High-Temperature Oxidation Resistance of NiAl Intermetallic Formed In Situ by Thermal Spraying
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Specimen
2.2. Test Analysis
3. Results
3.1. Analysis of the Phase Change Process of Coating after Heat Treatment
3.1.1. The Phase Change Process of the Al/Ni Comparative Specimen
3.1.2. The Phase Change Process of Al/Ni Composite Coating on the Surface of Ti Substrate
3.2. Effect of Heating Temperature and Time on the Formation of the Phase and the Depth of Phase Change Reaction
3.2.1. Effect of Heating Time on the Formation of Phase
3.2.2. The Effect of Heating Time on the Depth of Phase Change Reaction
3.2.3. Effect of Heating Temperature on the Depth of Phase Change Reaction
3.3. Analysis of the Relevant Theories of In-Situ Formation of NiAl Intermetallic Compounds
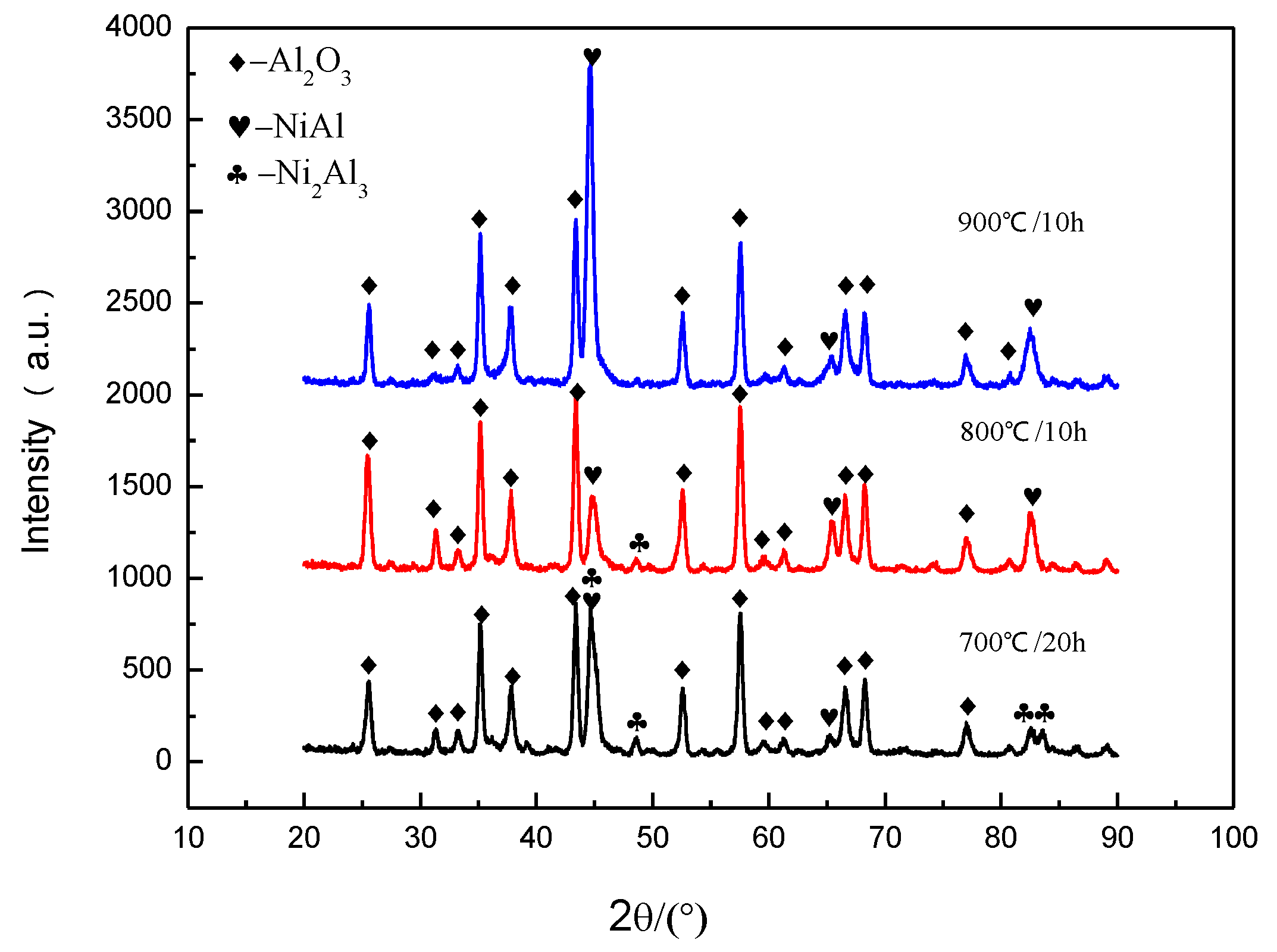
3.4. Study of Oxidation Resistance
4. Conclusions
- After heating at 973 K, metallurgical reactions took place between the Al/Ni composite coating. The NiAl3, Ni2Al3, and NiAl intermetallics were formed in sequence in the diffusion region, which advanced to the Ti substrate. However, NiAl3 and Ni2Al3 were all or partly dissolved in liquid Al, while only the high melting point NiAl intermetallic could exist stably, and a region (composed of the NiAl phase) that had a certain thickness was finally formed.
