Phase Composition, Thermal Conductivity, and Toughness of TiO2-Doped, Er2O3-Stabilized ZrO2 for Thermal Barrier Coating Applications
Abstract
:1. Introduction
2. Experimental Procedures
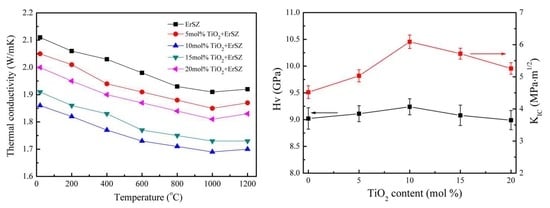
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Vaßen, R.; Stöver, D. Atmospheric plasma sprayed thick thermal barrier coatings with high segmentation crack density. Surf. Coat. Technol. 2004, 186, 353–363. [Google Scholar] [CrossRef]
- Vassen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C. Hot corrosion behavior of nanostructured Gd2O3 doped YSZ thermal barrier coating in presence of Na2SO4 + V2O5 molten salts. Prog. Nat. Sci. Mater. 2017, 27, 507–513. [Google Scholar] [CrossRef]
- Msuer, G.; Du, L.; Vassen, R. Atmospheric plasma spraying of single phase lanthanum zirconate thermal barrier coatings with optimized porosity. Coatings 2016, 6, 49. [Google Scholar]
- Zhang, W.; Li, G.; Zhang, Q.; Yang, G. Comprehensive damage evaluation of localized spallation of thermal barrier coatings. J. Adv. Ceram. 2017, 6, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Wei, L.; Guo, H.; Xu, H. Deposition mechanisms of yttria-stabilized zirconia coatings during plasma spray physical vapor deposition. Ceram. Int. 2016, 42, 5530–5536. [Google Scholar] [CrossRef]
- Tsipas, S.A. Effect of dopants on the phase stability of zirconia-based plasma sprayed thermal barrier coatings. J. Eur. Ceram. Soc. 2010, 30, 61–72. [Google Scholar] [CrossRef]
- Renteria, A.F.; Saruhan, B. Effect of ageing on microstructure changes in EB-PVD manufactured standard PYSZ top coat of thermal barrier coatings. J. Eur. Ceram. Soc. 2006, 26, 2249–2255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Microstructure and thermal properties of nanostructured gadolinia doped yttria-stabilized zirconia thermal barrier coatings produced by air plasma spraying. Ceram. Int. 2016, 42, 13047–13052. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Javed, A.; Zhu, C.; Liang, G. The effect of sintering temperature on the microstructure and phase transformation in tetragonal YSZ and LZ/YSZ composites. Ceram. Int. 2016, 42, 2456–2465. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Zhang, C.; Huang, X.; Ye, F. Dy2O3 Stabilized ZrO2 as a toughening agent for Gd2Zr2O7 ceramic. Mater. Lett. 2017, 188, 142–144. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Zhang, Y.; Ye, F. Improved toughness and thermal expansion of non-stoichiometry Gd2−xZr2+xO7+x/2 ceramics for thermal barrier coating application. J. Mater. Sci. Technol. 2016, 32, 28–33. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Cheng, Y.; Zhang, C.; He, S.; Zhang, Y.; Ye, F. Plasma sprayed nanostructured GdPO4 thermal barrier coatings: Preparation microstructure and CMAS corrosion resistance. J. Am. Ceram. Soc. 2017, 100, 4209–4218. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Gong, S. Thermal shock resistance and mechanical properties of La2Ce2O7 thermal barrier coatings with segmented structure. Ceram. Int. 2009, 35, 2639–2644. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, H.; Zhao, Y.; Li, G. Ce1−xSmxO2−x/2—A novel type of ceramic material for thermal barrier coatings. J. Adv. Ceram. 2016, 5, 244–252. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Xiang, H.; Feng, Z. Preparation, mechanical, and thermal properties of a promising thermal barrier material: Y4Al2O9. J. Adv. Ceram. 2015, 4, 94–102. [Google Scholar] [CrossRef]
- Rebollo, N.R.; Fabrichnaya, O.; Levi, C.G. Phase stability of Y + Gd co-doped zirconia. Zeitschrift für Metallkunde 2003, 94, 163–170. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Gross, J.R.; Dutton, R.E.; Wang, H. Phase stability, sintering, and thermal conductivity of plasma-sprayed ZrO2-Gd2O3 compositions for potential thermal barrier coating applications. Acta Mater. 2006, 54, 1615–1621. [Google Scholar] [CrossRef]
- Feng, J.; Ren, X.; Wang, X.; Zhou, R.; Pan, W. Thermal conductivity of ytterbia-stabilized zirconia. Scr. Mater. 2012, 66, 41–44. [Google Scholar] [CrossRef]
- Sun, L.; Guo, H.; Peng, H.; Gong, S.; Xu, H. Influence of partial substitution of Sc2O3 with Gd2O3 on the phase stability and thermal conductivity of Sc2O3-doped ZrO2. Ceram. Int. 2013, 39, 3447–3451. [Google Scholar] [CrossRef]
