Tunnel Oxides Formed by Field-Induced Anodisation for Passivated Contacts of Silicon Solar Cells
Abstract
:1. Introduction
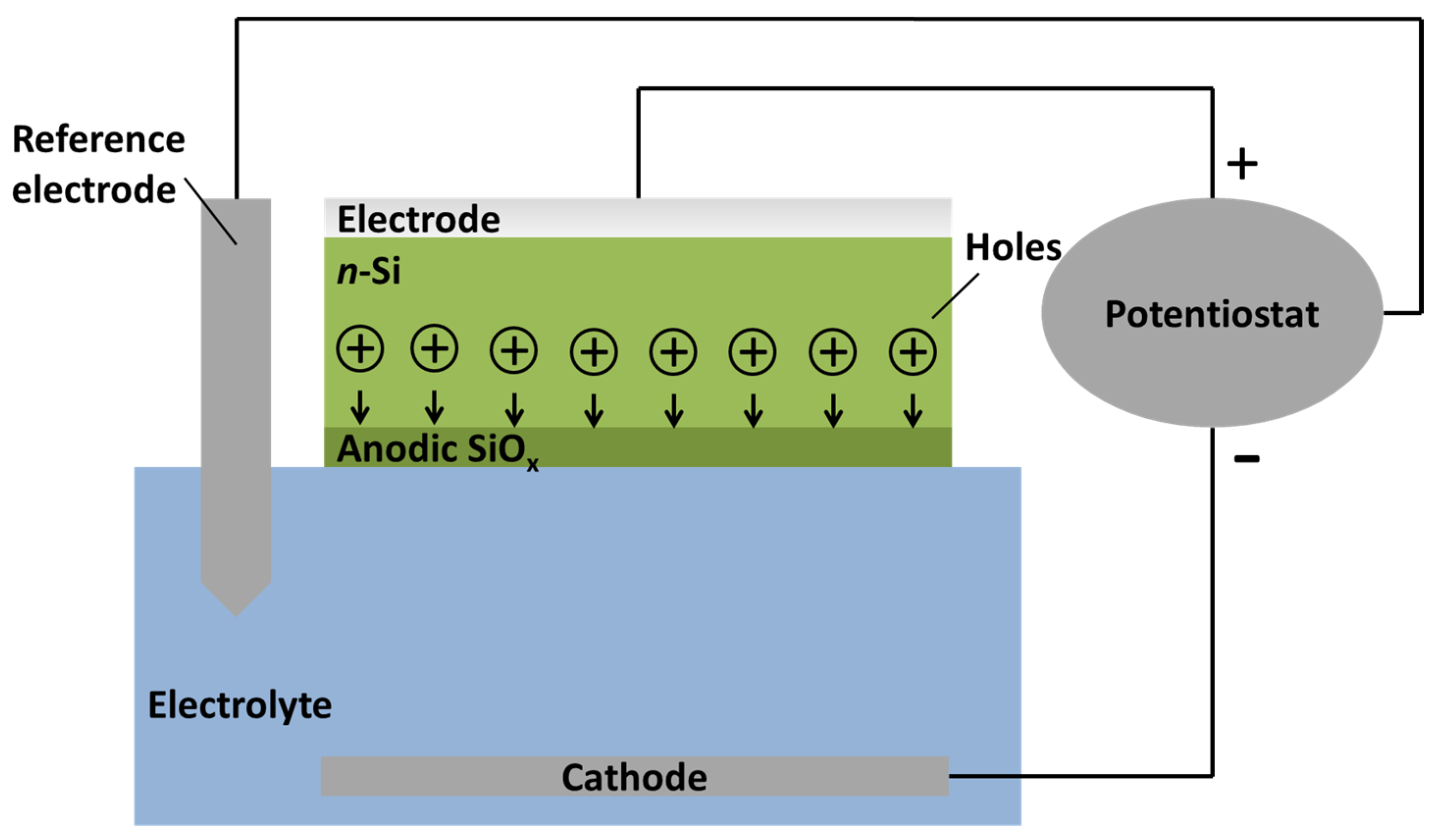
Overview of the FIA Process
2. Experimental
3. Results and Discussion
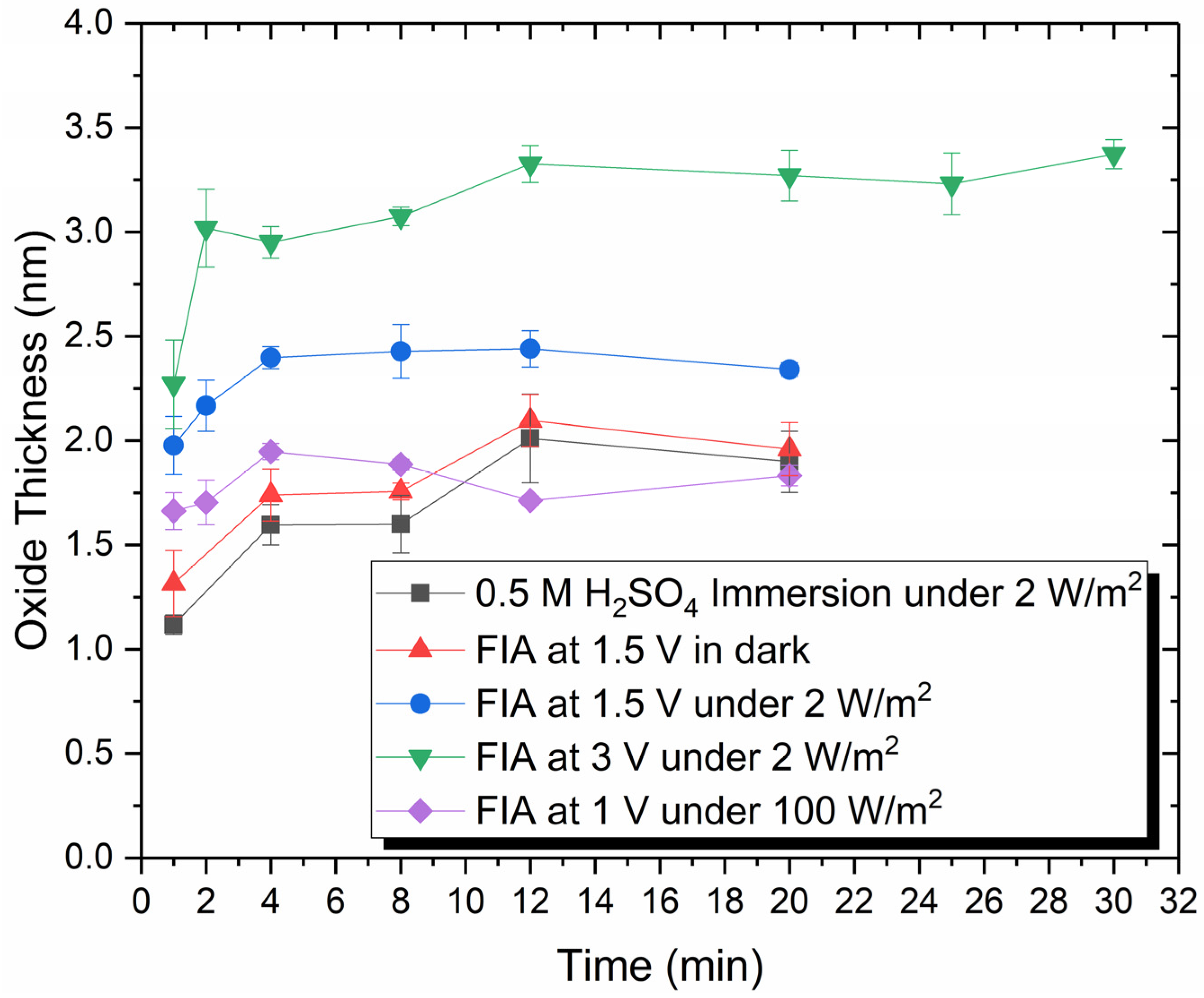
3.1. Ellipsometry Measurements of Oxide Thickness
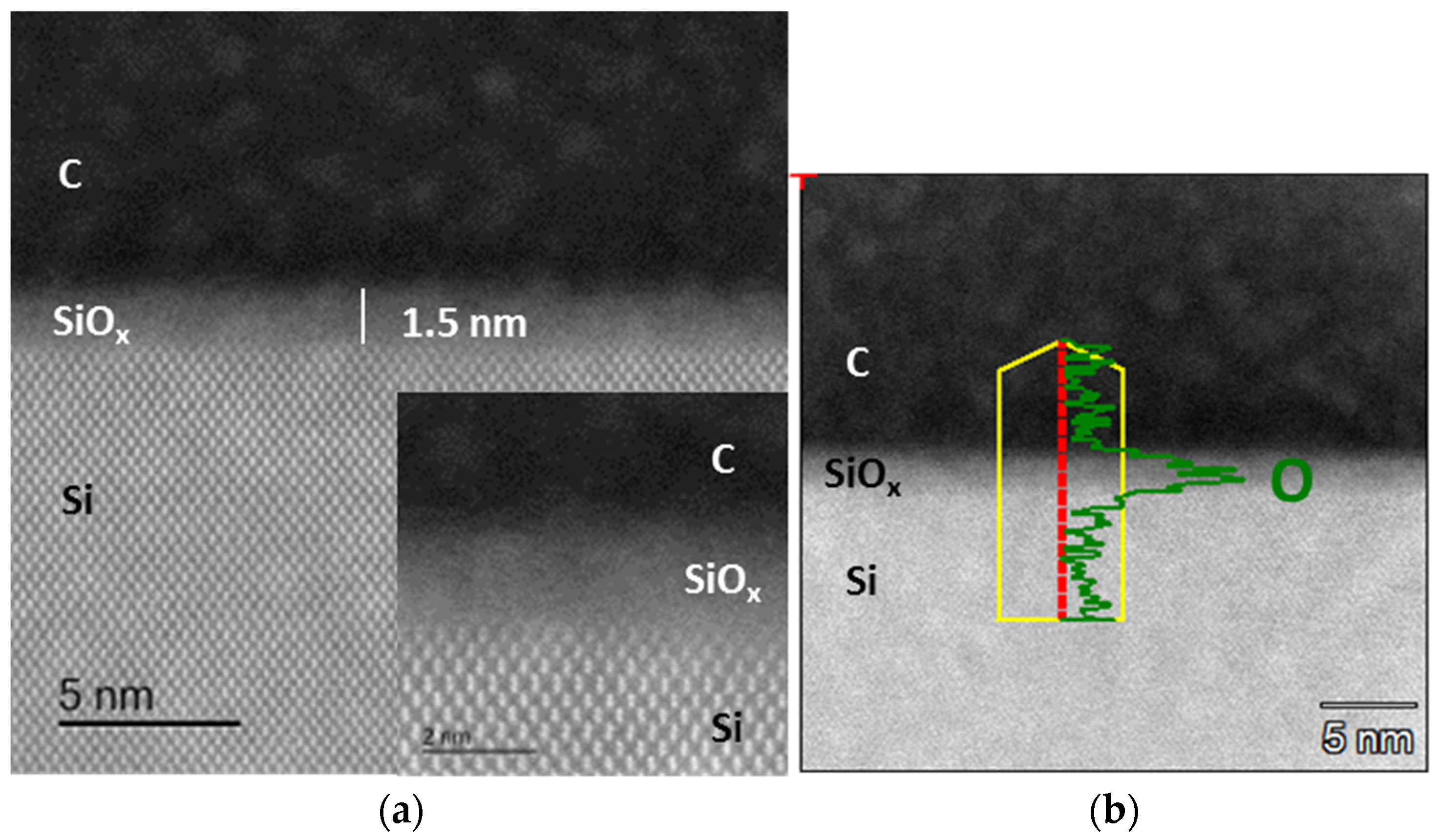
3.2. Microscopic and Spectroscopic Analyses
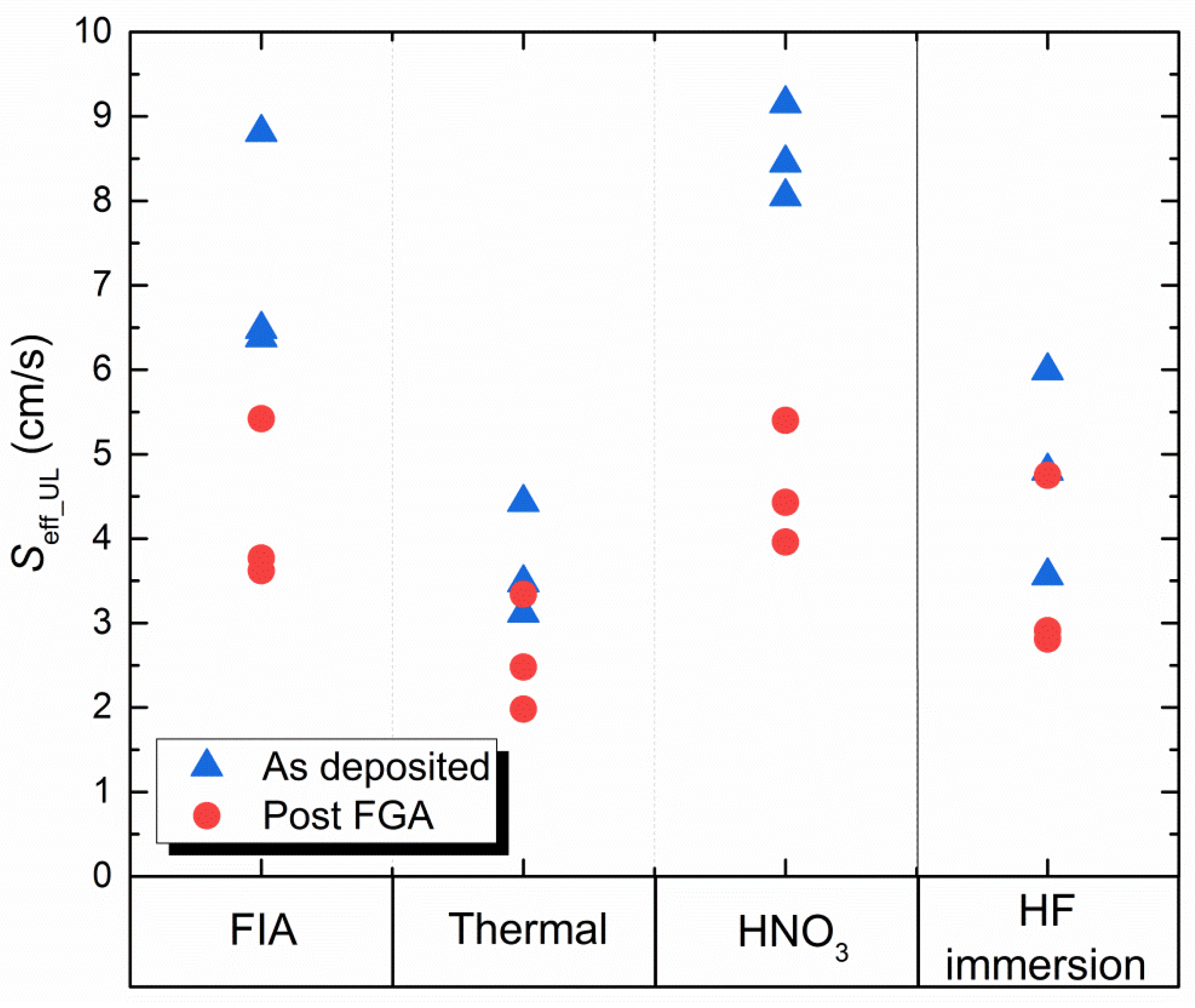
3.3. Surface Passivation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Green, M.A.; Godfrey, R. MIS solar cell—General theory and new experimental results for silicon. Appl. Phys. Lett. 1976, 29, 610–612. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, L.; Guo, W.; Wang, D.; Meng, F.; Liu, Z. Improved passivation effect at the amorphous/crystalline silicon interface due to ultrathin SiOx layers pre-formed in chemical solutions. Appl. Phys. Express 2014, 7, 065504. [Google Scholar] [CrossRef]
- Seif, J.P.; Descoeudres, A.; Filipič, M.; Smole, F.; Topič, M.; Holman, Z.C.; De Wolf, S.; Ballif, C. Amorphous silicon oxide window layers for high-efficiency silicon heterojunction solar cells. J. Appl. Phys. 2014, 115, 024502. [Google Scholar] [CrossRef]
- Ohdaira, K.; Oikawa, T.; Higashimine, K.; Matsumura, H. Suppression of the epitaxial growth of Si films in Si heterojunction solar cells by the formation of ultra-thin oxide layers. Curr. Appl. Phys. 2016, 16, 1026–1029. [Google Scholar] [CrossRef]