- The thickness of the NiAl phase increased as the reaction time prolonged. The thickness of the NiAl phase was 20 µm after heating at 973 K for 2 h and the thickness of NiAl phase was 130 µm when the heating time was extended to 20 h.
- The higher the temperature, the easier the diffusion from the Al to the Ni coating; the 150 μm-thick Ni coating was penetrated by elemental Al after about 20 h at 973 K, whereas the same thickness of Ni coating was penetrated by elemental Al after only 10 h at 1073 K. When the reaction temperature was raised to 1173 K, the elemental Al not only penetrated the 150 μm-thick Ni coating, but also diffused into the Ti substrate after 10 h.
- After the heat treatment, a ragged wave-like morphology was exhibited in the diffusion front of Al and a small amount of elemental Ni in the diffusion region did not participate in the reaction. The growth of the NiAl intermetallic in the diffusion region of the Al/Ni/Ti specimen was obviously slower compared with the Al/Ni specimen. Furthermore, when the Al coating on the surface of the specimen was thinner, all the elemental Al diffused into the Ni coating in a short time and the thickness of diffusion reaction layer did not increase with the increasing reaction time after the Al coating was depleted. However, the elemental Al could not only penetrate the Ni coating, but also reached the surface of the Ti substrate when the Al coating was thick enough. TiAl intermetallics which were rich in Al were produced during the further reaction between elemental Al and Ti.
- The specimen after heat treatment had a better high-temperature oxidation resistance than the pure Ti substrate without coating, as a certain thickness of NiAl intermetallic phase was produced.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riedl, H.; Koller, C.M.; Munnik, F.; Hutter, H.; Mendez Martin, F.; Rachbauer, R.; Kolozsvári, S.; Bartosik, M.; Mayrhofer, P.H. Influence of oxygen impurities on growth morphology, structure and mechanical properties of Ti-Al-N thin films. Thin Solid Films 2016, 603, 39–49. [Google Scholar] [CrossRef]
- Cui, H.Z.; Ma, L.; Cao, L.L.; Teng, F.L.; Cui, N. Effect of NiAl content on phases and microstructures of TiC-TiB2-NiAl composites fabricated by reaction synthesis. Trans. Nonferr. Met. Soc. China 2014, 24, 346–353. [Google Scholar] [CrossRef]
- Sina, H.; Surreddi, K.B.; Iyengar, S. Phase evolution during the reactive sintering of ternary Al-Ni-Ti powder compacts. J. Alloy Compd. 2016, 661, 294–305. [Google Scholar] [CrossRef]
- Vera, M.L.; Colaccio, Á.; Rosenberger, M.R.; Schvezov, C.E.; Ares, A.E. Influence of the Electrolyte Concentration on the Smooth TiO2 Anodic Coatings on Ti-6Al-4V. Coatings 2017, 7, 39. [Google Scholar] [CrossRef]
- O’Sullivan, C.; O’Hare, P.; Byrne, G.; O’Neill, L.; Ryan, K.B.; Crean, A.M. A Modified Surface on Titanium Deposited by a Blasting Process. Coatings 2011, 1, 53–71. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.R.; Bao, Z.B.; Shen, M.L.; Zhu, S.L.; Wang, F.H. A magnetron sputtered microcrystalline beta-NiAl coating for SC superalloys. Part II. Effects of a NiCrO diffusion barrier on oxidation behavior at 1100 degrees C. Appl. Surf. Sci. 2017, 407, 485–494. [Google Scholar] [CrossRef]
