Fabrication of Self-healing Superhydrophobic Surfaces from Water-Soluble Polymer Suspensions Free of Inorganic Particles through Polymer Thermal Reconstruction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Colloidal Polymer Spheres
2.3. Fabrication of Superhydrophobic Surfaces
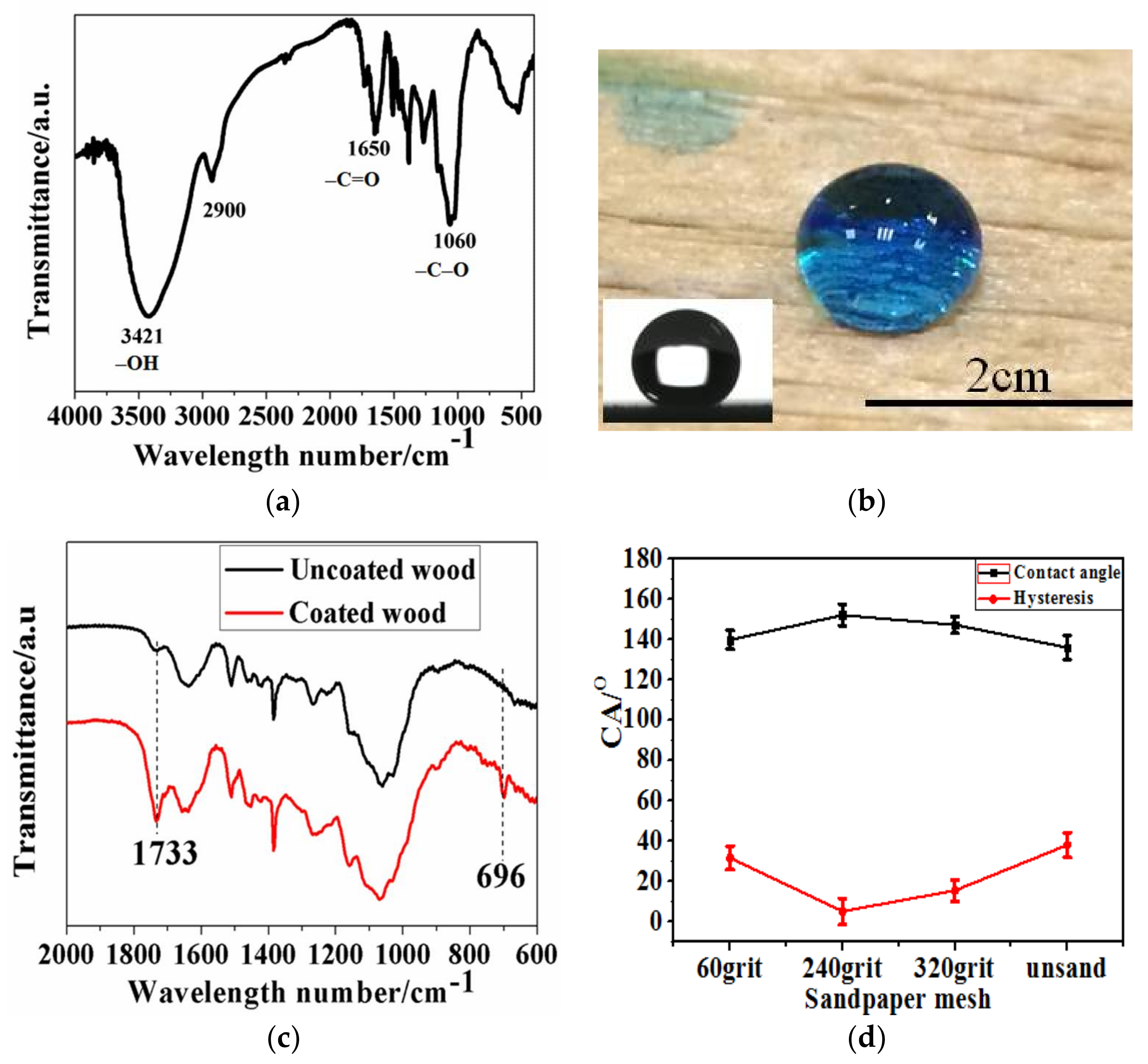
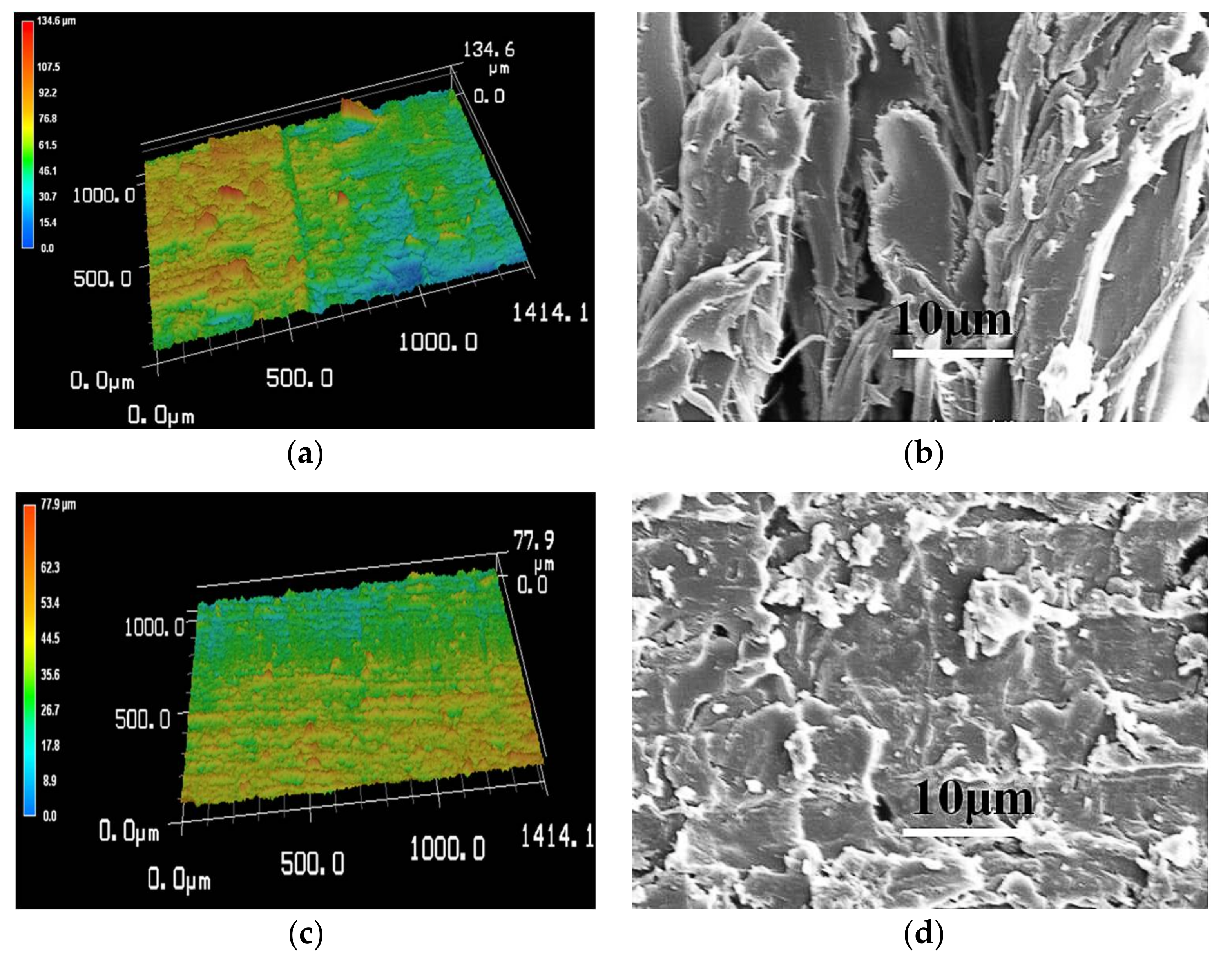
2.4. Characterization
3. Results and Discussion
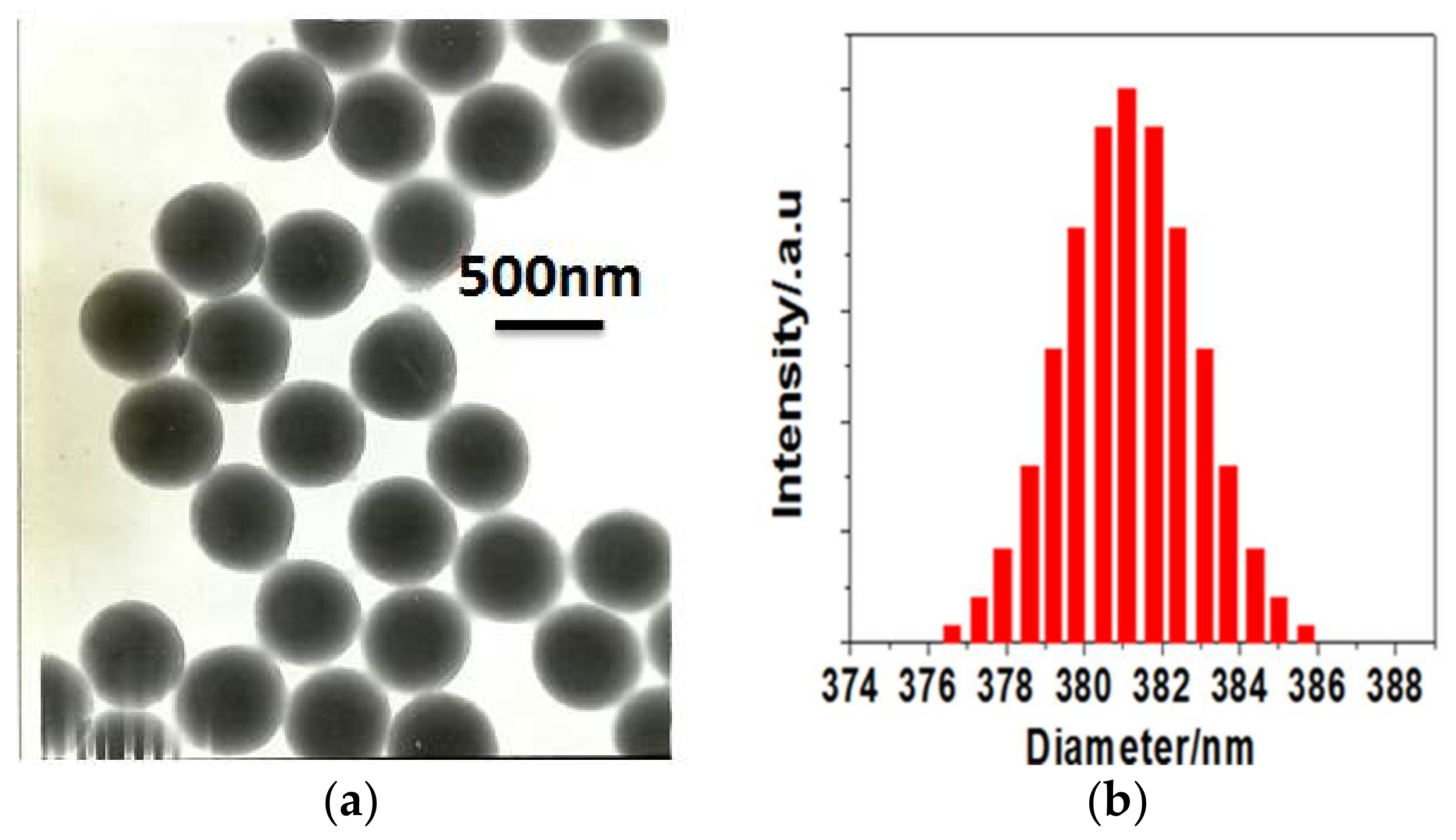
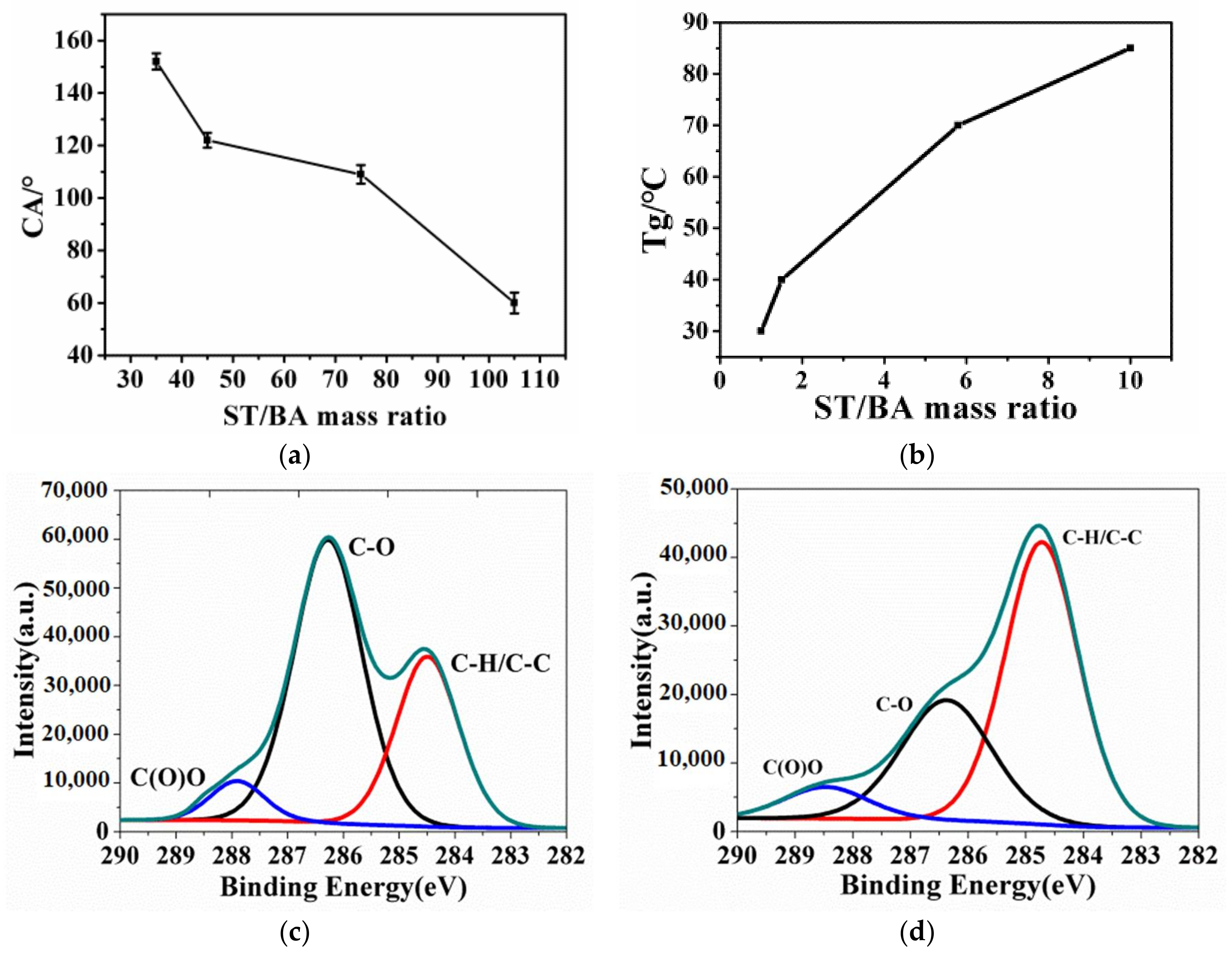
3.1. Synthesis and Morphology of Water-Soluble Amphiphilic Polymer Spheres
3.2. The fabrication of Superhydrophobic Surfaces through Polymer Thermal Reconstruction and the Effect of Surface Roughness on Wettability
3.3. Effect of ST/BA Mass Ratio on Wettability
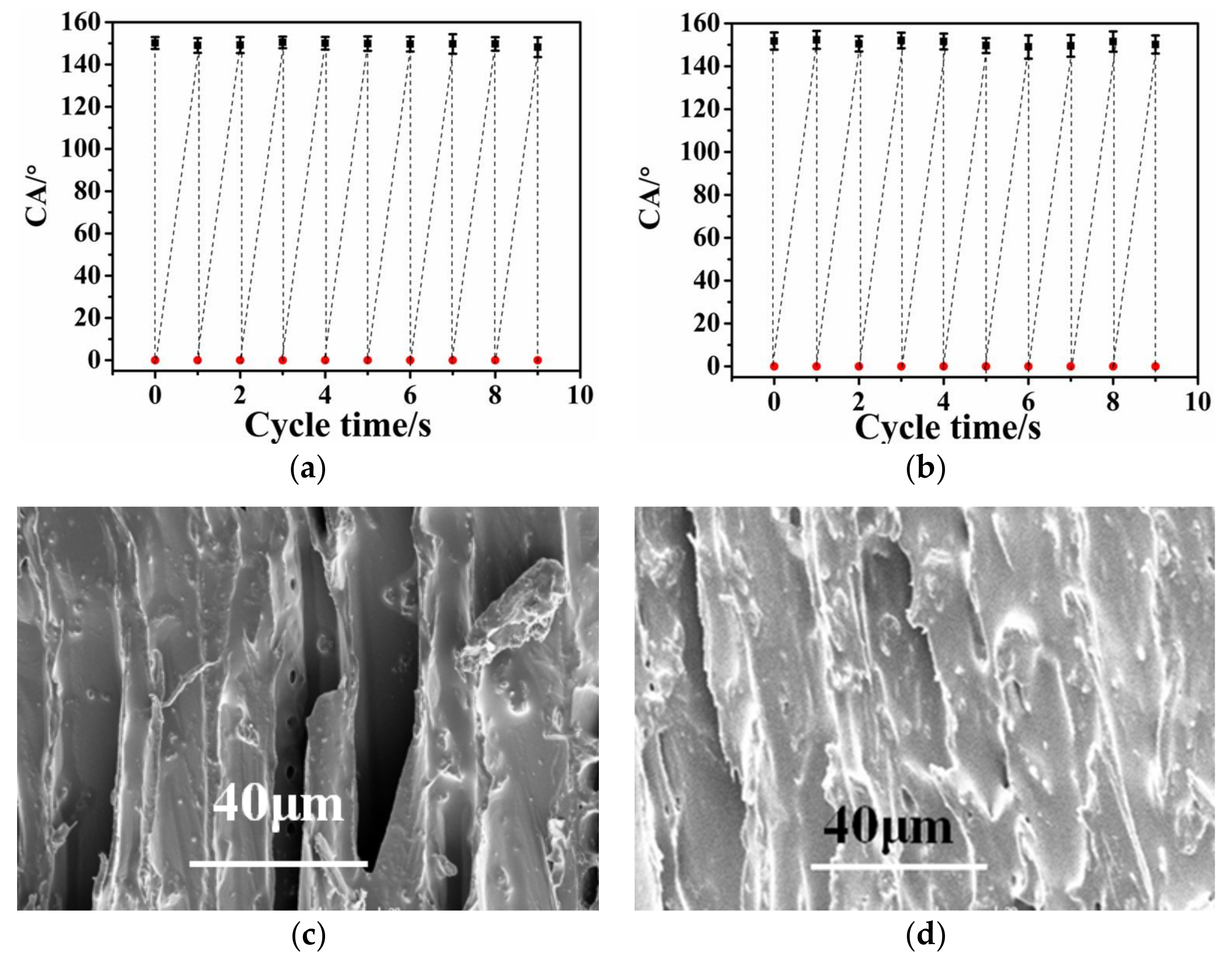
3.4. The Roubustness and Self-Replenishment of Superhydrophobic Surfaces
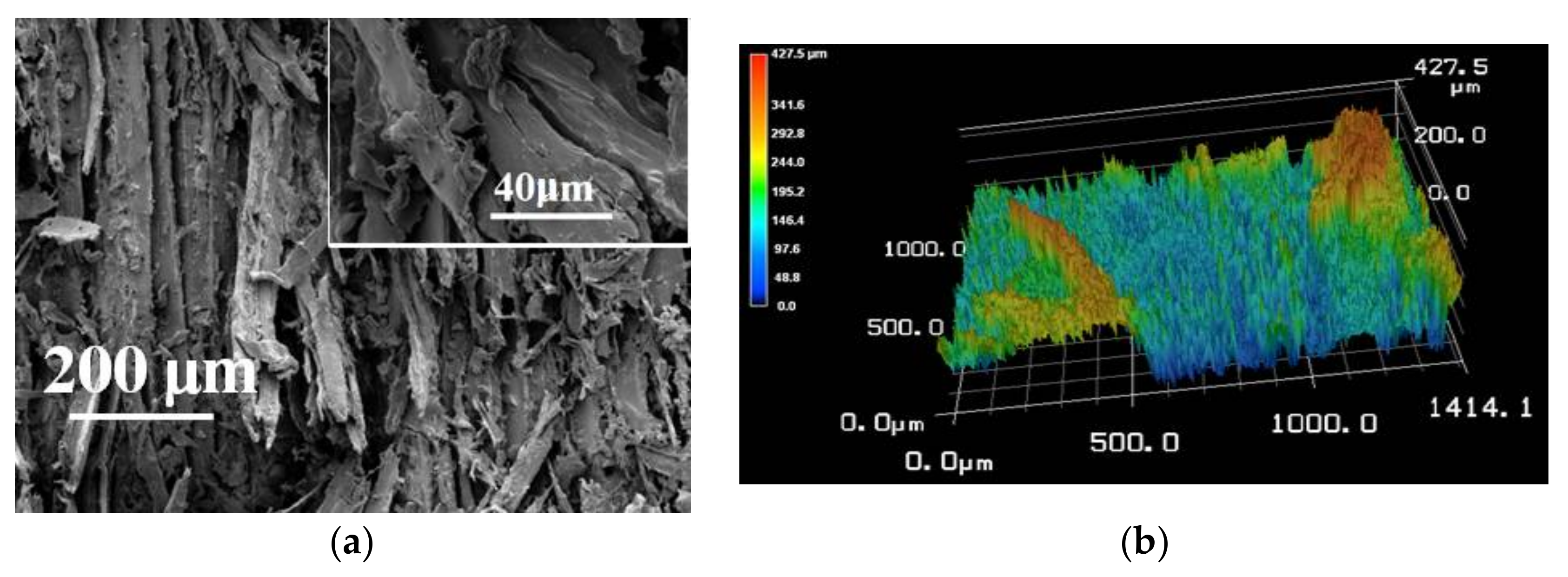
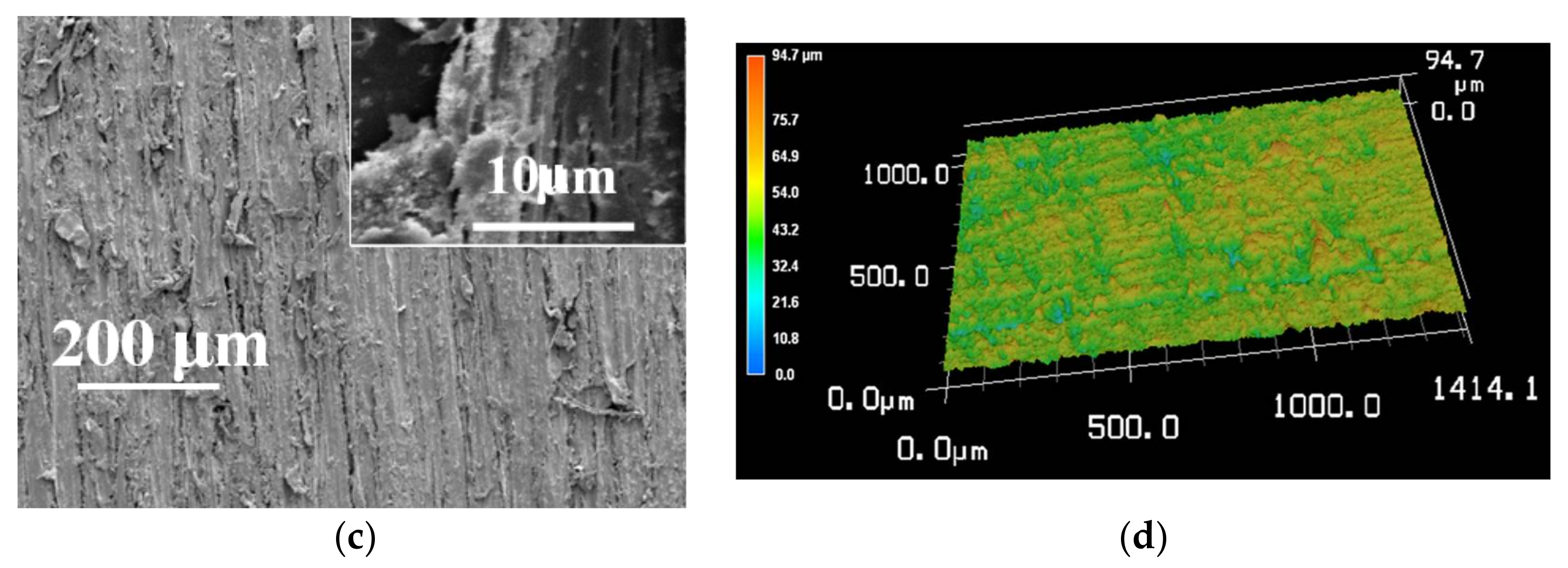
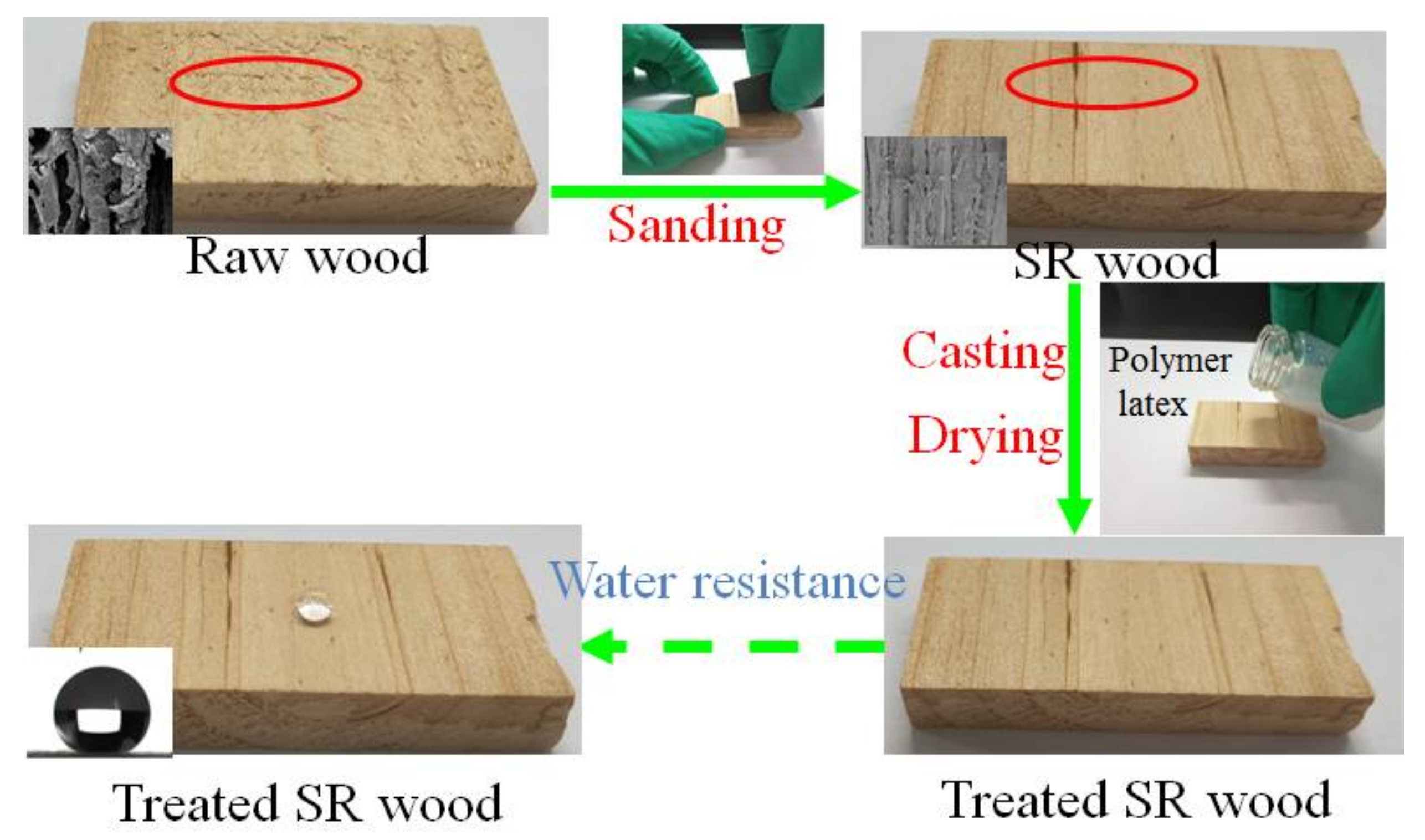
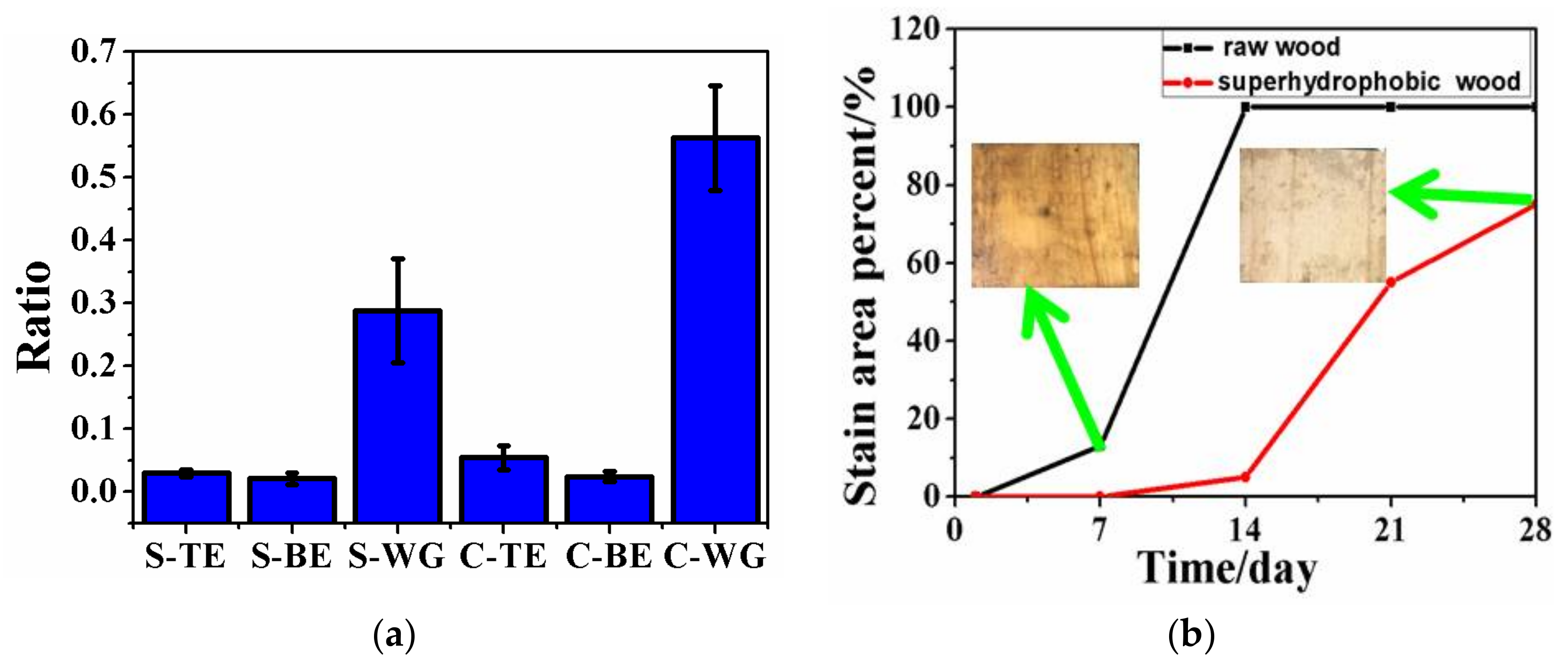
3.5. The Improved Water and Mildew Resistance of Wood with Superhydrophobic Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar] [CrossRef]
- Ellinas, K.; Kefallinou, D.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Is there a threshold in the antibacterial action of superhydrophobic surfaces? ACS Appl. Mater. Interfaces 2017, 9, 39781–39789. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levanen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Shi, Y.; Yu, G. Multifunctional superhydrophobic surfaces templated from innately microstructured hydrogel matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hu, Y.; Grinthal, A.; Khan, M.; Aizenberg, J. Liquid-based gating mechanism with tunable multiphase selectivity and antifouling behaviour. Nature 2015, 519, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Fei, B.; Hu, H.; Lai, C.; Xin, J. Bioinspired, stimuli-responsive, multifunctional superhydrophobic surface with directional wetting, adhesion, and transport of water. Adv. Funct. Mater. 2015, 25, 5047–5056. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired multifunctional foam with self-cleaning and oil/water separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Ruan, C.; Ai, K.; Li, X.; Lu, L. A Superhydrophobic sponge with excellent absorbency and flame retardancy. Angew. Chem. 2014, 126, 5556–5560. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Khudiyev, T.; Daglar, B.; Budunoglu, H.; Okyay, A.K.; Bayindir, M. Superhydrophobic and omnidirectional antireflective surfaces from nanostructured ormosil colloids. ACS Appl. Mater. Interfaces 2013, 5, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngblood, J.P.; McCarthy, T.J. Ultrahydrophobic polymer surfaces prepared by simultaneous ablation of polypropylene and sputtering of poly(tetrafluoroethylene) using radio frequency plasma. Macromolecules 1999, 32, 6800–6806. [Google Scholar] [CrossRef]