- Bullock, J.; Yan, D.; Cuevas, A.; Wan, Y.; Samundsett, C. n- and p-type silicon solar cells with molybdenum oxide hole contacts. Energy Procedia 2015, 77, 446–450. [Google Scholar] [CrossRef]
- Gad, K.M.; Vössing, D.; Richter, A.; Rayner, B.; Reindl, L.M.; Mohney, S.E.; Kasemann, M. Ultrathin titanium dioxide nanolayers by atomic layer deposition for surface passivation of crystalline silicon. IEEE J. Photovolt. 2016, 6, 649–653. [Google Scholar] [CrossRef]
- Feldmann, F.; Bivour, M.; Reichel, C.; Hermle, M.; Glunz, S.W. Passivated rear contacts for high-efficiency n-type Si solar cells providing high interface passivation quality and excellent transport characteristics. Sol. Energy Mater. Sol. Cells Part A 2014, 120, 270–274. [Google Scholar] [CrossRef]
- Glunz, S.; Feldmann, F.; Richter, A.; Bivour, M.; Reichel, C.; Steinkemper, H.; Benick, J.; Hermle, M. The irresistible charm of a simple current flow pattern—25% with a solar cell featuring a full-area back contact. In Proceedings of the 31st European Photovoltaic Solar Energy Conference and Exhibition, Hamburg, Germany, 14–18 September 2015; pp. 259–263. [Google Scholar]
- Yan, D.; Cuevas, A.; Bullock, J.; Wan, Y.; Samundsett, C. Phosphorus-diffused polysilicon contacts for solar cells. Sol. Energy Mater. Sol. Cells 2015, 142, 75–82. [Google Scholar] [CrossRef]