- Pettit, F.S. Oxidation mechanisms for nickel-aluminum alloys at temperatures between 900° and 1300 °C. Trans. Metall. Soc. AIME 1967, 239, 1296–1305. [Google Scholar]
- Meng, X.X.; Pei, Y.W.; Shao, W.; Qu, W.T.; Zhou, C.G. Cyclic oxidation behaviour of Co/Si co-doped beta-NiAl coating on nickel based superalloys. Corros. Sci. 2018, 133, 112–119. [Google Scholar] [CrossRef]
- Sato, N.; Fuji, M.; Kohiruimaki, N.; Fukumoto, M.; Hara, M. Preparation of Ni aluminide/Ni bilayer coating on Nb-W alloys by molten salt electrodeposition and oxidation resistance. Oxid. Met. 2016, 1, 29–38. [Google Scholar] [CrossRef]
- Wang, H.X.; Zhang, Y.; Cheng, J.L.; Li, Y.S. High temperature oxidation resistance and microstructure change of aluminized coating on copper substrate. Trans. Nonferr. Metal. Soc. China 2015, 25, 184–190. [Google Scholar] [CrossRef]
- Gong, X.; Chen, R.R.; Wang, Q.; Wang, Y.; Zhang, N.N.; Zhang, Z.L.; Fu, H.Z. Cyclic oxidation behavior and oxide scale adhesion of Al/NiCrAlY coating on pure titanium alloy. J. Alloy Compd. 2017, 729, 679–687. [Google Scholar] [CrossRef]
- Mitoraj-Krolikowska, M.; Godlewska, E. Silicide coatings on Ti-6Al-1Mn (at.%) alloy and their oxidation resistance. Surf. Coat. Technol. 2018, 334, 491–499. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.M.; Shen, Y.F. Oxidation behavior of a high Si content Al-Si composite coating fabricated on Ti–6Al–4V substrate by mechanical alloying method. J. Alloy Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Parlikar, C.; Alam, M.Z.; Sarkar, R.; Das, D.K. Effect of oxidation resistant Al3Ti coating on tensile properties of a near α-Ti alloy. Surf. Coat. Technol. 2013, 236, 107–117. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, F.Y.; Wang, A.M.; Yu, H.J.; Chen, C.Z. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Izumi, T.; Nishimoto, T.; Narita, T. Formation and oxidation behavior of Ni2Al3 coating on heat-resistant Ti-alloy. Intermetallics 2005, 13, 615–619. [Google Scholar] [CrossRef]
- Abe, T.; Sato, N.; Fukumoto, M.; Hara, M. Preparation of Ni aluminide/Ni or TiAl3 bilayer coating on TiAl by molten salt electrodeposition and cyclic oxidation resistance. J. Jpn. Inst. Met. Mater. 2013, 77, 245–252. [Google Scholar] [CrossRef]
- Sato, T.; Nezu, A.; Watanabe, T. Preparation of Ti-Al intermetallic compound by wire arc spraying. Trans. Mater. Res. Soc. Jpn. 2000, 25, 301–304. [Google Scholar]
- Kiselev, S.P.; Ryashin, N.S. Ti-Al intermetallic compounds synthesis in coatings deposited by cold spraying. Am. Inst. Phys. Conf. Ser. 2016, 1770, 3496–3502. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less-Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.S.; Chen, J.M.; Zhao, J.R.; Yu, Y.J.; Zhou, H. Improvement of the oxidation and wear resistance of pure Ti by laser cladding at elevated temperature. Surf. Coat. Technol. 2010, 205, 2142–2151. [Google Scholar] [CrossRef]
