Study of Calcium Ethoxide as a New Product for Conservation of Historical Limestone
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
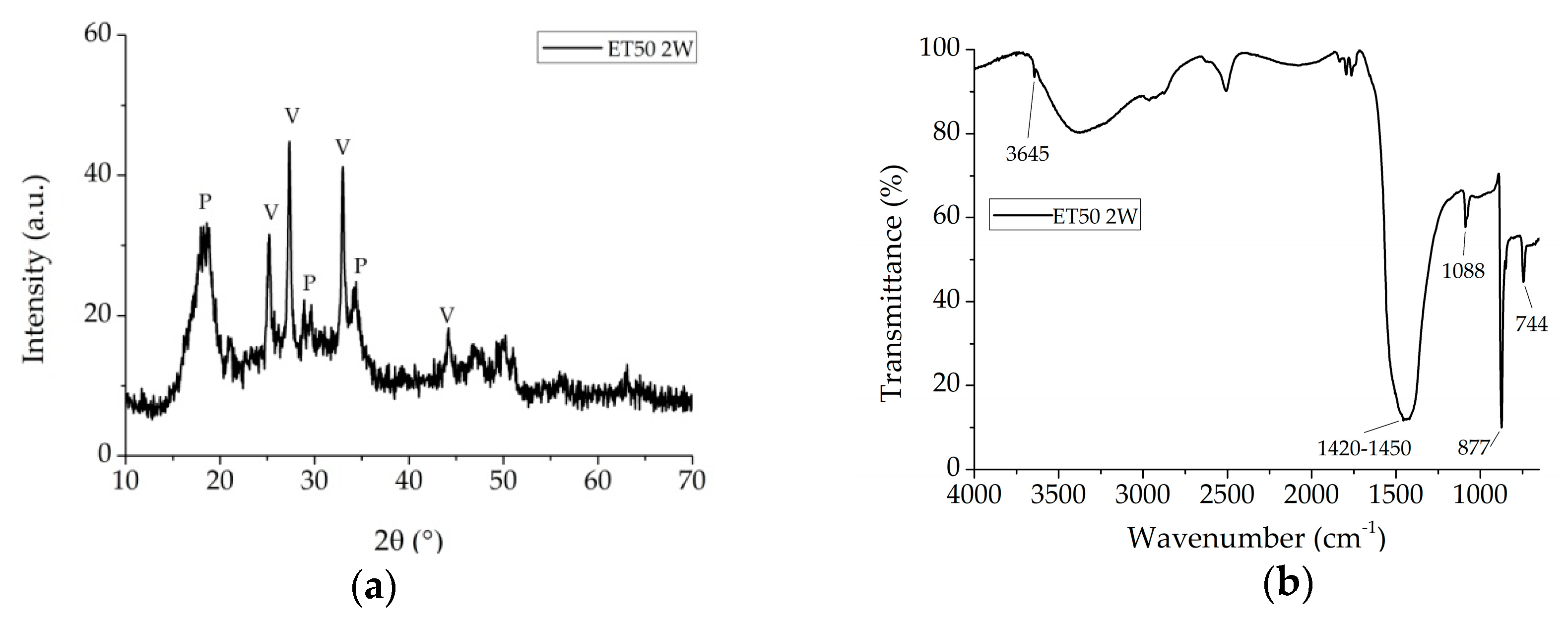
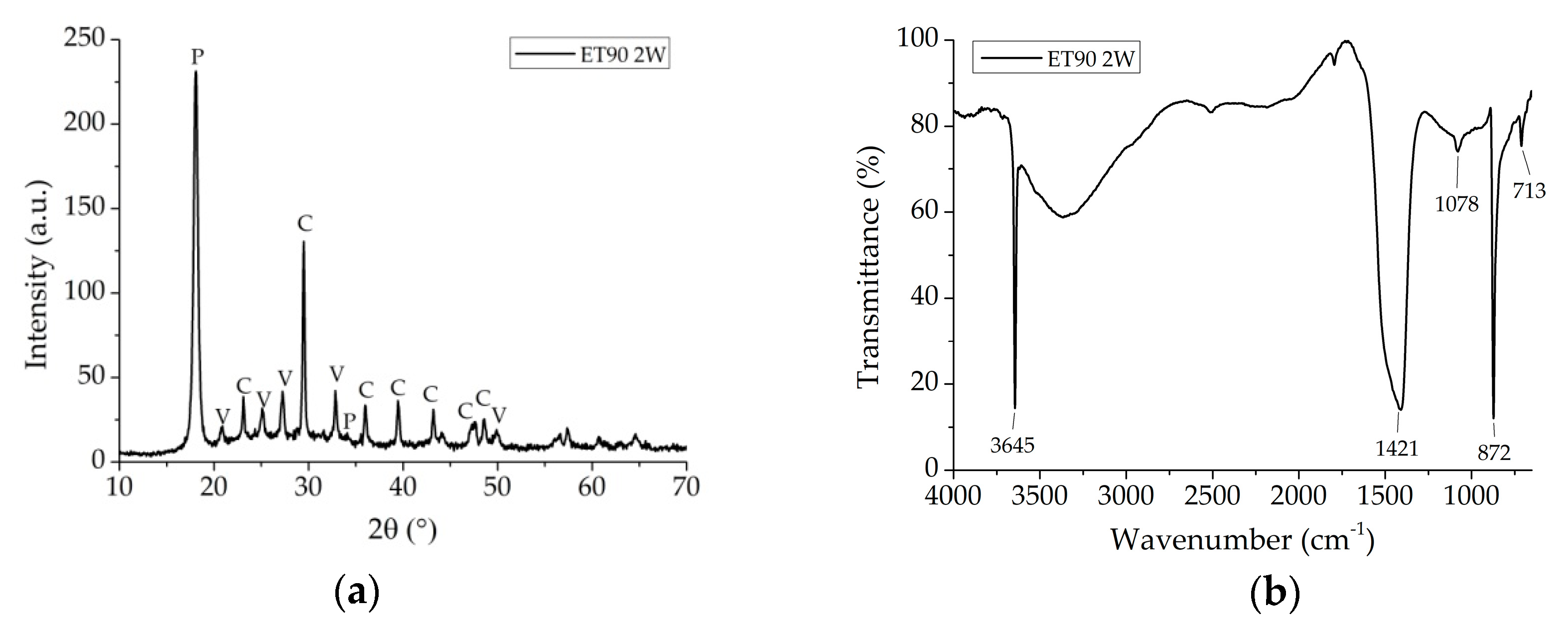
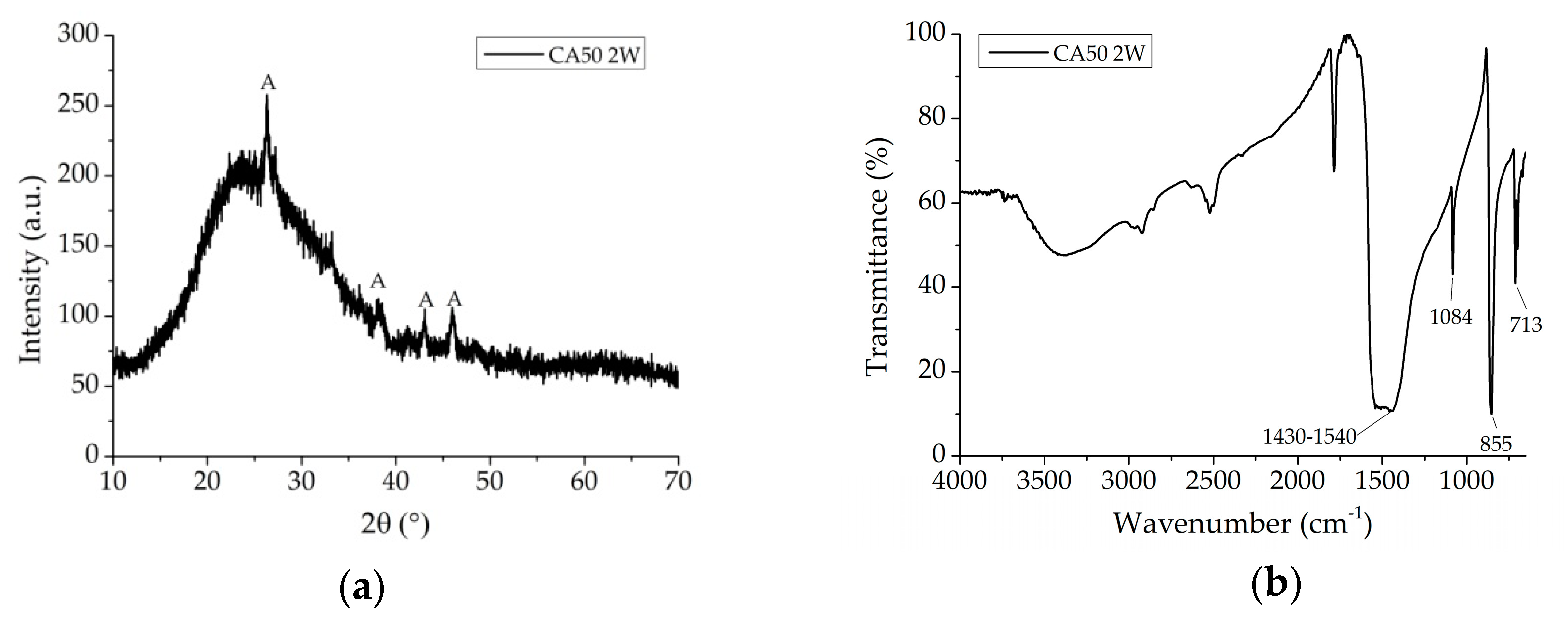
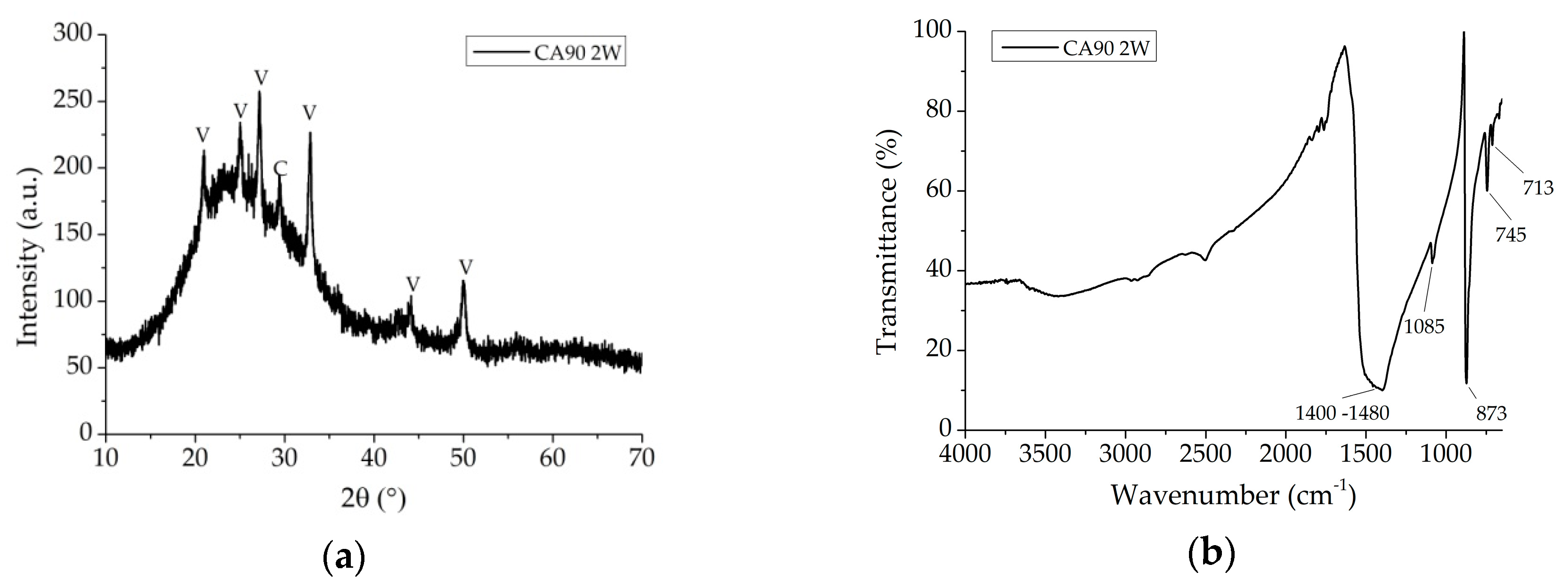
3.1. Kinetic and Reaction Pathway
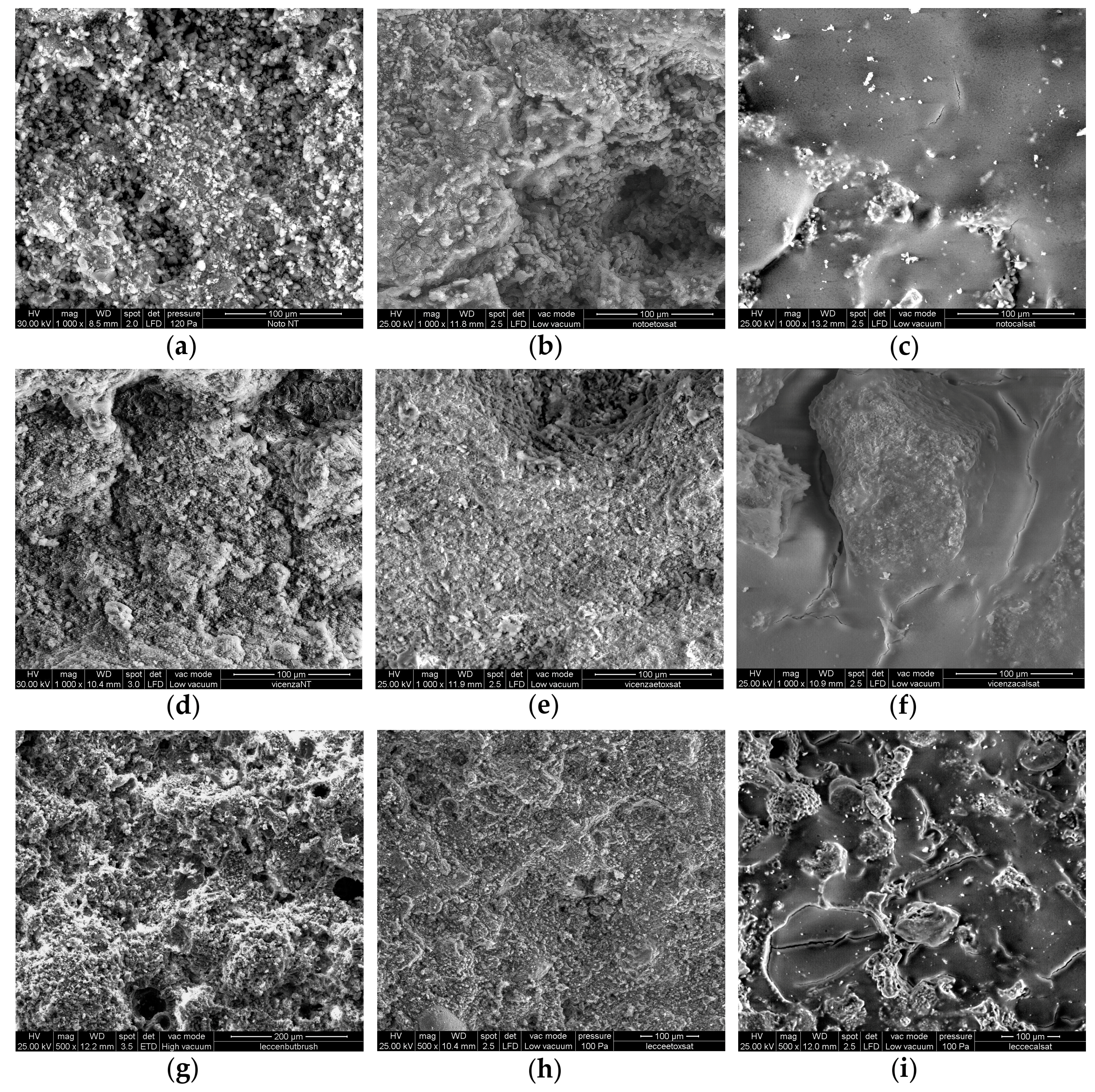
3.2. Coating Investigation
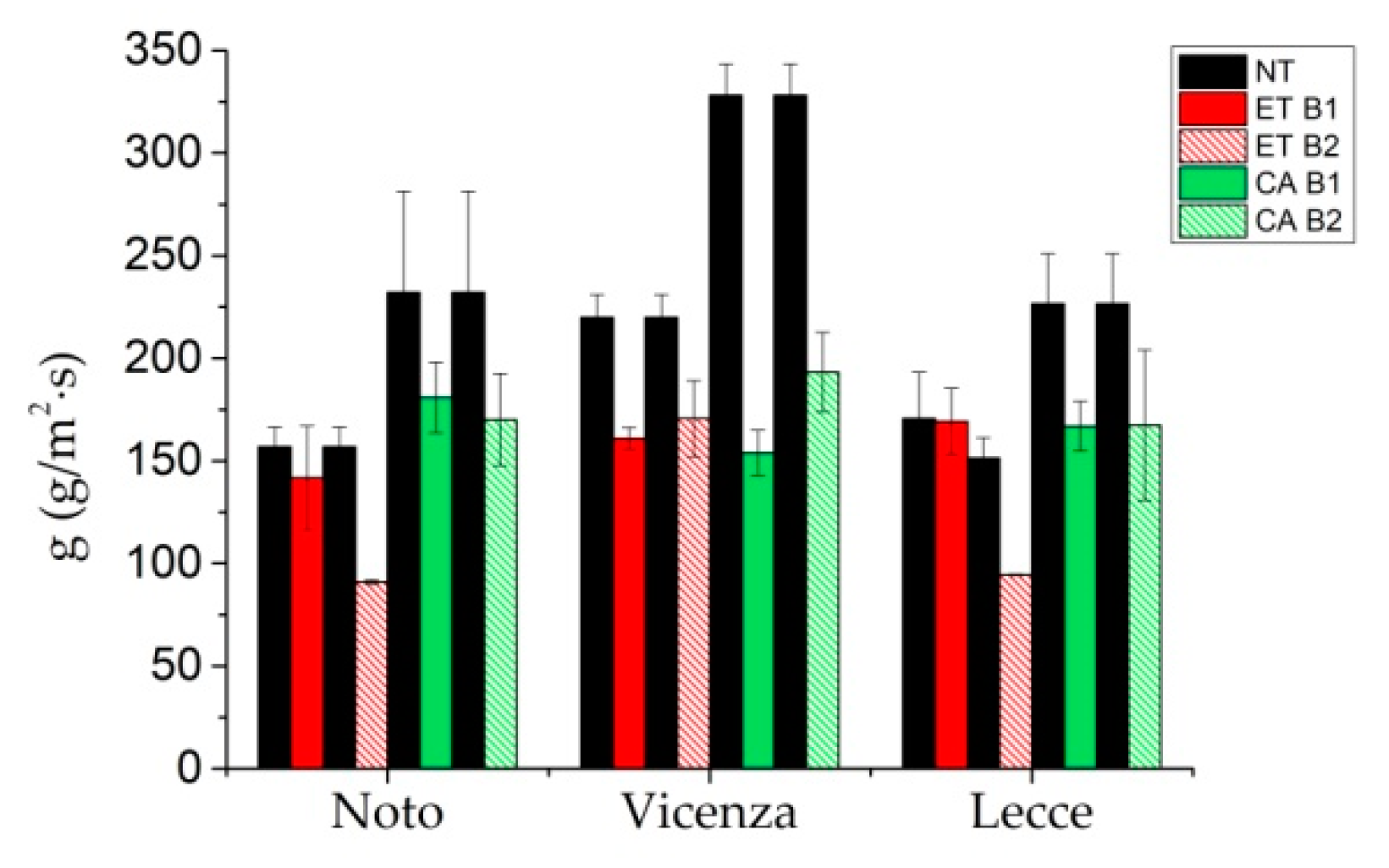
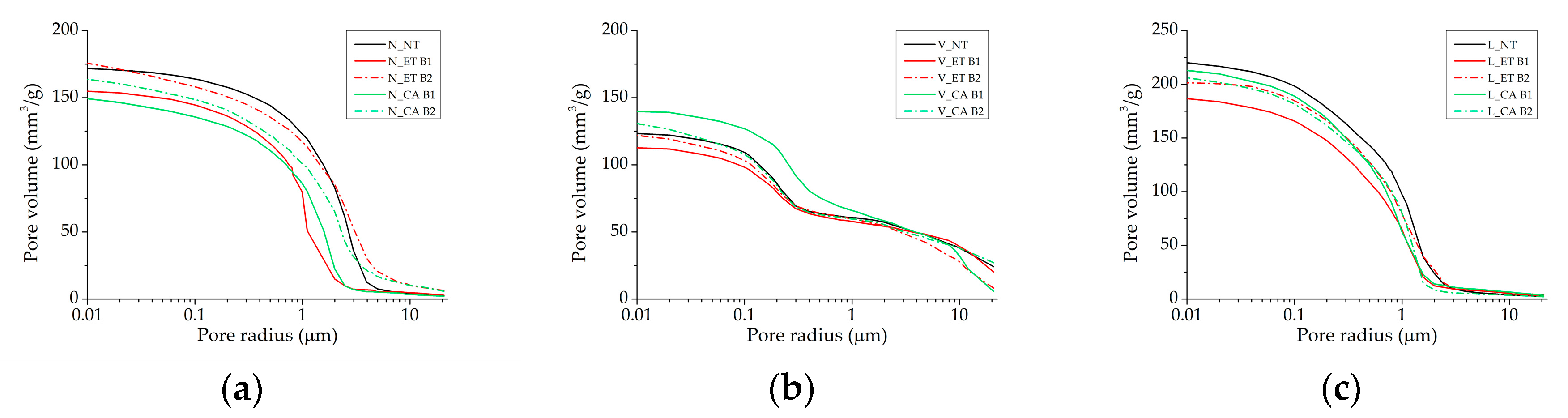
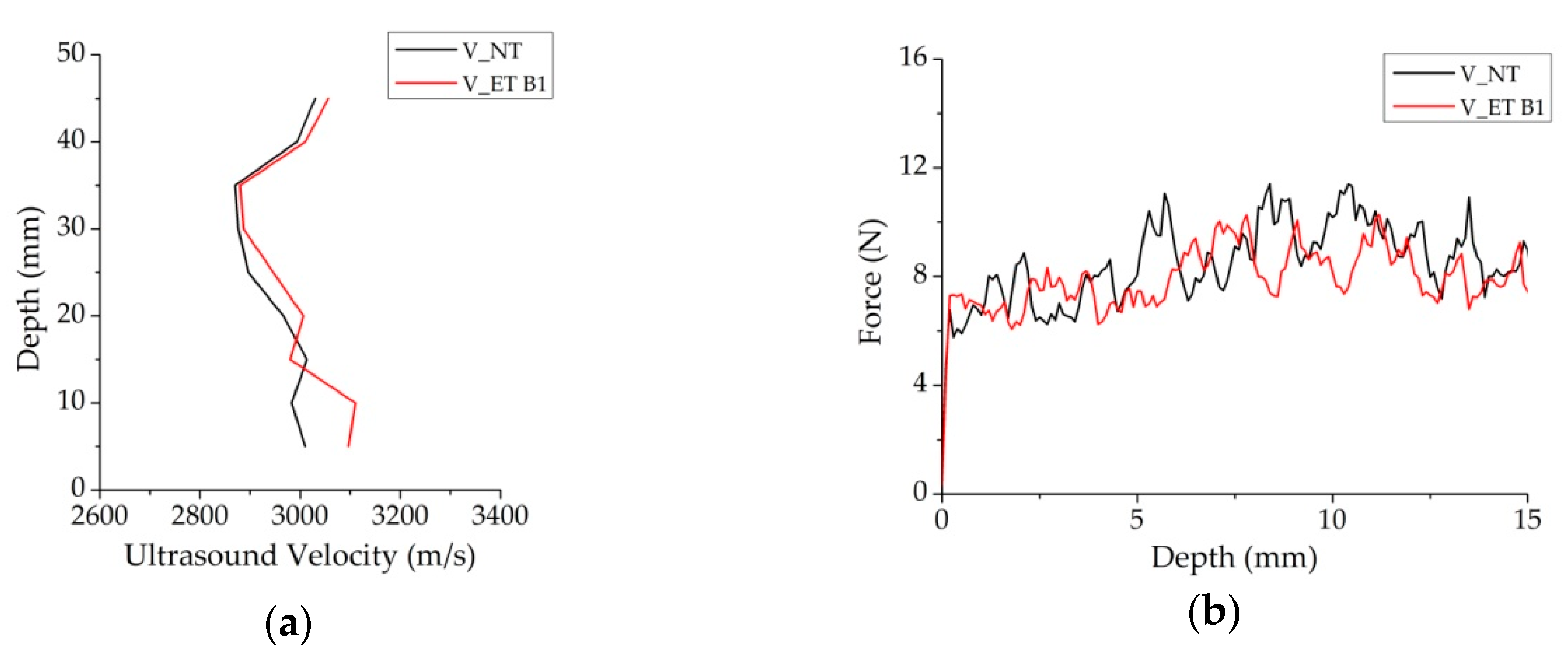
3.3. Performance of Consolidation Treatments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brimblecombe, P. History of air pollution and damage to the cultural heritage of European cities. In