- Upadhyaya, A.D.; Ok, Y.W.; Chang, E.; Upadhyaya, V.; Madani, K.; Tate, K.; Rounsaville, B.; Choi, C.J.; Chandrasekaran, V.; Yelundur, V.; et al. Ion-implanted screen-printed n-type solar cell with tunnel oxide passivated back contact. IEEE J. Photovolt. 2016, 6, 153–158. [Google Scholar] [CrossRef]
- Stodolny, M.K.; Lenes, M.; Wu, Y.; Janssen, G.J.M.; Romijn, I.G.; Luchies, J.R.M.; Geerligs, L.J. n-Type polysilicon passivating contact for industrial bifacial n-type solar cells. Sol. Energy Mater. Sol. Cells 2016, 158, 24–28. [Google Scholar] [CrossRef]
- Yang, G.; Ingenito, A.; Isabella, O.; Zeman, M. IBC c-Si solar cells based on ion-implanted poly-silicon passivating contacts. Sol. Energy Mater. Sol. Cells 2016, 158, 84–90. [Google Scholar] [CrossRef]
- Young, D.L.; Lee, B.G.; Fogel, D.; Nemeth, W.; LaSalvia, V.; Theingi, S.; Page, M.; Young, M.; Perkins, C.; Stradins, P. Gallium-doped poly-Si:Ga/SiO2 passivated emitters to n-Cz wafers with iV oc >730 mv. IEEE J. Photovolt. 2017, 7, 1640–1645. [Google Scholar] [CrossRef]
- Haase, F.; Kiefer, F.; Schäfer, S.; Kruse, C.; Krügener, J.; Brendel, R.; Peibst, R. Interdigitated back contact solar cells with polycrystalline silicon on oxide passivating contacts for both polarities. Jpn. J. Appl. Phys. 2017, 56, 08MB15. [Google Scholar] [CrossRef]
