A Ladder-Type Organosilicate Copolymer Gate Dielectric Materials for Organic Thin-Film Transistors
Abstract
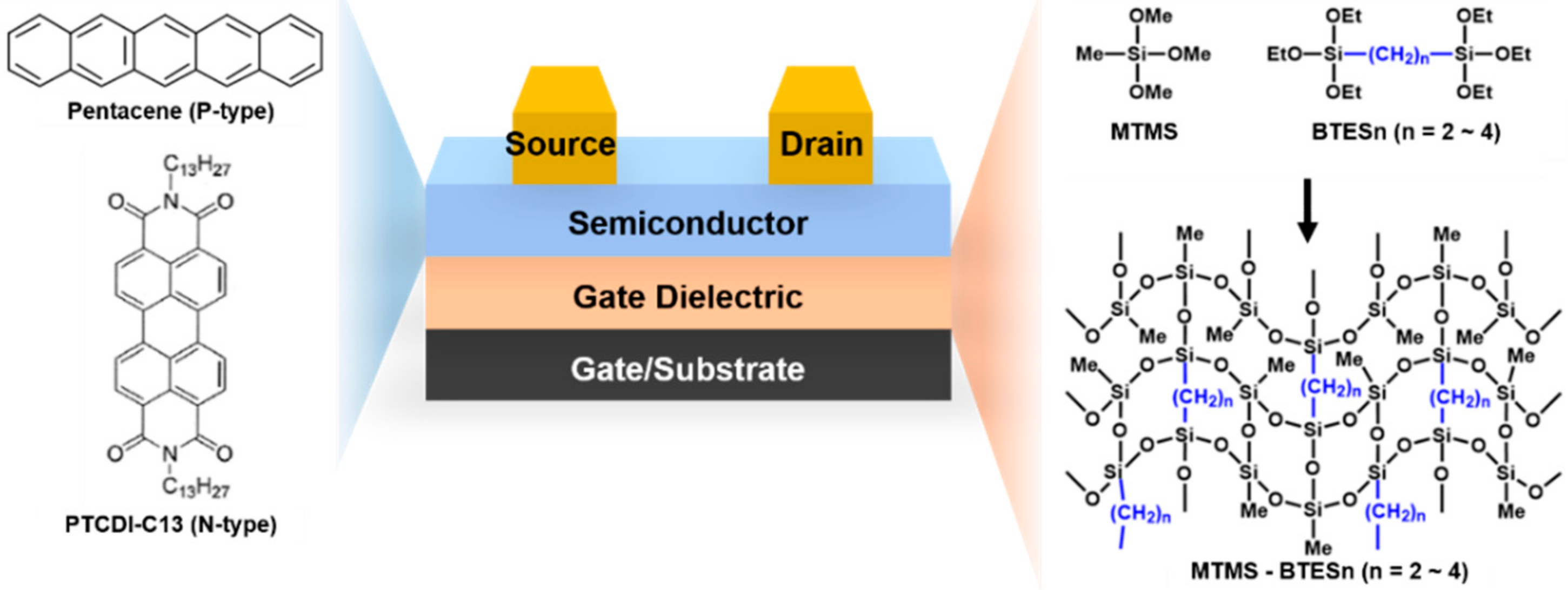
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Synthesis
2.2.1. Synthesis of MTMS-BTES2
2.2.2. Synthesis of MTMS-BTES3
2.2.3. Synthesis of MTMS-BTES4
2.3. Film Preparation and Device Fabrication
2.4. Film and Device Characterization
3. Results
3.1. Synthesis
3.2. Dielectric Characterization
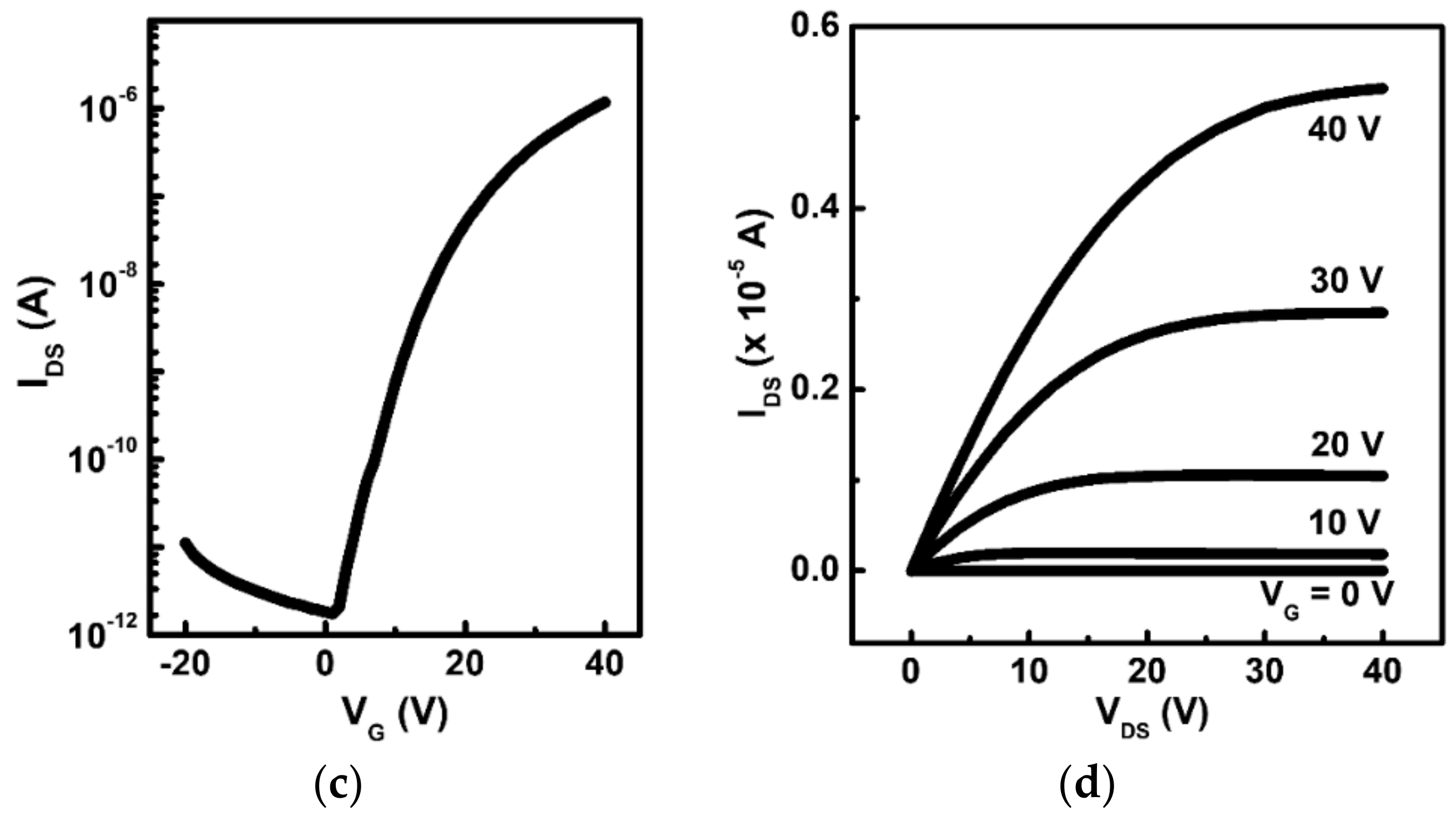
3.3. Thin-Film Transistor Fabrication and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ortiz, R.P.; Facchetti, A.; Marks, T.J. High-k Organic, Inorganic, and Hybrid Dielectrics for Low-Voltage Organic Field-Effect Transistors. Chem. Rev. 2010, 110, 205–239. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Lee, W.H.; Cho, K.W. Recent Advances in Organic Transistor Printing Processes. ACS Appl. Mater. Interfaces 2013, 5, 2302–2315. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.B. The path to ubiquitous and low-cost organic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.T.; Payne, M.M.; Anthony, J.E.; Podzorov, V. Ultra-flexible solution-processed organic field-effect transistors electronic appliances on plastic. Nat. Commun. 2012, 3, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Choi, D.H.; Kwon, G.H.; Zorlu, Y.; Cosut, B.; Kim, H.K.; Facchetti, A.; Kim, C.I.; Usta, H. Solution-Processable BODIPY-Based Small Molecules for Semiconducting Microfibers in Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2016, 8, 14077–14087. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.M.; Atoyo, J.; Carnie, M.J.; Baran, D.; Schroeder, B.C. Review—Organic Materials for Thermoelectric Energy Generation. ECS J. Solid State Sci. Technol. 2017, 6, 3080–3088. [Google Scholar] [CrossRef]
- Pierre, A.; Sadeghi, M.; Payne, M.M.; Facchetti, A.; Anthony, J.E.; Arias, A.C. All-Printed Flexible Organic Transistors Enabled by Surface Tension-Guided Blade Coating. Adv. Mater. 2014, 26, 5722–5727. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, H.M.; Marks, T.J.; Ratner, M.A. Molecular Donor—Bridge—Acceptor Strategies for High-Capacitance Organic Dielectric Materials J. Am. Chem. Soc. 2015, 137, 7189–7196. