Effects of Flow Rates and Density Matching on the Integrity of Solid Particles Coated by Water Phase Compound Droplets during the Transport Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
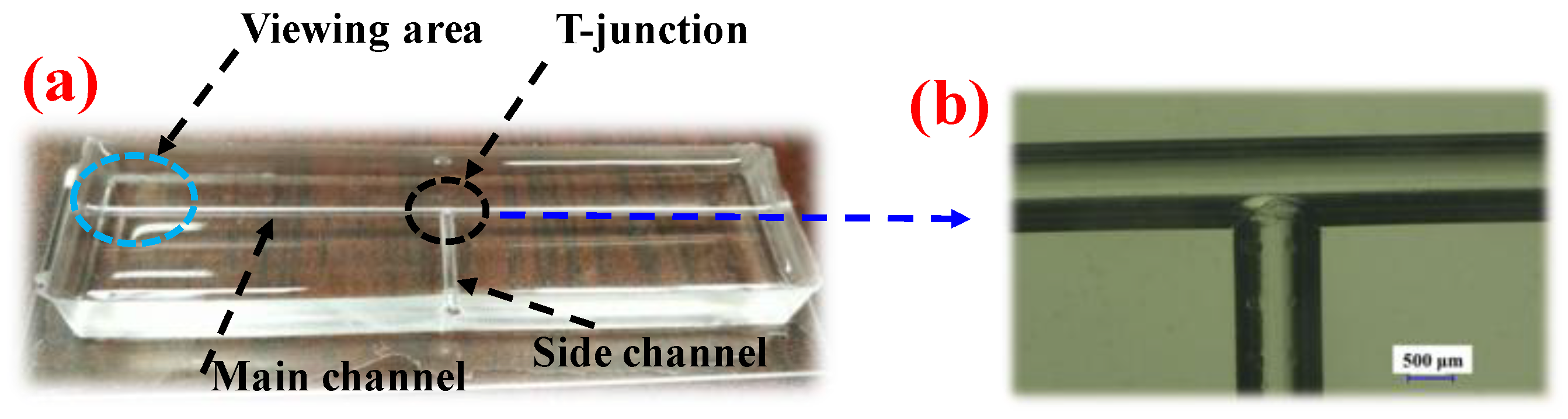
2.2. Experimental Setup and Methods
3. Results and Discussion
3.1. Analysis of the Transport of S/W/O Compound Droplets
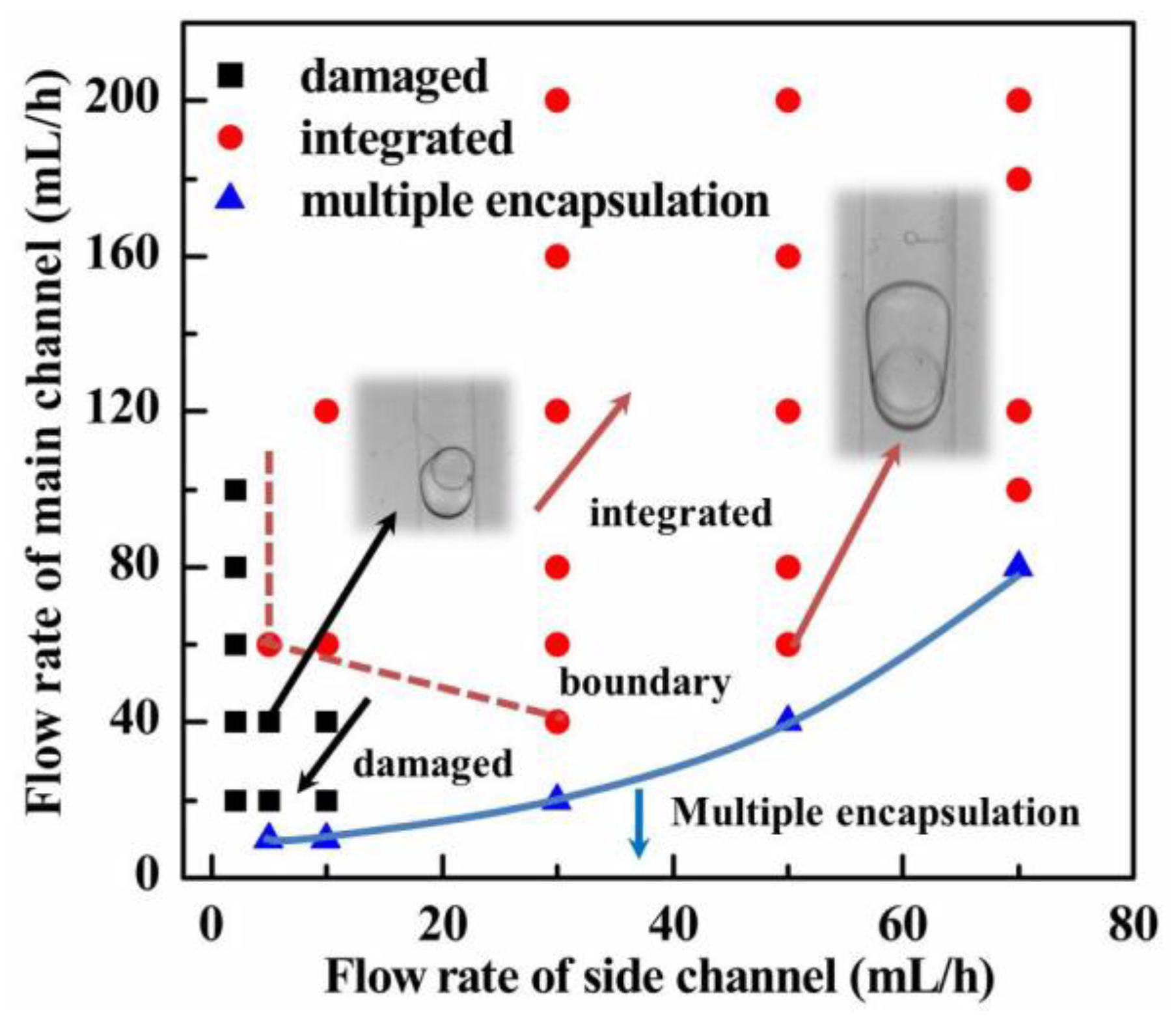
3.2. Effects of Flow Rates on the Integrity of S/W/O Compound Droplets
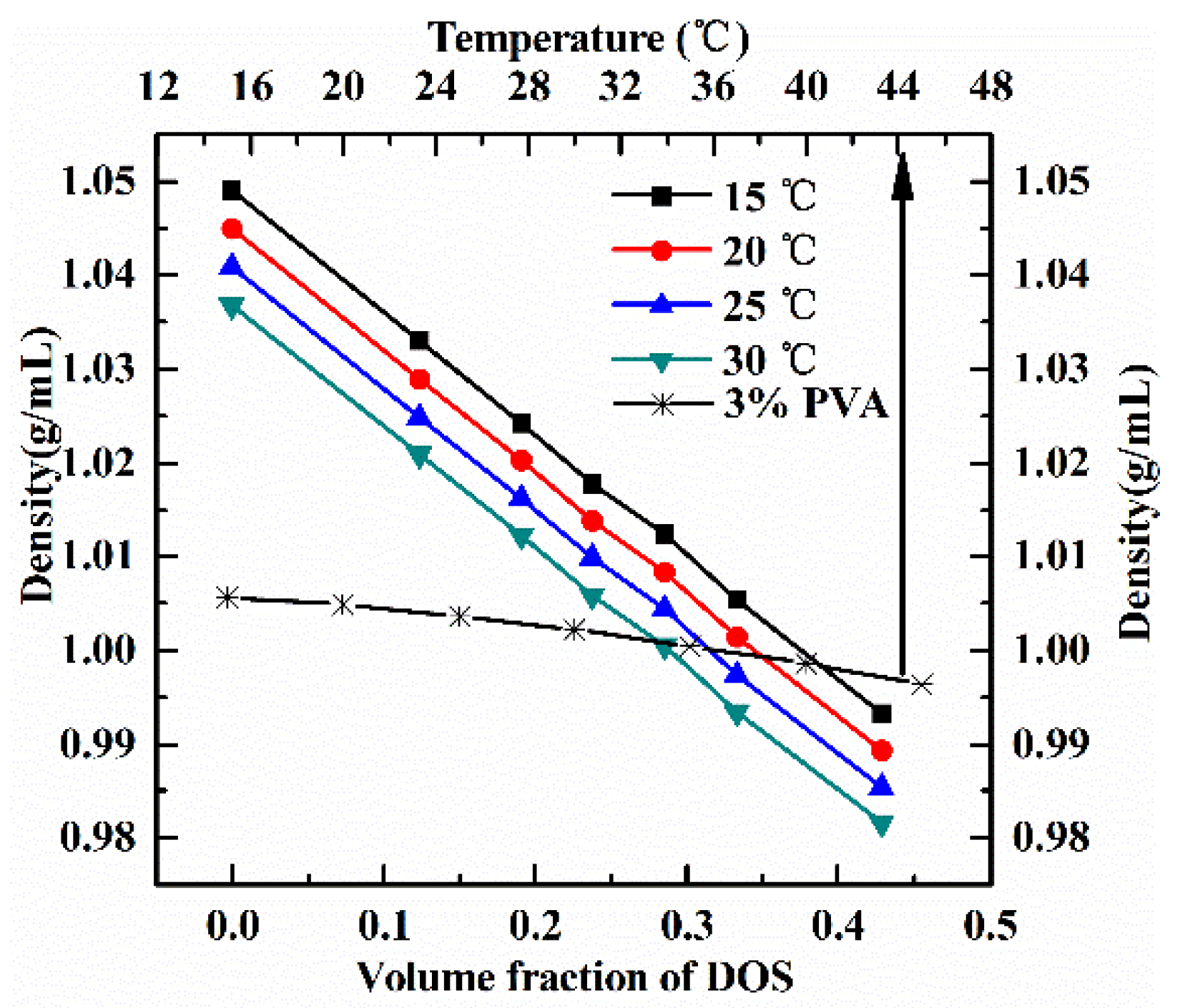
3.3. Effects of Density Matching on the Integrity of S/W/O Compound Droplets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Preparation of PS Shells

Appendix A.2. Fabrication of the T-Junction Device
Appendix A.3. Density Matching among Three Phases
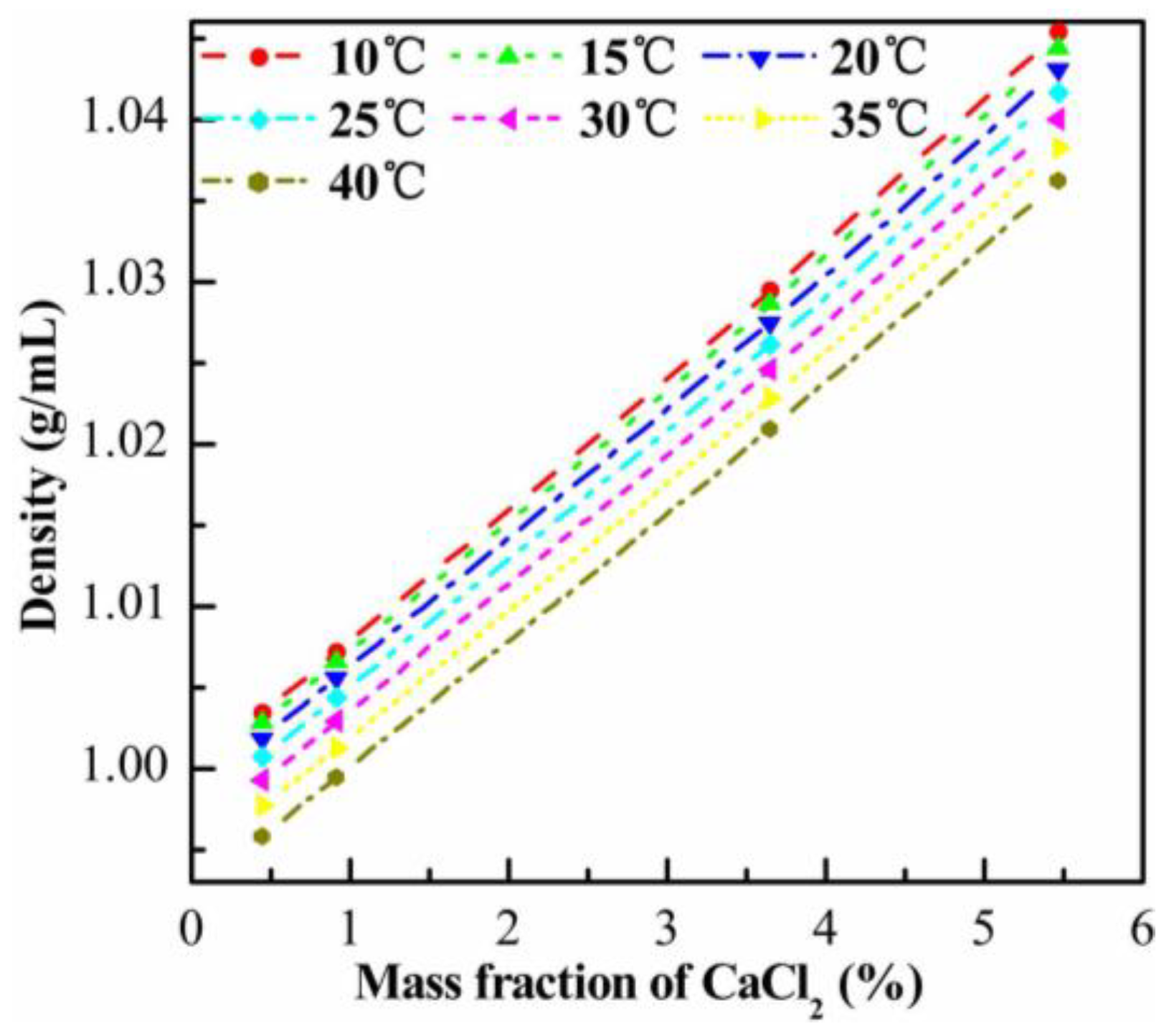
Appendix A.4. Optical Images of S/W/O Compound Droplets

References
- Sefton, M.V.; May, M.H.; Lahooti, S.; Babensee, J.E. Making microencapsulation work: Conformal coating, immobilization gels and in vivo performance. J. Controll. Release 2000, 65, 173–186. [Google Scholar] [CrossRef]
- Khademhosseini, A.; May, M.H.; Sefton, M.V. Conformal coating of mammalian cells immobilized onto magnetically driven beads. Tissue Eng. 2005, 11, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Taly, V.; Kelly, B.T.; Griffiths, A.D. Droplets as microreactors for high-throughput biology. ChemBioChem 2007, 8, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shum, H.C.; Bandyopadhyay, A.; Bose, S.; Weitz, D.A. Double emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatite. Chem. Mater. 2009, 21, 5548–5555. [Google Scholar] [CrossRef]