- Moldovan, A.; Feldmann, F.; Zimmer, M.; Rentsch, J.; Benick, J.; Hermle, M. Tunnel oxide passivated carrier-selective contacts based on ultra-thin SiO2 layers. Sol. Energy Mater. Sol. Cells 2015, 142, 123–127. [Google Scholar] [CrossRef]
- Feldmann, F.; Steinhauser, B.; Tutsch, L.; Kluska, S.; Hermle, M.; Glunz, S. Status and Industrial Perspectives of Topcon. Proceedings of Materials Research Society Fall Meetings & Exhibit, Boston, MA, USA, 26 November–1 December 2017. [Google Scholar]
- Ling, Z.P.; Xin, Z.; Ke, C.; Buatis, K.J.; Duttagupta, S.; Lee, J.S.; Lai, A.; Hsu, A.; Rostan, J.; Stangl, R. Comparison and characterization of different tunnel layers, suitable for passivated contact formation. Jpn. J. Appl. Phys. 2017, 56, 08MA01. [Google Scholar] [CrossRef]
- Fehlner, F.P. Formation of ultrathin oxide films on silicon. J. Electrochem. Soc. 1972, 119, 1723–1727. [Google Scholar] [CrossRef]
- Azman, A.; Ayub, R.; Arshad, M.M.; Norhafiezah, S.; Fathil, M.; Kamarudin, M.; Nurfaiz, M.; Hashim, U. Controlling growth rate of ultra-thin silicon dioxide layer by incorporating nitrogen gas during dry thermal oxidation. In Proceedings of the 2014 IEEE International Conference on Semiconductor Electronics (ICSE2014), Kuala Lumpur, Malaysia, 27–29 August 2014; pp. 392–395. [Google Scholar]