- Ellinas, K.; Pujari, S.P.; Dragatogiannis, D.A.; Charitidis, C.A.; Tserepi, A.; Zuilhof, H.; Gogolides, E. Plasma micro-nanotextured, scratch, water and hexadecane resistant, superhydrophobic, and superamphiphobic polymeric surfaces with perfluorinated monolayers. ACS Appl. Mater. Interfaces 2014, 6, 6510–6524. [Google Scholar] [CrossRef] [PubMed]
- Shon, Y.-S.; Lee, S.; Colorado, R.; Perry, S.S.; Lee, T.R. Spiroalkanedithiol-based sams reveal unique insight into the wettabilities and frictional properties of organic thin films. J. Am. Chem. Soc. 2000, 122, 7556–7563. [Google Scholar] [CrossRef]
- Miyauchi, M.; Kieda, N.; Hishita, S.; Mitsuhashi, T.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Reversible wettability control of TiO2 surface by light irradiation. Surf. Sci. 2002, 511, 401–407. [Google Scholar] [CrossRef]
- Mutel, B.; Taleb, A.B.; Dessaux, O.; Goudmand, P.; Gengembre, L.; Grimblot, J. Characterization of mixed zinc-oxidized zinc thin films deposited by a cold remote nitrogen plasma. Thin Solid Films 1995, 266, 119–128. [Google Scholar] [CrossRef]
- Bayer, I.S.; Davis, A.J.; Loth, E.; Steele, A. Water jet resistant superhydrophobic carbonaceous films by flame synthesis and tribocharging. Mater. Today Commun. 2015, 3, 57–68. [Google Scholar] [CrossRef]