Science, Technology and European Cultural Heritage; Baer, N.S., Sabbioni, C., Sors, A.I., Eds.; Butterworth-Heinemann: Oxford, UK, 1991; pp. 51–66. [Google Scholar]
- Maravelaki, P.; Bertoncello, R.; Biscontin, G.; Battaglin, G.; Zendri, E.; Tondello, E. Investigation of the surface processes on exposed limestones. In Symposium J–Materials Issues in Art and Archaeology III; Vandiver, P.B., Ed.; Materials Research Society: Pittsburgh, PA, USA, 1992; Volume 267, p. 943. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.; Benavente, D.; Gomez-Heras, M.; Marco-Castaño, L.; García-Del-Cura, M.Á. Non-linear decay of building stones during freeze-thaw weathering processes. Constr. Build. Mater. 2013, 38, 443–454. [Google Scholar] [CrossRef]
- Cardell, C.; Delalieux, F.; Roumpopoulos, K.; Moropoulou, A.; Auger, F.; Van Grieken, R. Salt-induced decay in calcareous stone monuments and buildings in a marine environment in SW France. Constr. Build. Mater. 2003, 17, 165–179. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Favaro, M.; Tomasin, P.; Ossola, F.; Vigato, P.A. A novel approach to consolidation of historical limestone: The calcium alkoxides. Appl. Organomet. Chem. 2008, 22, 698–704. [Google Scholar] [CrossRef]
- Ossola, F.; Tomasin, P.; De Zorzi, C.; El Habra, N.; Chiurato, M.; Favaro, M. New Calcium Alkoxides for Consolidation of Carbonate Rocks. Influence of Precursors’ characteristics on morphology, crystalline phase and consolidation effects. New J. Chem. 2012, 36, 2618–2624. [Google Scholar] [CrossRef]