- Adams, A.; Smith, T.; Chang, C. The growth and characterization of very thin silicon dioxide films. J. Electrochem. Soc. 1980, 127, 1787–1794. [Google Scholar] [CrossRef]
- Kobayashi, A.H.; Maida, O.; Takahashi, M.; Iwasa, H. Nitric acid oxidation of Si to form ultrathin silicon dioxide layers with a low leakage current density. J. Appl. Phys. 2003, 94, 7328–7335. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imamura, K.; Kim, W.B.; Im, S.S. Nitric acid oxidation of Si (NAOS) method for low temperature fabrication of SiO2/Si and SiO2/SiC structures. Appl. Surf. Sci. 2010, 256, 5744–5756. [Google Scholar] [CrossRef]
- Grant, N.E.; McIntosh, K.R. Low surface recombination velocity on (100) silicon by electrochemically grown silicon dioxide annealed at low temperature. IEEE Electron Device Lett. 2010, 31, 1002–1004. [Google Scholar] [CrossRef]
- Grant, N.E.; McIntosh, K.R. Passivation of a (100) silicon surface by silicon dioxide grown in nitric acid. IEEE Electron Device Lett. 2009, 30, 922–924. [Google Scholar] [CrossRef]
- Lennon, A.; Yeo, J.; Lu, P.H.; Sun, Y.; Wang, X.; Lu, Z.; Li, Y.; Vais, V. Field-induced anodization for silicon solar cell passivation. In Proceedings of the 2013 IEEE 39th Photovoltaic Specialists Conference (PVSC), Tampa, FL, USA, 16–21 June 2013; pp. 1257–1259. [Google Scholar]
- Vais, V.; Lennon, A.J.; Wenham, S.R.; Ji, J.J.; Wenham, A.M. Metal contact scheme for solar cells. WO Patent WO/2012/000015, 12 11 2012. [Google Scholar]
