Significantly Reduced Secondary-Electron-Yield of Aluminum Sheet with Fluorocarbon Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Fluorocarbon (FC) Coatings
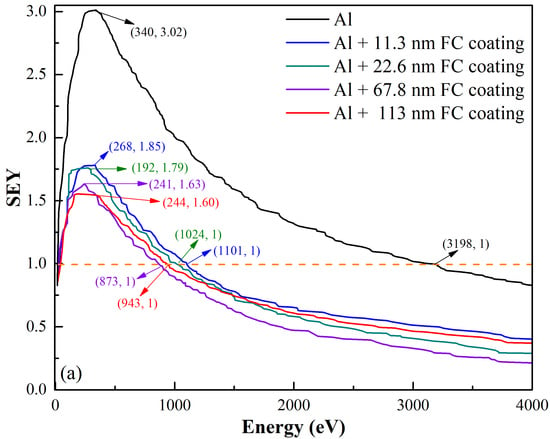
3.2. Secondary Electron Yield (SEY) Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasch, J.; Anderson, D.; Semenov, V.E. Multipactor breakdown in microwave pulses. J. Phys. D Appl. Phys. 2013, 46, 505201. [Google Scholar] [CrossRef] [Green Version]
- González-Iglesias, D.; Pérez, A.M.; Monerris, O. Recent advances of the multipactor RF breakdown in RF satellite microwave passive devices. In Proceedings of the Progress in Electromagnetic Research Symposium (PIERS), Shanghai, China, 8–11 August 2016; pp. 4401–4405. [Google Scholar]
- Woode, A.; Petit, J. Investigations into multipactor breakdown in satellite microwave payloads. ESA J. 1990, 14, 467–478. [Google Scholar]
- Sattler, J.M.; Coutu, R.A.; Lake, R.; Laurvick, T.; Back, T.; Fairchild, S. Modeling micro-porous surfaces for secondary electron emission control to suppress multipactor. J. Appl. Phys. 2017, 122, 055304. [Google Scholar] [CrossRef]
- He, Y.N.; Peng, W.B.; Cui, W.Z.; Ye, M.; Zhao, X.L.; Wang, D.; Hu, T.C.; Wang, R.; Li, Y. Thermal evaporated hyperbranched Ag nanostructure as an effective secondary-electron trapping surface coating. AIP Adv. 2016, 6, 025122. [Google Scholar] [Green Version]
- Zhang, N.; Wang, R.; Li, Y.; Cui, W.Z. Simulation of metal surfaces secondary electron emission in multipactor. In Proceedings of the 2011 International Conference on Electronics, Communications and Control (ICECC), Ningbo, China, 9–11 September 2011; pp. 4520–4522. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Fukuma, H.; Shibata, K.; Tsukuba, J.M.; Pivi, L.; Wang, S. Experimental studies on grooved surfaces to suppress secondary electron emission. In Proceedings of the 1st International Particle Accelerator Conference (IPAC’10), Kyoto, Japan, 23–28 May 2010; pp. 2021–2023. [Google Scholar]
- Fuentes, G.G.; Rodríguez, R.J.; García, M.; Galán, L.; Montero, I.; Segovia, J.L.D. Spectroscopic investigations of Cr, CrN and TiCr anti-multipactor coatings grown by cathodic-arc reactive evaporation. Appl. Surf. Sci. 2007, 253, 7627–7631. [Google Scholar] [CrossRef]
- Pivi, M.; King, F.K.; Kirby, R.E.; Raubenheimer, T.O.; Stupakov, G.; Le Pimpec, F. Sharp reduction of the secondary electron emission yield from grooved surfaces. J. Appl. Phys. 2008, 104, 104904. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; He, Y.N.; Hu, S.G.; Wang, R.; Hu, T.C.; Yang, J.; Cui, W.Z. Suppression of secondary electron yield by micro-porous array structure. J. Appl. Phys. 2013, 113, 074904. [Google Scholar] [CrossRef]
- Ye, M.; He, Y.N.; Hu, S.G.; Yang, J.; Wang, R.; Hu, T.C.; Peng, W.B.; Cui, W.Z. Investigation into anomalous total secondary electron yield for micro-porous Ag surface under oblique incidence conditions. J. Appl. Phys. 2013, 114, 104905. [Google Scholar]
- Cui, W.; Wang, R.; Hu, T.; Yang, J.; He, Y.N. Improvement multipactor discharge of microwave components by micro-porous surface. In Proceedings of the 16th International Symposium on Antenna Technology and Applied Electromagnetics (ANTEM), Victoria, BC, Canada, 13–16 July 2014; pp. 1–2. [Google Scholar] [CrossRef]
- Yang, J.; Cui, W.Z.; Li, Y.; Xie, G.B.; Zhang, N.; Wang, R. Investigation of argon ion sputtering on the secondary electron emission from gold samples. Appl. Surf. Sci. 2016, 382, 88–92. [Google Scholar] [CrossRef]
- Hu, X.-C.; Cao, M.; Cui, W.-Z. Influence of surface topography on the secondary electron yield of clean copper samples. Micron 2016, 90, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Huang, H.J.; Liu, G.Z.; Chen, C.H.; Hou, Q.; Fang, J.Y. The effect of grooved surface on dielectric multipactor. J. Appl. Phys. 2009, 105, 391–392. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, N.; Hu, T.-C.; Wang, F.; Cui, W.-Z. Secondary electron emission from rough metal surfaces: A multi-generation model. J. Phys. D Appl. Phys. 2015, 48, 055501. [Google Scholar] [CrossRef]