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, L.B.; Zhou, Y.; Han, S. T; Zhou, L.; Sun, Q.; Zhuang, J.; Peng, H.; Yan, H.; Roy, V.A.L. Surface Decoration on Polymeric Gate Dielectrics for Flexible Organic Field-Effect Transistors via Hydroxylation and Subsequent Monolayer Self-Assembly. ACS Appl. Mater. Interfaces 2015, 7, 23464–23471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, Y.; Liu, Y. Engineering of Amorphous Polymeric Insulators for Organic Field-Effect Transistors. Adv. Electron. Mater. 2017, 3, 1700157. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Reddy, M.R.; Kim, H.S.; Choi, D.H.; Kim, C.I.; Seo, S.Y. Benzothiadiazole-Based Small-Molecule Semiconductors for Organic Thin-Film Transistors and Complementary-like Inverters. ChemPlusChem 2017, 82, 742–749. [Google Scholar] [CrossRef]
- Ozdemir, M.; Choi, D.H.; Zorlu, Y.; Cosut, B.; Kim, H.S.; Kim, C.I.; Usta, H. A new rod-shaped BODIPY-acetylene molecule for solution-processed semiconducting microribbons in n-channel organic field-effect transistors. New J. Chem. 2017, 41, 6232–6240. [Google Scholar] [CrossRef]
- Ozdemir, R.; Choi, D.H.; Ozdemir, M.; Kim, H.K.; Kostakoglu, S.T.; Erkartal, M.; Kim, H.S.; Kim, C.I.; Usta, H. A Solution-Processable Liquid-Crystalline Semiconductor for Low-Temperature-Annealed Air-Stable N-Channel Field-Effect Transistors. ChemPhysChem 2017, 18, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Vegiraju, S.; He, G.Y.; Kim, C.I.; Priyanka, P.; Chiu, Y.J.; Liu, C.W.; Huang, C.Y.; Ni, J.S.; Wu, Y.W.; Chen, Z.; et al. Solution-Processable Dithienothiophenoquinoid (DTTQ) Structures for Ambient-Stable n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2017, 27, 1606761. [Google Scholar] [CrossRef]
- Ozdemir, M.; Kim, S.W.; Kim, H.S.; Kim, M.G.; Kim, B.J.; Kim, C.I.; Usta, H. Semiconducting Copolymers Based on meso-Substituted BODIPY for Inverted Organic Solar Cells and Field-Effect Transistors. Adv. Electron. Mater. 2017, 1700354. [Google Scholar] [CrossRef]
- Ho, D.I.; Jeon, M.S.; Kim, H.K.; Gidron, O.; Kim, C.I.; Seo, S.Y. Solution-processable dithieno [3,2-b:2′,3′-d] thiophene derivatives for organic thin-film transistors and complementary-like inverters. Org. Electron. 2018, 52, 356–363. [Google Scholar] [CrossRef]