- Cairney, J.M.; Rebollo, N.R.; Rühle, M.; Levi, C.G. Phase stability of thermal barrier oxides: A comparative study of Y and Yb additions. Int. J. Mater. Res. 2007, 98, 1177–1187. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Zhao, X.; Wang, C.; Ye, F. Toughening effect of Yb2O3 stabilized ZrO2 doped in Gd2Zr2O7 ceramic for thermal barrier coatings. Mater. Sci. Eng. A Struct. 2015, 648, 385–391. [Google Scholar] [CrossRef]
- Schaedler, T.A.; Leckie, R.M.; Krämer, S.; Evans, A.G.; Levi, C.G. Toughening of nontransformable t′-YSZ by addition of titania. J. Am. Ceram. Soc. 2007, 90, 3896–3901. [Google Scholar] [CrossRef]
- Chen, T.D.; Tekeli, S.; Dillona, R.P.; Mecartney, M.L. Phase stability, microstructural evolution and room temperature mechanical properties of TiO2 doped 8 mol % Y2O3 stabilized ZrO2 (8Y-CSZ). Ceram. Int. 2008, 34, 365–370. [Google Scholar] [CrossRef]
- Zhao, M.; Pan, W. Effect of lattice defects on thermal conductivity of Ti-doped, Y2O3-stabilized ZrO2. Acta Mater. 2013, 61, 5496–5503. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.; Xu, L.; Li, M.; Wang, Q.; Ye, F.; Dan, C.; Ji, V. Effects of TiO2 doping on the defect chemistry and thermo-physical properties of Yb2O3 stabilized ZrO2. J. Eur. Ceram. Soc. 2017, 37, 4163–4169. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Alcock, C.B.; Spencer, P.J. Materials Thermochemistry, 6th ed.; Pergamon Press: Oxford, UK, 1993; pp. 254–326. [Google Scholar]
- Wu, J.; Wei, X.Z.; Padture, N.P.; Klemens, P.G.; Gell, M.; Garcia, E.; Miranzo, P.; Osendi, M.I. Low-thermal-conductivity rare-earth zirconates for potential thermal-barrier-coating applications. J. Am. Ceram. Soc. 2002, 85, 3031–3035. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture toughness determinations by indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Berman, R. Thermal Conduction in Solids; Clarendon Press: Oxford, UK, 1976; pp. 1–101. [Google Scholar]
- Lehmann, H.; Pitzer, D.; Pracht, G.; Vassen, R.; Stover, D. Thermal conductivity and thermal expansion coefficients of the lanthanum rare-earth-element zirconate system. J. Am. Ceram. Soc. 2003, 86, 1338–1344. [Google Scholar] [CrossRef]
- Wan, C.; Qu, Z.; Du, A.; Pan, W. Influence of B site substituent Ti on the structure and thermophysical properties of A2B2O7-type pyrochlore Gd2Zr2O7. Acta Mater. 2009, 57, 4782–4789. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, F.; Xiao, P. Role and determining factor of substitutional defects on thermal conductivity: A study of La2(Zr1−xBx)2O7 (B = Hf, Ce, 0 ≤ x ≤ 0.5) pyrochlore solid solutions. Acta Mater. 2014, 68, 106–115. [Google Scholar] [CrossRef]
- Schulz, U. Phase transformation in EB-PVD yttria partially stabilized zirconia thermal barrier coatings during annealing. J. Am. Ceram. Soc. 2000, 83, 904–910. [Google Scholar] [CrossRef]
- Curry, N.; VanEvery, K.; Snyder, T.; Markoscan, N. Thermal conductivity analysis and lifetime testing of suspension plasma-sprayed thermal barrier coatings. Coatings 2014, 4, 630–650. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, X.; Pan, W. Effect of lattice distortion and disordering on the mechanical properties of titania-doped yttria-stabilized zirconia. J. Am. Ceram. Soc. 2014, 97, 1566–1571. [Google Scholar] [CrossRef]
- Mercer, C.; Williams, J.R.; Clarke, D.R.; Evans, A.G. On a ferroelastic mechanism governing the toughness of metastable tetragonal-prime (t′) yttria-stabilized zirconia. Proc. R. Soc. A 2007, 463, 1393–1408. [Google Scholar] [CrossRef]
- Evans, A.G. Perspective on the development of high-toughness ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]






| TiO2 Content (mol %) | c | t1′ + t2′ | m |
|---|---|---|---|
| 10 | 7.8 | 88.7 | 3.5 |
| 15 | 0 | 91.8 | 8.2 |
| 20 | 0 | 87.6 | 12.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Guo, L.; Yan, Z.; Ye, F. Phase Composition, Thermal Conductivity, and Toughness of TiO2-Doped, Er2O3-Stabilized ZrO2 for Thermal Barrier Coating Applications. Coatings 2018, 8, 253. https://doi.org/10.3390/coatings8070253
Wang Q, Guo L, Yan Z, Ye F. Phase Composition, Thermal Conductivity, and Toughness of TiO2-Doped, Er2O3-Stabilized ZrO2 for Thermal Barrier Coating Applications. Coatings. 2018; 8(7):253. https://doi.org/10.3390/coatings8070253
Chicago/Turabian StyleWang, Qi, Lei Guo, Zheng Yan, and Fuxing Ye. 2018. "Phase Composition, Thermal Conductivity, and Toughness of TiO2-Doped, Er2O3-Stabilized ZrO2 for Thermal Barrier Coating Applications" Coatings 8, no. 7: 253. https://doi.org/10.3390/coatings8070253