- Norimatsu, T.; Nagai, K.; Yamanaka, T.; Endo, T.; Yoshida, H.; Sakagami, Y. Activities on target fabrication and injection toward laser fusion energy in Japan. Fusion Eng. Des. 2002, 63–64, 587–596. [Google Scholar] [CrossRef]
- Letts, S.A.; Fearon, E.M.; Buckley, S.R.; Saculla, M.D.; Allison, L.M.; Cook, R. Fabrication of polymer shells using a depolymerizable mandrel. Fusion. Sci. Technol. 1995, 28, 1797–1802. [Google Scholar] [CrossRef]
- Norimatsu, T.; Takagi, M.; Takaki, T.; Morimoto, K.; Izawa, Y.; Mima, K. Recent research on target fabrication for up-coming projects. Fusion Eng. Des. 1999, 44, 449–459. [Google Scholar] [CrossRef]
- Alan, K.B.; John, Z.G.; Eben, M. Fabrication of Polyvinyl alcohol coated polystyrene shells. J. Vac. Sci. Technol. A 1987, 6, 3417–3421. [Google Scholar]
- Esmaeilzadeh, H.; Zheng, K.; Su, J.; Mead, J.; Sobkowicz, M.J.; Sun, H. Experimental study of drag reduction on superhydrophobic surfaces using quartz crystal microbalance. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Tampa, FL, USA, 3–9 November 2017. [Google Scholar]
- Su, J.; Esmaeilzadeh, H.; Zhang, F.; Yu, Q.; Cernigliaro, G.; Xu, J.; Sun, H. An ultrasensitive micropillar-based quartz crystal microbalance device for real-time measurement of protein immobilization and protein-protein interaction. Biosens. Bioelectron. 2018, 99, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, H.; Cernigliaro, G.; Su, J.; Gong, L.; Mirzaee, I.; Charmchi, M.; Sun, H. The effects of material properties on pillar-based QCM sensors. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015. [Google Scholar]