- Cui, J.; Wang, X.; Opila, R.; Lennon, A. Light-induced anodisation of silicon for solar cell passivation. J. Appl. Phys. 2013, 114, 184101. [Google Scholar] [CrossRef]
- Tong, J.; Wang, X.; Ouyang, Z.; Lennon, A. Ultra-thin tunnel oxides formed by field-induced anodisation for carrier-selective contacts. Energy Procedia 2015, 77, 840–847. [Google Scholar] [CrossRef]
- Cui, J.; Grant, N.; Lennon, A. Effective surface passivation of p-type crystalline silicon with silicon oxides formed by light-induced anodisation. Appl. Surf. Sci. 2014, 323, 40–44. [Google Scholar] [CrossRef]
- Tong, J.; Wang, X.; Lennon, A. Requirements to achieve field-induced anodisation for silicon surface passivation. Energy Procedia 2014, 55, 855–864. [Google Scholar] [CrossRef]
- Kern, W. Cleaning solutions based on hydrogen peroxide for use in silicon semiconductor technology. RCA Rev. 1970, 31, 187–206. [Google Scholar]
- Sze, S.M. Semiconductor Devices: Physics and Technology, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Richter, A.; Glunz, S.W.; Werner, F.; Schmidt, J.; Cuevas, A. Improved quantitative description of auger recombination in crystalline silicon. Phys. Rev. B 2012, 86, 165202. [Google Scholar] [CrossRef]