- Reddy, M.R.; Kim, H.S.; Kim, C.I.; Seo, S.Y. 2-Thiopene [1] benzothieno [3,2-b] benzothiophene derivatives as solution processable organic semiconductors for organic thin-film transistors. Synth. Met. 2018, 235, 153–159. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, X. Flexible and Stretchable Devices. Adv. Mater. 2016, 18, 4177–4179. [Google Scholar] [CrossRef] [PubMed]
- Nela, L.; Tang, J.; Cao, Q.; Tulevski, G.; Han, S.J. Large-Area High-Performance Flexible Pressure Sensor with Carbon Nanotube Active Matrix for Electronic Skin. Nano Lett. 2018, 18, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Pochorovski, I.; Bao, Z. Separation of Semiconducting Carbon Nanotubes for Flexible and Stretchable Electronics Using Polymer Removable Method. Acc. Chem. Res. 2017, 50, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Joe, D.J.; Kim, S.J.; Park, J.H.; Park, D.Y.; Lee, H.E.; Im, T.H.; Choi, I.S.; Ruoff, R.S.; Lee, K.J. Laser-Material Interactions for Flexible Applications. Adv. Mater. 2017, 29, 1606586. [Google Scholar] [CrossRef] [PubMed]
- Myny, K. The development of flexible integrated circuits based on thin-film transistors. Nat. Electron. 2018, 1, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.J.; Sonar, P.; Dodabalapur, A. Improved Performance in Diketo pyrrolopyrrole-Based Transistors with Bilayer Gate Dielectrics. ACS Appl. Mater. Interfaces 2014, 6, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Tardy, J.; Erouel, M.; Deman, A.L.; Gagnaire, A.; Teodorescu, V.; Blanchin, M.G.; Canut, B.; Barau, A.; Zaharescu, M. Organic thin film transistors with HfO2 high-k gate dielectric grown by anodic oxidation or deposited by sol-gel. Microelectron. Reliab. 2007, 47, 372–377. [Google Scholar] [CrossRef]
- Li, S.X.; Feng, L.R.; Guo, X.J.; Zhang, Q. Application of thermal azide-alkyne cycloaddition(TAAC) reaction as a low temperature cross-linking method in polymer gate dielectrics for organic field-effect transistors. J. Mater. Chem. C 2014, 2, 3517–3520. [Google Scholar] [CrossRef]
- Puigdollers, J.; Voz, C.; Orpella, A.; Quidant, R.; Martin, I.; Vetter, M.; Alcubilla, R. Pentacene thin-film transistors with polymeric gate dielectric. Org. Electron. 2004, 5, 67–71. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.; Chen, S.C.; Dai, S.; Huang, J.; Zheng, Q. Binary polymer composite dielectrics for flexible low-voltage organic field effect transistors. Org. Electron. 2018, 53, 205–212. [Google Scholar] [CrossRef]