- Su, J.; Esmaeilzadeh, H.; Sun, H. Study of frequency response of quartz crystal microbalance to different wetting states of micropillar surfaces. In Proceedings of the ASME Fluids Engineering Division Summer Meeting, Waikoloa, HI, USA, 30 July–3 August 2017. [Google Scholar]
- Su, J.; Inalpolat, M.; Ge, T.; Esmaeilzadeh, H.; Sun, H. Experimental study and analysis of dropwise condensation using quartz crystal microbalance. In Proceedings of the ASME Heat Transfer Summer Conference, Washington, DC, USA, 10–14 July 2016. [Google Scholar]
- Thorsen, T. Dynamic pattern formation in a vesicle-generation microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip. 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Li, F.C.; Yang, J.C.; Kinoshita, H.; Oishi, M.; Oshima, M. Study on the mechanism of droplet formation in T-junction microchannel. Chem. Eng. Sci. 2012, 69, 340–351. [Google Scholar] [CrossRef]
- De Menech, M.; Garstecki, P.; Jousse, F.; Stone, H.A. Transition from squeezing to dripping in a microfluidic T-shaped junction. J. Fluid Mech. 2008, 595, 141–161. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of breakup. Lab Chip. 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Engl, W.; Roche, M.; Colin, A.; Panizza, P. Droplet traffic at a simple junction at low capillary number. Phys. Rev. Lett. 2005, 95, 208304. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tice, J.D.; Ismagilov, R. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Bretherton, F.P. The motion of long bubbles in tubes. J. Fluid Mech. 1961, 10, 166–188. [Google Scholar] [CrossRef]