- Parkhutik, V. Modeling of the electrochemical anodization of silicon: State-of-the-art and perspectives. In Proceedings of the Third International Symposium on Pits and Pores: Formation, Properties and Significance for Advanced Luminescent Materials, Honolulu, HI, USA, 6 October 2004. [Google Scholar]
- Tong, J.; To, A.; Lennon, A.; Hoex, B. Unintentional consequences of dual mode plasma reactors: Implications for upscaling lab-record silicon surface passivation by silicon nitride. Jpn. J. Appl. Phys. 2017, 56, 08MB12. [Google Scholar] [CrossRef]


| Oxide | Thickness (nm) | Si1+ | Si2+ | Si3+ | Si1+/(Si2+ + Si3+) |
|---|---|---|---|---|---|
| FIA (1.0 V) | 1.8 | 1.28 | 0.46 | 0.35 | 1.58 |
| Thermal | 1.6 | 0.48 | 0.47 | 0.36 | 0.58 |
| HNO3 | 1.6 | 1.49 | – | 0.65 | 2.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, J.; Lim, S.; Lennon, A. Tunnel Oxides Formed by Field-Induced Anodisation for Passivated Contacts of Silicon Solar Cells. Coatings 2018, 8, 81. https://doi.org/10.3390/coatings8020081
Tong J, Lim S, Lennon A. Tunnel Oxides Formed by Field-Induced Anodisation for Passivated Contacts of Silicon Solar Cells. Coatings. 2018; 8(2):81. https://doi.org/10.3390/coatings8020081
Chicago/Turabian StyleTong, Jingnan, Sean Lim, and Alison Lennon. 2018. "Tunnel Oxides Formed by Field-Induced Anodisation for Passivated Contacts of Silicon Solar Cells" Coatings 8, no. 2: 81. https://doi.org/10.3390/coatings8020081