- Song, Y.H.; Park, J.S.; Kim, J.M.; Yi, S.H. Oxidation behaviors of pure Ti thermal plasma spray coated Mo-Si-B alloys. Mater. Sci. Forum 2011, 695, 365–368. [Google Scholar] [CrossRef]
- Kusabiraki, K.; Kuroda, N.; Motohira, I.; Ooka, T. High-Temperature Oxidation of Pure Titanium in Co2 and Ar-10%CO2 Atmospheres. Oxid. Met. 1997, 48, 289–302. [Google Scholar] [CrossRef]
- Biswas, A.; Roy, S.K.; Gurumurthy, K.R.; Prabhu, N.; Banerjee, S. A study of self-propagating high-temperature synthesis of NiAl in thermal explosion mode. Acta Mater. 2002, 50, 757–773. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.G.; Ai, Y.L.; Li, J.L.; Min, J.; Chu, Da.; Zhao, J.; Chen, J. In situ fabrication of α-Al2O3 and Ni2Al3 reinforced aluminum matrix composites in an Al–Ni2O3 system. Adv. Powder Technol. 2011, 22, 629–633. [Google Scholar] [CrossRef]
- Cao, J.; Song, X.G.; Wu, L.Z.; Qi, J.L.; Feng, J.C. Characterization of Al/Ni multilayers and their application in diffusion bonding of TiAl to TiC cermet. Thin Soid Films 2012, 520, 3528–3531. [Google Scholar] [CrossRef]
- Moon, J.H.; Han, J.G.; Kim, Y.J. Performance of an atmospheric plasma torch with various inlet angles. Surf. Coat. Technol. 2005, 193, 94–100. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Izumi, T.; Nishimoto, T.; Narita, T. Superior long-term oxidation resistance of Ni-Al coated TiAl alloys. Intermetallics 2005, 13, 727–732. [Google Scholar] [CrossRef]
- Alimadadi, H.; Kjartansdóttir, C.; Burrows, A.; Kasama, T.; Møller, P. Nickel-aluminum diffusion: A study of evolution of microstructure and phase. Mater. Charact. 2017, 130, 105–112. [Google Scholar] [CrossRef]










| Materials | Voltage/V | Current/A | Distance/mm | Feeding Powder Amount/(L·min−1) |
|---|---|---|---|---|
| Ni | 40 | 500 | 100 | 7 |
| Materials | Voltage/V | Current/A | Atomization Compressed Air Supply Pressure/MPa | Distance/mm |
|---|---|---|---|---|
| Al | 31 | 180 | 0.6 | 150 |
| Elements | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Al | 76.31 | 62.52 | 42.32 | 60.02 | 41.62 | 0 | 61.83 | 42.73 | 59.05 | 41.60 |
| Ni | 23.69 | 37.48 | 57.68 | 39.98 | 58.38 | 100 | 38.17 | 57.27 | 40.95 | 58.40 |
| Specimens | 973 K/2 h | 973 K/5 h | 973 K/10 h | 973 K/20 h |
|---|---|---|---|---|
| Al/Ni | 80 | 110 | 160 | 250 |
| Al/Ni/Ti | 20 | 40 | 80 | 130 |
| Points | Al | Ti |
|---|---|---|
| A | 73.83 | 26.17 |
| B | 65.00 | 35.00 |
| C | 50.39 | 49.61 |
| D | 22.14 | 77.86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; Li, D.; Li, S.; Zhang, Z.; Zhang, N. High-Temperature Oxidation Resistance of NiAl Intermetallic Formed In Situ by Thermal Spraying. Coatings 2018, 8, 292. https://doi.org/10.3390/coatings8080292
Jia Q, Li D, Li S, Zhang Z, Zhang N. High-Temperature Oxidation Resistance of NiAl Intermetallic Formed In Situ by Thermal Spraying. Coatings. 2018; 8(8):292. https://doi.org/10.3390/coatings8080292
Chicago/Turabian StyleJia, Qianqian, Deyuan Li, Shumei Li, Zhuang Zhang, and Nannan Zhang. 2018. "High-Temperature Oxidation Resistance of NiAl Intermetallic Formed In Situ by Thermal Spraying" Coatings 8, no. 8: 292. https://doi.org/10.3390/coatings8080292