- Bayer, I.S.; Brandi, F.; Cingolani, R.; Athanassiou, A. Modification of wetting properties of laser-textured surfaces by depositing triboelectrically charged teflon particles. Colloid Polym. Sci. 2013, 291, 367–373. [Google Scholar] [CrossRef]
- Gu, Z.-Z.; Uetsuka, H.; Takahashi, K.; Nakajima, R.; Onishi, H.; Fujishima, A.; Sato, O. Structural color and the lotus effect. Angew. Chem. Int. Ed. 2003, 42, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, Y.; Feng, X.; Song, Y.; Jiang, L. Control over the wettability of colloidal crystal films by assembly temperature. Macromol. Rapid Commun. 2006, 27, 188–192. [Google Scholar] [CrossRef]
- Wang, J.; Wen, Y.; Hu, J.; Song, Y.; Jiang, L. Fine control of the wettability transition temperature of colloidal-crystal films: From superhydrophilic to superhydrophobic. Adv. Funct. Mater. 2007, 17, 219–225. [Google Scholar] [CrossRef]
- Latthe, S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol-gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Geng, Z.; He, J.; Xu, L.; Yao, L. Rational design and elaborate construction of surface nano-structures toward highly antireflective superamphiphobic coatings. J. Mater. Chem. A 2013, 1, 8721–8724. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Hang, J.; Jin, L.; Shang, D.; Shi, L. Large-scale fabrication of robust superhydrophobic coatings with high rigidity and good flexibility. Adv. Mater. Interfaces 2016, 3, 500718. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef] [PubMed]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S.; Krishnan, K.G.; Robison, R.; Loth, E.; Berry, D.H.; Farrell, T.E.; Crouch, J.D. Thermal alternating polymer nanocomposite (TAPNC) coating designed to prevent aerodynamic insect fouling. Sci. Rep. 2016, 6, 38459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Sun, J. Bioinspired self-healing superhydrophobic coatings. Angew. Chem. Int. Ed. 2010, 49, 6129–6133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, Y.; Ding, J.; Feng, L.; Wang, X.; Lin, T. Durable, self-healing superhydrophobic and superoleophobic surfaces from fluorinated-decyl polyhedral oligomeric silsesquioxane and hydrolyzed fluorinated alkyl silane. Angew. Chem. Int. Ed. 2011, 50, 11433–11436. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.C.C.; Luo, Y.; Put, M.W.P.V.D.; Carcouët, C.C.M.; With, G.D. Self-replenishing dual structured superhydrophobic coatings prepared by drop-casting of an all-in-one dispersion. Adv. Funct. Mater. 2014, 24, 986–992. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wu, M.; Sun, J. All spraying processes for the fabrication of robust, self-healing, superhydrophobic coatings. Adv. Mater. 2014, 26, 3344–3348. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, C.; Xie, Q.; Zhang, G. Self-repairing silicone coatings for marine anti-biofouling. J. Mater. Chem. A 2017, 5, 15855–15861. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, S.; Yang, S.; Wu, L. Fabrication of all-water-based sel-repairing superhydrophobic coatings based on UV-responsive microcapsules. Adv. Funct. Mater. 2015, 25, 1035–1041. [Google Scholar] [CrossRef]
- Xue, C.H.; Bai, X.; Jia, S.T. Robust, self-healing superhydrophobic fabrics prepared by one-step coating of pdms and octadecylamine. Sci. Rep. 2016, 6, 27262. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Chem. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Chen, C.M.; Yang, S. Directed water shedding on high-aspect-ratio shape memory polymer micropillar arrays. Adv. Mater. 2014, 26, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Salazar, J.; Vahabi, H.; Joshiimre, A.; Voit, W.E.; Kota, A.K. Metamorphic superomniphobic surfaces. Adv. Mater. 2017, 29, 1700295. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Lynn, D.M. Restoration of superhydrophobicity in crushed polymer films by treatment with water: Self-healing and recovery of damaged topographic features aided by an unlikely source. Adv. Mater. 2013, 25, 5104–5108. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yang, Y.; Lu, F.; Bao, B.; You, B. Self-assembly of binary particles and application as structural colors. Polym. Chem. 2012, 3, 2495–2501. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, Y.; Lu, F.; Bao, B.; You, B.; Shi, L. Self-assembly of colloidal spheres and application as solvent responding polymer film. J. Colloid Interface Sci. 2013, 389, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Liu, D.; Yang, Y.; Shen, Z.; You, B. Self-assembly of ternary particles for tough colloidal crystals with vivid structure colors. J. Nanomater. 2013, 2013, 8–21. [Google Scholar] [CrossRef]
- Cai, P.; Bai, N.; Xu, L.; Tan, C.; Li, Q. Fabrication of superhydrophobic wood surface with enhanced environmental adaptability through a solution-immersion process. Surf. Coat. Technol. 2015, 277, 262–269. [Google Scholar] [CrossRef]
- Chang, H.; Tu, K.; Wang, X.; Liu, J. Fabrication of mechanically durable superhydrophobic wood surfaces using polydimethylsiloxane and silica nanoparticles. RSC Adv. 2015, 5, 30647–30653. [Google Scholar] [CrossRef]
- Wang, S.; Shi, J.; Liu, C.; Cheng, X.; Wang, C. Fabrication of a superhydrophobic surface on a wood substrate. Appl. Surf. Sci. 2011, 257, 9362–9365. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Surface Roughness Standard (JIS B0601:1994). Available online: https://us.misumi-ec.com/pdf/tech/mech/US2010_fa_p3541_3542.pdf (accessed on 11 March 2018).
- Plötze, M.; Niemz, P. Porosity and pore size distribution of different wood types as determined by mercury intrusion porosimetry. Eur. J. Wood Wood Prod. 2011, 69, 649–657. [Google Scholar] [CrossRef]