- Ruzic, D.; Moore, R.; Manos, D.; Cohen, S. Secondary electron yields of carbon-coated and polished stainless steel. J. Vac. Sci. Technol. 1982, 20, 1313–1316. [Google Scholar] [CrossRef] [Green Version]
- Nistor, V.; González, L.A.; Aguilera, L.; Montero, I.; Galán, L.; Wochner, U. Multipactor suppression by micro-structured gold/silver coatings for space applications. Appl. Surf. Sci. 2014, 315, 445–453. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Wei, Q.; Wu, S.; Hua, X.; Zhang, J. Electron-Induced Secondary Electron Emission Properties of MgO/Au Composite Thin Film Prepared by Magnetron Sputtering. J. Electron. Mater. 2017, 46, 1466–1475. [Google Scholar] [CrossRef]
- Diaz, N.; Casraneda, S.; Ripalda, J.M.; Montero, I.; Galan, L.; Feltham, S.; Rabosa, D.; Rueda, F. Materials of Low Secondary Electron Emission to Prevent the Multipactor Effect in High-Power RF Devices. In Proceedings of the 6th Spacecraft Charging Conference, Hanscom AFB, MA, USA, 2–6 November 1998; pp. 205–209. [Google Scholar]
- Zhang, N.; Kang, Y.; Wang, R.; Cui, W.Z. A new surface treatment for suppressing multipactor in microwave components. In Proceedings of the 2012 International Conference on Microwave and Millimeter Wave Technology (ICMMT), Shenzhen, China, 5–8 May 2012; pp. 1–3. [Google Scholar]
- Wang, J.; Wang, Y.; Xu, Y.H.; Zhang, X.Y.; Zhang, B.; Wei, W. Secondary electron emission characteristics of graphene films with copper substrate. Chin. Phys. C 2016, 40, 180–183. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.-Z.; Wang, H.-G. Simulation investigation of multipactor in metal components for space application with an improved secondary emission model. Phys. Plasmas 2015, 22, 053108. [Google Scholar] [CrossRef]
- Nagesh, S.K.; Revannasiddiah, D.; Shastry, S.V.K. Investigation of multipactor breakdown in communication satellite microwave co-axial systems. Pramana 2005, 64, 95–110. [Google Scholar] [CrossRef]
- Piccinini, F.; Levi, M.; Turri, S. Photoactive sol–gel hybrid coatings from modified fluorocarbon polymers and amorphous titania. Prog. Org. Coat. 2013, 76, 1265–1272. [Google Scholar] [CrossRef]
- Yasuoka, H.; Yoshida, M.; Sugita, K.; Ohdaira, K.; Murata, H.; Matsumura, H. Fabrication of PTFE thin films by dual catalytic chemical vapor deposition method. Thin Solid Films 2008, 516, 687–690. [Google Scholar] [CrossRef]
- Stelmashuk, V.; Biederman, H.; Slavínská, D.; Zemek, J.; Trchová, M. Plasma polymer films RF sputtered from PTFE under various argon pressures. Vacuum 2005, 77, 131–137. [Google Scholar] [CrossRef]
- Jafari, R.; Menini, R.; Farzaneh, M. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar] [CrossRef] [Green Version]
- Nagayama, Y.; Iwamori, S.; Yamada, Y. Mechanical properties of polytetrafluoroethylene (PTFE) thin film sputtered on the metal substrates. Shinku 2003, 46, 827–834. [Google Scholar] [CrossRef]
- Martinelli, E.; Sarvothaman, M.K.; Alderighi, M.; Galli, G.; Mielczarski, E.; Mielczarski, J.A. PDMS network blends of amphiphilic acrylic copolymers with poly(ethylene glycol)-fluoroalkyl side chains for fouling-release coatings. I. Chemistry and stability of the film surface. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2677–2686. [Google Scholar] [CrossRef]
- Fowkes, F.M. Additivity of Intermolecular Forces at Interfaces. I. Determination of the contribution to surface and interfacial tensions of dispersion forces in various liquids. J. Phys. Chem. 1963, 67, 2538–2541. [Google Scholar] [CrossRef]
- Qun, W. Research on the Measurement Device of Secondary Electron Emission Coefficient. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2016. (In Chinese). [Google Scholar]
- Holysz, L.; Chibowski, E. Surface free energy components of alpha-alumina from thin-layer wicking. Langmuir 1992, 8, 717–721. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Y.; Di, J.; Du, W. Morphology and structure of polymer fluorocarbon coatings on polyimide by sputtering. Surf. Coat. Technol. 2007, 201, 5522–5525. [Google Scholar] [CrossRef]