- Noh, Y.Y.; Sirringhaus, H. Ultra-thin polymer gate dielectrics for top-gate polymer field-effect transistors. Org. Electron. 2009, 10, 174–180. [Google Scholar] [CrossRef]
- Cheng, X.; Caironi, M.; Noh, Y.Y.; Wang, J.; Newman, C.; Yan, H.; Facchetti, A.; Sirringhaus, H. Air Stable Cross-Linked Cytop Ultrathin Gate Dielectric for High Yield Low-Voltage Top-Gate Organic Field-Effect Transistors. Chem. Mater. 2010, 22, 1559–1566. [Google Scholar] [CrossRef]
- Ha, Y.G.; Jeong, S.H.; Wu, J.S.; Kim, M.G.; Dravid, V.P.; Facchetti, A.; Marks, T.J. Flexible Low-Voltage Organic Thin-Film Transistors Enabled by Low-Temperature, Ambient Solution-Processable Inorganic/Organic Hybrid Gate Dielectrics. J. Am. Chem. Soc. 2010, 132, 17426–17434. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, K.P.; Jang, M.; Jang, J.Y.; Anthony, J.E.; Yang, H.; Park, C.E. Photo-Curable Polymer Blend Dielectrics for Advancing Organic Field-Effect Transistor Applications. Adv. Mater. 2010, 22, 4809–4813. [Google Scholar] [CrossRef] [PubMed]
- Vidor, F.F.; Meyers, T.; Hilleringmann, U. Inverter Circuits Using ZnO Nanoparticle Based Thin-Film Transistors for Flexible Electronic Applications. Nanomaterials 2016, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Q. The dielectric properties of low temperature thermally cross-linked polystyrene and poly(methyl methacrylate) thin films. RSC Adv. 2015, 5, 28980. [Google Scholar] [CrossRef]
- Li, S.; Feng, L.; Zhao, J.; Guo, X.; Zhang, Q. Low temperature cross-linked, high performance polymer gate dielectrics for solution-processed organic field-effect transistors. Polym. Chem. 2015, 6, 5884–5890. [Google Scholar] [CrossRef]
- Xu, W.; Guo, C.; Rhee, S.W. High performance organic field-effect transistors using cyanoethyl pullulan (CEP) high-k polymer cross-linked with trimethylolpropane triglycidyl ether (TTE) at low temperatures. J. Mater. Chem. C 2013, 1, 3955–3960. [Google Scholar] [CrossRef]
- Yoon, M.H.; Yan, H.; Facchetti, A.; Marks, T.J. Low-Voltage Organic Field-Effect Transistors and Inverters Enabled by Ultrathin Cross-Linked Polymers as Gate Dielectrics. J. Am. Chem. Soc. 2005, 127, 10388–10395. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, W.; Zhang, W.; Guo, X.; Zhang, Q. Cross-linked Polymer-Blend Gate Dielectrics through Thermal Click Chemistry. Chem. Eur. J. 2015, 21, 17762–17768. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Cosseddu, P.; Zucca, A.; Loi, A.; Bonfiglio, A. Combining inkjet printing and chemical vapor deposition for fabricating low voltage, organic field-effect transistors on flexible substrates. Thin Solid Films 2017, 631, 124–131. [Google Scholar] [CrossRef]
