterization and dyeing potential of colorant-bearing plants of the Mayan area in Yucatan Peninsula, Mexico. J. Clean. Prod. 2015, 91, 191–200. [Google Scholar] [CrossRef]
- Goktas, O.; Duru, M.E.; Yeniocak, M.; Ozen, E. Determination of the color stability of an environmentally friendly wood stain derived from laurel (Laurus nobilis L.) leaf extracts under UV exposure. For. Prod. J. 2008, 58, 77–80. [Google Scholar]
- Neeraj, N.; Neera, M.; Sayan, C. Microbial pigments with health benefits—A mini review. Trends Biosci. 2011, 4, 157–160. [Google Scholar]
- Hamano, P.; Kilikian, B. Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Braz. J. Chem. Eng. 2006, 23, 443–449. [Google Scholar] [CrossRef]
- Robinson, S.; Hinsch, E.; Weber, G.; Freitas, S. Method of extraction and resolubilisation of pigments from Chlorociboria aeruginosa and Scytalidium cuboideum, two prolific spalting fungi. Color. Technol. 2014, 130, 221–225. [Google Scholar] [CrossRef]
- Venil, C.; Zakaria, Z.; Ahmad, W. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Khandare, R.; Govindwar, S. Phytoremediation of textile dyes and effluents: Current scenario and future prospects. Biotechnol. Adv. 2015, 33, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Kim, M.-J.; Park, J.-S.; Karthikeyan, K.; Lakshmanaperumalsamy, P.; Lee, K.-J.; Park, Y.-J.; Oh, B.-T. Dyeing of cotton yarn with five water soluble fungal pigments obtained from five fungi. Fibers Polym. 2010, 11, 598–605. [Google Scholar] [CrossRef]
- Chadni, Z.; Rahaman, M.; Jerin, I.; Hoque, K.; Reza, M. Extraction and optimization of red pigment production as secondary metabolites from Talaromyces verruculosus and its potential use in textile industries. Mycology 2017, 8, 48–57. [Google Scholar] [CrossRef]
- Weber, G.; Chen, H.; Hinsch, E.; Freitas, S.; Robinson, S. Pigments extracted from the wood-staining fungi Chlorociboria aeruginosa, Scytalidium cuboideum, and S. ganodermophthorum show potential for use as textile dyes. Color. Technol. 2014, 130, 445–452. [Google Scholar] [CrossRef]
- Hinsch, E.; Robinson, S. Mechanical color reading of wood-staining fungal pigment textile dyes: An alternative method for determining colorfastness. Coatings 2016, 6, 25. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide To Methods and Applications; Innis, M.A., Ed.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Altschul, S.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 99 Some Aromatic Amines, Organic Dyes, and Related Exposures; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef] [PubMed]
- AATCC TM61-2013 Colorfastness to Laundering, Home & Commercial: Accelerated; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2013.
- Visagie, C.; Frisvad, J.; Houbraken, J.; Seifert, K.; Samson, R.; Jacob, K. Five new Talaromyces species with ampulliform-like phialides and globose rough walled conidia resembling T. verruculosus. Mycoscience 2015, 56, 486–502. [Google Scholar] [CrossRef]
- Frisvad, J.; Samson, R.; Stolk, A. Disposition of recently described species of Penicillium. Persoonia 1990, 14, 209–232. [Google Scholar]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- Eisenman, H.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, E.; Weber, G.; Chen, H.; Robinson, S. Colorfastness of Extracted Wood-staining Fungal Pigments on Fabrics: A new potential for textile dyes. J. Text. Appar. Technol. Manag. 2015, 9, 1–11. [Google Scholar]
- Morales-Oyervides, L.; Oliverira, J.; Sousa-Gallagher, M.; Mendez-Zavala, A.; Montañez, J. Assessment of the Dyeing Properties of the Pigments Produced by Talaromyces spp. J. Fungi 2017, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gupta, C.; Aggarwal, S.; Naqpal, N. Pigment extraction from fungus for textile dyeing. Indian J. Fibre Text. Res. 2012, 37, 68–73. [Google Scholar]
| Isolate | GenBank Name | Identity % (blast) | Access Number | Sequence Size (pb) |
|---|---|---|---|---|
| 1 | Penicillium murcianum | 100 | NR138358 | 575 |
| 2 | Talaromyces australis | 99 | NR147431 | 509 |
| 3 | Trichoderma spirale | 99 | KP009301 | 549 |
| 4 | Talaromyces sp. | 100 | LT558966 | 522 |
| 5 | Fusarium oxysporum | 100 | KR997535 | 479 |
| Fungi | Dyes Yield (g·L−1) | Color |
|---|---|---|
| Talaromyces australis | 0.22 | Red * |
| Penicillium murcianum | 0.28 | Yellow * |
| Talaromyces sp. | 0.10 | Orange |
| Trichoderma spirale | 0.24 | Yellow |
| Fusarium oxysporum | 0.25 | Purple |
| Wool Fabric | CIE L*a*b* Color | ∆E | |
|---|---|---|---|
| Before Washing | After Washing | ||
| Red | L* = 52 (0.2), a* = 24.5 (1.6), b* = 17 (0.5) | L* = 52.5 (0.7), a* = 23.1 (0.3), b* = 6.2 (0.1) | 2.1 (0.9) |
| Yellow | L* = 54.4 (1.0), a* = 6.3 (0), b* = 28.5 (0.2) | L* = 54.4 (0.7), a* = 6.4 (0.2), b* = 25.6 (0.7) | 2.9 (0.5) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, V.A.; Galleguillos, F.A.; Sagredo, N.; Machuca, Á. A Note on the Dyeing of Wool Fabrics Using Natural Dyes Extracted from Rotten Wood-Inhabiting Fungi. Coatings 2018, 8, 77. https://doi.org/10.3390/coatings8020077
Hernández VA, Galleguillos FA, Sagredo N, Machuca Á. A Note on the Dyeing of Wool Fabrics Using Natural Dyes Extracted from Rotten Wood-Inhabiting Fungi. Coatings. 2018; 8(2):77. https://doi.org/10.3390/coatings8020077
Chicago/Turabian StyleHernández, Vicente A., Felipe A. Galleguillos, Nicole Sagredo, and Ángela Machuca. 2018. "A Note on the Dyeing of Wool Fabrics Using Natural Dyes Extracted from Rotten Wood-Inhabiting Fungi" Coatings 8, no. 2: 77. https://doi.org/10.3390/coatings8020077