- Sperry, P.R.; Snyder, B.S.; O’Dowd, M.L.; Lesko, P.M. Role of water in particle deformation and compaction in latex film formation. Langmuir 1994, 10, 2619–2628. [Google Scholar] [CrossRef]
- Ruhs, C. Developing environmentally benign and effective organic wood preservatives by understanding the biocidal and non-biocidal properties of extractives in naturally durable heartwood. Holzforschung 2008, 62, 264–269. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Development of environmentally-benign wood preservatives based on the combination of organic biocides with antioxidants and metal chelators. Phytochemistry 2002, 61, 555–560. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Wu, Y.; Shen, Z.; Chen, H. Fabrication of Self-healing Superhydrophobic Surfaces from Water-Soluble Polymer Suspensions Free of Inorganic Particles through Polymer Thermal Reconstruction. Coatings 2018, 8, 144. https://doi.org/10.3390/coatings8040144
Shen Y, Wu Y, Shen Z, Chen H. Fabrication of Self-healing Superhydrophobic Surfaces from Water-Soluble Polymer Suspensions Free of Inorganic Particles through Polymer Thermal Reconstruction. Coatings. 2018; 8(4):144. https://doi.org/10.3390/coatings8040144
Chicago/Turabian StyleShen, Yalun, Yitian Wu, Zhehong Shen, and Hao Chen. 2018. "Fabrication of Self-healing Superhydrophobic Surfaces from Water-Soluble Polymer Suspensions Free of Inorganic Particles through Polymer Thermal Reconstruction" Coatings 8, no. 4: 144. https://doi.org/10.3390/coatings8040144