- Duchêne, S.; Detalle, V.; Favaro, M.; Ossola, R.; Tomasin, P.; De Zorzi, C.; El Habra, N. Nanomaterials for the consolidation of marble and wall paintings. In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone Columbia University, New York, NY, USA, 21–25 October 2012; pp. 1–9. [Google Scholar]
- Bourguignon, E.; Tomasin, P.; Detalle, V.; Vallet, J.; Labouré, M.; Olteanu, I.; Chiurato, M.A.; Bernardi, A.; Becherini, F. Calcium alkoxides as alternative consolidants for wall paintings: Evaluation of their performance in laboratory and on site, on model and original samples, in comparison to conventional products. J. Cult. Herit. 2017, 29, 54–66. [Google Scholar] [CrossRef]
- Natali, I.; Tomasin, P.; Becherini, F.; Bernardi, A.; Ciantelli, C.; Favaro, M.; Favoni, O.; Pérez, V.J.F.; Olteanu, I.D.; Dolores, M.; et al. Innovative consolidating products for stone materials: Field exposure tests as a valid approach for assessing durability. Herit. Sci. 2015, 3, 1–13. [Google Scholar] [CrossRef]
- Favaro, M.; Chiurato, M.; Tomasin, P.; Ossola, F.; Habra, E.; Svensson, I.; Beckers, E.; Bernardi, A. Calcium and magnesium alkoxides for conservation treatment of stone and wood in built heritage. In Proceedings of the Built Heritage 2013: Monitoring Conservation Management, Milan, Italy, 18–20 November 2013; pp. 1296–1303. [Google Scholar]
- Favaro, M.; Chiurato, M.; Tomasin, P.; Ossola, F.; Habra, E.; Brianese, N.; Svensson, I.; Beckers, E.; Perez, V.J.F.; Romero Sánchez, M.D.; et al. Alkaline earth alkoxides for conservation treatment of stone and wood in built heritage. In Proceedings of the 3rd European Workshop on Cultural Heritage Preservation, EWCHP 2013, Bozen, Italy, 15–17 September 2013; pp. 105–111. [Google Scholar]
- Final Report Summary—NANOMATCH (Nano-Systems for the Conservation of Immoveable and Moveable Polymaterial Cultural Heritage in a Changing Environment); Consiglio Nazionale delle Ricerche: Rome, Italy, 2015; Available online: https://cordis.europa.eu/result/rcn/163221_en.html (accessed on 12 March 2018).