- Ratulowski, J.; Chang, H.C. Transport of gas bubbles in capillaries. Phys. Fluids 1989, 1, 1642–1655. [Google Scholar] [CrossRef]
- Hodges, S.R.; Jensen, O.E.; Rallison, J.M. The motion of a viscous drop through a cylindrical tube. J. Fluid Mech. 2004, 501, 279–301. [Google Scholar] [CrossRef]
- Schwartz, L.W.; Princen, H.M.; Kiss, A.D. On the motion of bubbles in capillary tubes. J. Fluid Mech. 1986, 172, 259–275. [Google Scholar] [CrossRef]
- Reinelt, D.A.; Saffman, P.G. The penetration of a finger into a viscous fluid in a channel and tube. SIAM. J. Sci. Comput. 1985, 6, 542–561. [Google Scholar] [CrossRef]
- Hazel, A.L.; Heil, M. The steady propagation of a semi-infinite bubble into a tube of elliptical or rectangular cross-section. J. Fluid Mech. 2002, 470, 91–114. [Google Scholar] [CrossRef]
- Adzima, B.J.; Velankar, S.S. Pressure drop for droplets flows in microfluidic channels. J. Micromech. Microeng. 2006, 16, 1504–1510. [Google Scholar] [CrossRef]
- Jousse, F.; Lian, G.P.; Janes, R.; Melrose, J. Compact model for multi-phase liquid–liquid flows in micro-fluidic devices. Lab Chip. 2005, 5, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Fuerstman, M.J.; Lai, A.; Thurlow, M.E.; Shevkoplyas, S.S.; Stone, H.A.; Whitesides, G.M. The pressure drop along rectangular microchannels containing bubbles. Lab Chip. 2007, 7, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, F.; Prat, L.; Miceli, N.D.; Cristobal, G.; Link, D.R.; Weitz, D.A. Mixing characterization inside microdroplets engineered on a microcoalescer. Chem. Eng. Sci. 2007, 62, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, H.; Kaneda, S.; Fujii, T.; Oshima, M. Three dimensional measurement and visualization of internal flow of a moving droplet using confocal micro-PIV. Lab Chip. 2007, 7, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Lindken, R.; Rossi, M.; Westerweel, J. Micro-particle image velocimetry (mPIV): Recent developments, applications, and guidelines. Lab Chip. 2009, 9, 2551–2567. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liu, X.D.; Shi, M.H. Hydrodynamics of double emulsion droplet in shear flow. Appl. Phys. Lett. 2013, 102, 051609. [Google Scholar] [CrossRef]
- Pan, D.W.; Liu, M.F.; Li, F.; Chen, Q.; Liu, X.D.; Liu, Y.Y.; Zhang, Z.W.; Huang, W.X.; Li, B. Formation mechanisms of solid in water in oil compound droplets in a horizontal T-junction device. Chem. Eng. Sci. 2018, 176, 254–263. [Google Scholar] [CrossRef]
- Staben, M.E.; Galvin, K.P.; Davis, R.H. Low-Reynolds-number motion of a heavy sphere between the two parallel plane walls. Chem. Eng. Sci. 2006, 61, 1932–1945. [Google Scholar] [CrossRef]
- Staben, M.E.; Davis, R.H. Particle transport in Poiseuille flow in narrow channels. Int. J. Multiph. Flow. 2005, 31, 529–547. [Google Scholar] [CrossRef]
- Liu, M.F.; Chen, S.F.; Qi, X.B.; Li, B.; Shi, R.T.; Liu, Y.Y.; Chen, Y.P.; Zhang, Z.W. Improvement of wall thickness uniformity of thick-walled polystyrene shells by density matching. Chem. Eng. J. 2014, 241, 466–476. [Google Scholar] [CrossRef]
- Shao, T.; Feng, X.; Jin, Y.; Cheng, Y. Controlled production of compound droplets in dual-coaxial capillaries device for millimeter-scale hollow polymer spheres. Chem. Eng. Sci. 2013, 104, 55–63. [Google Scholar] [CrossRef]