- Leppaniemi, J.; Huttunen, O.H.; Majumdar, H.; Alastalo, A. Flexography-Printed In2O3 Semiconductor Layers for High Mobility Thin-Film Transistors on Flexible Plastic Substrate. Adv. Mater. 2015, 27, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Vidor, F.F.; Wirth, G.I.; Hilleringmann, U. Low temperature fabrication of a ZnO nanoparticle thin-film transistor suitable for flexible electronics. Microelectron. Reliab. 2014, 54, 2760–2765. [Google Scholar] [CrossRef]
- Jung, S.Y.; Albariqi, M.; Gruntz, G.; Al-Hathal, T.; Peinado, A.; Garcia-Caurel, E.; Nicolas, Y.; Toupance, T.; Bonnassieux, Y.; Horowitz, G. A TIPS-TPDO-tetraCN-Based n-Type Organic Field-Effect Transistor with a Cross-linked PMMA Polymer Gate Dielectric. ACS Appl. Mater. Interfaces 2016, 8, 14701–14708. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.G.; Emery, J.D.; Bedzyk, M.J.; Usta, H.; Facchetti, A.; Marks, T.J. Solution-Deposited Organic-Inorganic Hybrid Multilayer Gate Dielectrics. Design, Synthesis, Microstructures, and Electrical Properties with Thin-Film Transistors. J. Am. Chem. Soc. 2011, 133, 10239–10250. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Carlo, G.D.; Turrisi, R.; Zeng, L.; Stallings, K.; Huang, W.; Bedzyk, M.L.; Beverina, L.; Marks, T.J.; Facchetti, A. The Dipole Moment Inversion Effects in Self-Assembled Nanodielectrics for Organic Transistors. Chem. Mater. 2017, 29, 9974–9980. [Google Scholar] [CrossRef]
- Kang, W.G.; An, G.I.; Kim, M.J.; Lee, W.H.; Lee, D.Y.; Kim, H.J.; Cho, J.H. Ladder-Type Silsesquioxane Copolymer Gate Dielectrics for High-Performance Organic Transistors and Inverters. J. Phys. Chem. C 2016, 120, 3501–3508. [Google Scholar] [CrossRef]
- Kim, Y.T.; Roh, J.K.; Kim, J.H.; Kang, C.M.; Kang, I.N.; Jung, B.J.; Lee, C.H.; Hwang, D.H. Photocurable propyl-cinnamate-functionalized polyhedral oligomeric silsesquioxane as a gate dielectric for organic thin film transistors. Org. Electron. 2013, 14, 2315–2323. [Google Scholar] [CrossRef]