- CaLoSiL®. Colloidal nano-particles of lime for stone and plaster consolidation. Technical Leaflet. IBZ-Salzchemie GmbH & Co. KG.: Halsbrücke, Germany. Available online: https://ibz-freiberg.de/downloads/pdf/produkte/tm/eng/CaLoSiL_E_IP_NP.pdf (accessed on 12 March 2018).
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 89. [Google Scholar] [CrossRef]
- Gordillo, J.R.; Pérez, M.P.S. Comportamiento físico del mármol blanco de Macael (España) por oscilación térmica de bajo y medio rango Performance of Spanish white Macael marble exposed to narrow- and medium-range temperature cycling. Mater. Constr. 2010, 60, 127–141. [Google Scholar] [CrossRef]
- NORMAL 4/80 Distribuzione del Volume dei Pori in Funzione del Loro Diametro (Italian Normative on Stone Material—Distribution of Pores Volume vs Their Diameter); Commissione Beni Culturali UNI NORMAL: Rome, Italy, 1980.
- UNI EN 10859:2000 Beni Culturali Materiali Lapidei Naturali ed Artificiali: Determinazione Dell’assorbimento D’acqua per Capillarità; Commissione Beni Culturali UNI NORMAL: Rome, Italy, 2010.
- DIN 52615 Testing of Thermal Insulating Material; Determination of Water Vapour Permeability of Construction and Insulating Materials; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 1987.
- NORMAL 43/93 Misure Colorimetriche di Superfici Opache (Italian Normative on Stone Material—Colorimetric Measurement of Opaque Surfaces); Commissione Beni Culturali UNI NORMAL: Rome, Italy, 1994.
- Adler, H.H.; Kerr, P.F. Infrared absorption frequency trends for anhydrous normal carbonates. Am. Mineral. 1963, 48, 124–137. [Google Scholar]
- Hazen, R.M.; Downs, R.T.; Jones, A.P.; Kah, L. Carbon Mineralogy and Crystal Chemistry. Rev. Mineral. Geochem. 2013, 75, 7–46. [Google Scholar] [CrossRef]
- Essabir, H.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. A comparison between bio and mineral calcium carbonate on the properties of polypropylene composites. Constr. Build. Mater. 2017, 134, 549–555. [Google Scholar] [CrossRef]
- Wei, H.; Shen, Q.; Zhao, Y.; Wang, D.; Xu, D. Influence of polyvinylpyrrolidone on the precipitation of calcium carbonate and on the transformation of vaterite to calcite. J. Cryst. Growth 2003, 250, 516–524. [Google Scholar] [CrossRef]
- Meiron, O.E.; Bar-David, E.; Aflalo, E.D.; Shechter, A.; Stepensky, D.; Berman, A.; Sagi, A. Solubility and Bioavailability of Stabilized Amorphous Calcium Carbonate. J. Bone Miner. Res. 2011, 26, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.A.; Brečević, L. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Suzuki, A.; Ruiz-Agudo, E. Alcohol Dispersions of Calcium Hydroxide Nanoparticles for Stone Conservation. Langmuir 2013, 29, 11457–11470. [Google Scholar] [CrossRef] [PubMed]
- Cumaran Arunasalam, V.; Baxter, I.; Darr, J.A.; Drake, S.R.; Hursthouse, M.B.; Abdul Malik, K.M.; Mingos, D.M.P. Insertion reactions of small molecules into group 2 metal alkoxides; structural characterization of [Mg9(μ5-CO3)(O2COMe)8(μ3-OMe)8(MeOH)13]∙MeOH∙C7H8. Polyhedron 1998, 17, 641–657. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, Y.; Xue, J.; Wu, Q. Role of E. coli—Secretion and Melamine in Selective Formation of CaC2O4·H2O and CaC2O4·2H2O Crystals. Cryst. Growth Des. 2014, 14, 450–458. [Google Scholar] [CrossRef]
- Zhang, X.; Glasser, F.P.; Scrivener, K.L. Reaction kinetics of dolomite and portlandite. Cem. Concr. Res. 2014, 66, 11–18. [Google Scholar] [CrossRef]
- Chang, J.; Li, Y.; Cao, M.; Fang, Y. Influence of magnesium hydroxide content and fineness on the carbonation of calcium hydroxide. Constr. Build. Mater. 2014, 55, 82–88. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technol. 2010, 189, 64–69. [Google Scholar] [CrossRef]