| O Phase | W Phase | S Phase | ΔρO-W (g/cm3) | ΔρS-W (g/cm3) |
|---|---|---|---|---|
| DBP:DOS = 144:66 | 3% PVA | Pure water | 0.00024 | 0.00024 |
| DBP:DOS = 150:60 | 3% PVA | Pure water | 0.00532 | 0.00024 |
| DBP:DOS = 170:40 | 3% PVA | Pure water | 0.01718 | 0.00024 |
| DBP | 3% PVA | Pure water | 0.04198 | 0.00024 |
| O Phase | W Phase | S Phase | ΔρO-W (g/cm3) | ΔρS-W (g/cm3) |
|---|---|---|---|---|
| DBP:DOS = 144:66 | 3% PVA | Pure water | 0.000243 | 0.00024 |
| DBP:DOS = 144:66 | 3% PVA | 0.5% CaCl2 | 0.000243 | 0.00366 |
| DBP:DOS = 144:66 | 3% PVA | 1.0% CaCl2 | 0.000243 | 0.004838 |
| DBP:DOS = 144:66 | 3% PVA | 2.0% CaCl2 | 0.000243 | 0.011905 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, D.; Liu, M.; Chen, Q.; Huang, W.; Li, B. Effects of Flow Rates and Density Matching on the Integrity of Solid Particles Coated by Water Phase Compound Droplets during the Transport Process. Coatings 2018, 8, 191. https://doi.org/10.3390/coatings8050191
Pan D, Liu M, Chen Q, Huang W, Li B. Effects of Flow Rates and Density Matching on the Integrity of Solid Particles Coated by Water Phase Compound Droplets during the Transport Process. Coatings. 2018; 8(5):191. https://doi.org/10.3390/coatings8050191
Chicago/Turabian StylePan, Dawei, Meifang Liu, Qiang Chen, Weixing Huang, and Bo Li. 2018. "Effects of Flow Rates and Density Matching on the Integrity of Solid Particles Coated by Water Phase Compound Droplets during the Transport Process" Coatings 8, no. 5: 191. https://doi.org/10.3390/coatings8050191