- Lee, D.H.; Jeong, H.D. Solution-Processed Gate Insulator of Ethylene-Bridged Silsesquioxnae for Organic Field-Effect Transistor. J. Chosun Nat. Sci. 2010, 3, 7–18. [Google Scholar]
- Hamada, T.; Nagase, T.; Kobayashi, T.; Matsukawa, K.; Naito, H. Effective control of surface property on poly(silsesquioxane) films by chemical modification. Thin Solid Films 2008, 517, 1335–1339. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Lee, H.S.; Jeon, H.Y.; Baek, K.Y.; Choi, D.H.; Hwang, S.S. Synthesis and Characterization of UV-Curable Ladder-Like Polysilsesquioxane. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5012–5018. [Google Scholar] [CrossRef]
- Park, E.S.; Ro, H.W.; Nguyen, C.V.; Jaffe, R.L.; Yoon, D.Y. Infrared Spectroscopy Study of Microstructures of Poly-(silsesquioxane)s. Chem. Mater. 2008, 20, 1548–1554. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S. A Novel Photocrosslinkable Polyhedral Oligomeric Silsesquioxane and Its Nanocomposites with Poly(vinyl cinnamate). Chem. Mater. 2004, 16, 5141–5148. [Google Scholar] [CrossRef]




| Copolymer | Thickness (nm) | J (A·cm−2) at 1 MV·cm−1 | Ci (nF·cm−2) | k |
|---|---|---|---|---|
| MTMS-BTES2 | 140 | 8.11 × 10−8 | 23.0 | 3.60 |
| 100 | 1.24 × 10−8 | 30.8 | 3.47 | |
| 80 | 1.38 × 10−7 | 33.6 | 3.33 | |
| MTMS-BTES3 | 135 | 3.91 × 10−8 | 23.3 | 3.50 |
| 102 | 1.41 × 10−8 | 29.5 | 3.33 | |
| 90 | 1.20 × 10−8 | 32.2 | 3.27 | |
| MTMS-BTES4 | 150 | 5.27 × 10−8 | 20.5 | 3.47 |
| 113 | 6.55 × 10−8 | 29.1 | 3.31 | |
| 100 | 1.83 × 10−8 | 29.4 | 3.32 |
| Type | Semi-Conductor | µsat 1(cm2·V−1·s−1) | Ion/Ioff | VT (V) |
|---|---|---|---|---|
| p-type | P5 | 0.15 | 2.24 × 105 | −10.5 |
| n-type | PTCDI-C13 | 0.09 | 3.00 × 106 | 9.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, C. A Ladder-Type Organosilicate Copolymer Gate Dielectric Materials for Organic Thin-Film Transistors. Coatings 2018, 8, 236. https://doi.org/10.3390/coatings8070236
Kim D, Kim C. A Ladder-Type Organosilicate Copolymer Gate Dielectric Materials for Organic Thin-Film Transistors. Coatings. 2018; 8(7):236. https://doi.org/10.3390/coatings8070236
Chicago/Turabian StyleKim, Dongkyu, and Choongik Kim. 2018. "A Ladder-Type Organosilicate Copolymer Gate Dielectric Materials for Organic Thin-Film Transistors" Coatings 8, no. 7: 236. https://doi.org/10.3390/coatings8070236