- Nan, Z.; Chen, X.; Yang, Q.; Wang, X.; Shi, Z.; Hou, W. Structure transition from aragonite to vaterite and calcite by the assistance of SDBS. J. Colloid Interface Sci. 2008, 325, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. R. Soc. Chem. 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite—calcite—vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Gabrielli, C.; Jaouhari, R.; Joiret, S.; Maurin, G. In situ Raman spectroscopy applied to electrochemical scaling. Determination of the structure of vaterite. J. Raman Spectrosc. 2000, 31, 497–501. [Google Scholar] [CrossRef]
- López-Arce, P.; Gómez-Villalba, L.S.; Martínez-Ramírez, S.; Alvarez de Buergo, M.; Fort, R. Influence of relative humidity on the carbonation of calcium hydroxide nanoparticles and the formation of calcium carbonate polymorphs. Powder Technol. 2011, 205, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Villalba, L.S.; López-Arce, P.; De Buergo, M.A.; Fort, R. Structural stability of a colloidal solution of Ca(OH)2 nanocrystals exposed to high relative humidity conditions. Appl. Phys. A Mater. Sci. Process. 2011, 104, 1249–1254. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Sasse, H.S.; Snethlage, R. Methods for the evaluation of stone conservation treatments. In Saving Our Architectural Heritage. The Conservation of Historic Stone Structures: Report of Dahlem Workshop on Saving our Architectural Heritage, Berlin, Germany, March 3–8, 1996; Baer, N.S., Snethlage, R., Eds.; John Wiley & Sons: New York, NY, USA, 1997; p. 225. [Google Scholar]
- Mimoso, J.; Costa, D. The DRMS drilling technique with pilot holes. In Proceedings of the Heritage Weather. Conservation Conference HWC-2006, Madrid, Spain, 21–24 June 2006; Volume 2, pp. 651–655. [Google Scholar]
- Delgado Rodrigues, J.; Costa, D. A new interpretation methodology for microdrilling data from soft mortars. J. Cult. Herit. 2016, 22, 951–955. [Google Scholar] [CrossRef]


| Stone | Quantity of Product Retained (kg/m2) | |||
|---|---|---|---|---|
| ET B1 | ET B2 | CA B1 | CA B2 | |
| Noto | 0.028–0.208 | 0.017–0.051 | 0.017–0.122 | 0.010–0.025 |
| Vicenza | 0.011–0.060 | 0.019–0.053 | 0.027–0.210 | 0.014–0.062 |
| Lecce | 0.016–0.115 | 0.019–0.114 | 0.012–0.097 | 0.010–0.022 |
| Stone | Reduction in CWAC % | |||
|---|---|---|---|---|
| ET B1 | ET B2 | CA B1 | CA B2 | |
| Noto | 28.8 | 23.1 | 15.8 | 15.9 |
| Vicenza | 36.9 | 30.2 | 29.3 | 25.5 |
| Lecce | 6.5 | 17.6 | 20.2 | 7.9 |
| Stone | ET B1 | CA B1 | ||||
|---|---|---|---|---|---|---|
| L* Before | L* After | ΔE* | L* Before | L* After | ΔE* | |
| Noto Vicenza Lecce | 82.4 | 84.2 | 6.3 ± 0.2 | 83.6 | 86.9 | 9.3 ± 0.8 |
| 88.1 | 88.7 | 2.6 ± 0.2 | 88.1 | 88.3 | 2.3 ± 1.0 | |
| 80.9 | 82.8 | 6.9 ± 0.5 | 81.4 | 85.4 | 11.9 ± 0.1 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuena, M.; Tomasin, P.; Costa, D.; Delgado-Rodrigues, J.; Zendri, E. Study of Calcium Ethoxide as a New Product for Conservation of Historical Limestone. Coatings 2018, 8, 103. https://doi.org/10.3390/coatings8030103
Zuena M, Tomasin P, Costa D, Delgado-Rodrigues J, Zendri E. Study of Calcium Ethoxide as a New Product for Conservation of Historical Limestone. Coatings. 2018; 8(3):103. https://doi.org/10.3390/coatings8030103
Chicago/Turabian StyleZuena, Martina, Patrizia Tomasin, Dória Costa, José Delgado-Rodrigues, and Elisabetta Zendri. 2018. "Study of Calcium Ethoxide as a New Product for Conservation of Historical Limestone" Coatings 8, no. 3: 103. https://doi.org/10.3390/coatings8030103