- Alfonso, E.; Olaya, J.; Cubillos, G. Thin Film Growth through Sputtering Technique and Its Applications. In Crystallization Science and Technology; Andreeta, M., Ed.; IntechOpen Limited: London, UK, 2012; pp. 397–432. [Google Scholar] [CrossRef]
- Biederman, H. Organic films prepared by polymer sputtering. J. Vac. Sci. Technol. A 2000, 18, 1642–1648. [Google Scholar] [CrossRef]
- Mathias, E.; Miller, G. The decomposition of polytetrafluoroethylene in a glow discharge. J. Phys. Chem. 1967, 71, 2671–2675. [Google Scholar] [CrossRef]
- Biederman, H.; Stelmashuk, V.; Kholodkov, I.; Choukourov, A.; Slavı́Nská, D. RF sputtering of hydrocarbon polymers and their derivatives. Surf. Coat. Technol. 2003, 174–175, 7–32. [Google Scholar] [CrossRef]
- Shao, J.J.; Ren, Z.; Yang, Y.; Xiao, Y.; Yuan, Y.; Huang, J. Low temperature super-hydrophobicity of magnetron sputtered polytetrafluoroethylene coatings. Chin. J. Vac. Sci. Technol. 2017, 37, 154–160. (In Chinese) [Google Scholar]
- Sessler, G.M. Electrets; Springer: Berlin, Germany, 1987. [Google Scholar]
- Cao, M.; Zhang, X.S.; Liu, W.H.; Wang, H.G.; Li, Y.D. Secondary electron emission of graphene-coated copper. Diam. Relat. Mater. 2017, 73, 199–203. [Google Scholar] [CrossRef]
- Cazaux, J. Calculated influence of work function on SE escape probability and secondary electron emission yield. Appl. Surf. Sci. 2012, 257, 1002–1009. [Google Scholar] [CrossRef]
- Babich, A.V.; Pogosov, V.V. Electron work function and the surface tension of a metallic surface with an insulating coating. Phys. Met. Metallogr. 2008, 106, 332–340. [Google Scholar] [CrossRef]
- Abraham, F.; Ford, W.E.; Scholz, F.; Nelles, G.; Sandford, G.; von Wrochem, F. Surface Energy and Work Function Control of AlOx/Al Surfaces by Fluorinated Benzylphosphonic Acids. ACS Appl. Mater. Interfaces 2016, 8, 11857–11867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Waele, S.; Lejaeghere, K.; Sluydts, M.; Cottenier, S. Error estimates for density-functional theory predictions of surface energy and work function. Phys. Rev. B 2016, 94, 235418. [Google Scholar] [CrossRef]
- Cheng, S.; Tan, C.M.; Deng, T.; He, F.; Zhang, S.; Su, H. Investigation of work function and surface energy of aluminum: An ab-initio study. In Proceedings of the 2013 IEEE 5th International Nanoelectronics Conference (INEC), Sentosa, Singapore, 2–4 January 2013; pp. 473–475. [Google Scholar] [CrossRef]
- Heiler, H. Secondary electron emission in the scanning electron microscope. J. Appl. Phys. 1983, 54, R1–R18. [Google Scholar] [CrossRef]
- Montero, I.; Aguilera, L.; Dávila, M.E.; Nistor, V.C.; González, L.A.; Galán, L. Secondary electron emission under electron bombardment from graphene nanoplatelets. Appl. Surf. Sci. 2014, 291, 74–77. [Google Scholar] [CrossRef] [Green Version]






| Coating Thickness (nm) | WCA (°) | (mN/m) | (mN/m) | / | (mN/m) | Ra (nm) |
|---|---|---|---|---|---|---|
| 0 | 101.6 | 5.6 | 37.3765 | 6674 | 37.3821 | 3.3 |
| 11.3 | 110.5 | 330.3 | 17.8590 | 54 | 18.1893 | 11.8 |
| 22.6 | 112.1 | 251.4 | 16.7772 | 66 | 17.0286 | 16.7 |
| 67.8 | 116.2 | 52.4 | 16.0315 | 305 | 16.0839 | 35.2 |
| 113 | 120.9 | 7.8 | 13.3788 | 1715 | 13.3866 | 48.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhao, Q.; Li, J.; Wang, K.; Huang, Z.; Cui, W. Significantly Reduced Secondary-Electron-Yield of Aluminum Sheet with Fluorocarbon Coating. Coatings 2018, 8, 249. https://doi.org/10.3390/coatings8070249
Wang F, Zhao Q, Li J, Wang K, Huang Z, Cui W. Significantly Reduced Secondary-Electron-Yield of Aluminum Sheet with Fluorocarbon Coating. Coatings. 2018; 8(7):249. https://doi.org/10.3390/coatings8070249
Chicago/Turabian StyleWang, Feipeng, Qi Zhao, Jian Li, Kaizheng Wang, Zhengyong Huang, and Wanzhao Cui. 2018. "Significantly Reduced Secondary-Electron-Yield of Aluminum Sheet with Fluorocarbon Coating" Coatings 8, no. 7: 249. https://doi.org/10.3390/coatings8070249