Diesel Exhaust After-Treatment by Silicon Carbide Fiber Filter
Abstract
:1. Introduction

2. Numerical Method
2.1. X-ray CT Technique

2.2. SiC Fiber Filter
2.3. Lattice Boltzmann Method










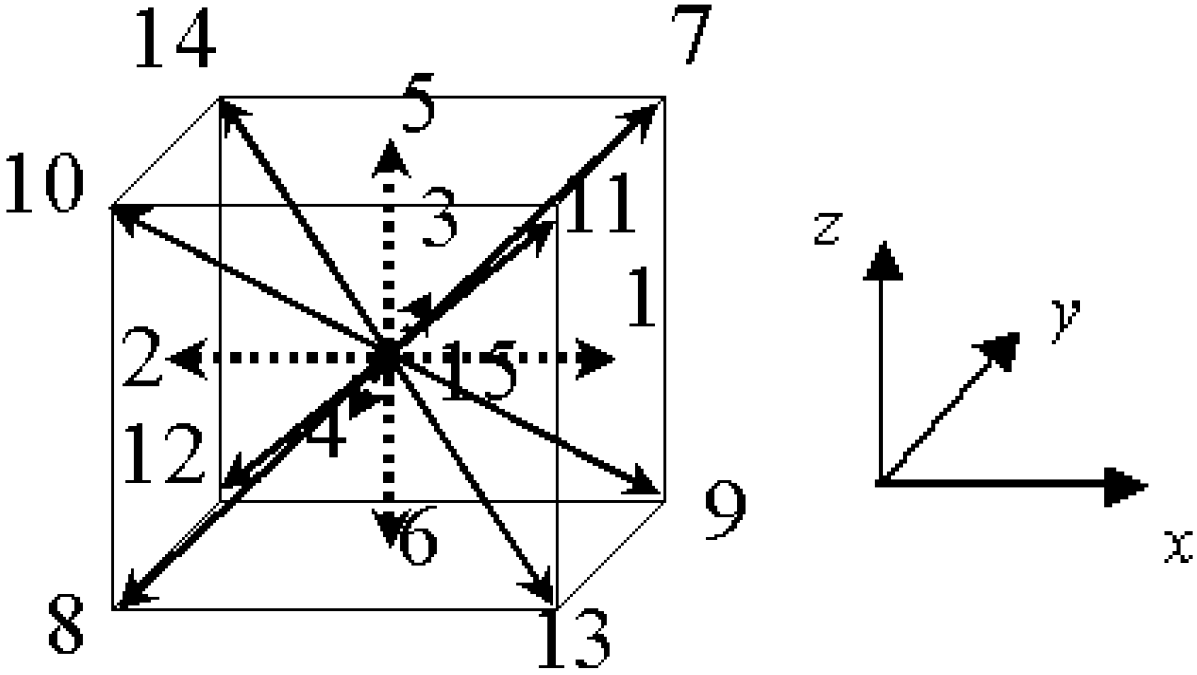
2.4. Calculation Domain and Boundary Conditions

| Case | Inflow velocity (m/s) | Filter temperature (°C) | CO2 (%) | Soot mass fraction |
|---|---|---|---|---|
| 1 | 0.05–2.0 | 400 | 10 | 0 |
| 2 | 1.0 | 700 | 10 | 0.01 |
| 3 | 1.0 | 1200 | 10 | 0.01 |
| 4 | 1.0 | 1400 | 10 | 0.01 |
| 5 | 1.0 | 700 | 20 | 0.01 |
| 6 | 1.0 | 1200 | 20 | 0.01 |
| 7 | 1.0 | 1400 | 20 | 0.01 |
3. Results and Discussion
3.1. Flow Field inside the Filter







3.2. Soot Oxidation




4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Kennedy, I.M. The health effects of combustion-generated aerosols. Proc. Combust. Inst. 2007, 31, 2757–2770. [Google Scholar] [CrossRef]
- Regulation (EC) No. 595/2009 of the European Parliament and the Council. Off. J. Eur. Union 2009, 1–13.
- Johnson, T. Review of diesel emissions and control. SAE Int. J. Fuels Lubr. 2010, 3, 16–29. [Google Scholar] [CrossRef]
- Clerc, J.C. Catalytic diesel exhaust aftertreatment. Appl. Catal. B 1996, 10, 99–115. [Google Scholar] [CrossRef]
- Wirojsakunchai, E.; Schroeder, E.; Kolodziej, C.; Foster, D.; Schmidt, N.; Root, T.; Kawai, T.; Suga, T.; Nevius, T.; Kusaka, T. Detailed diesel exhaust particulate characterization and real-time DPF filtration efficiency measurements during PM filling process. SAE Tech. Pap. 2007, 1, 320. [Google Scholar] [CrossRef]
- Bensaid, S.; Marchisio, D.L.; Russo, N.; Fino, D. Experimental investigation of soot deposition in diesel particulate filters. Catal. Today 2009, 147S, S295–S300. [Google Scholar]
- Yamamoto, K.; Oohori, S.; Yamashita, H.; Daido, S. Simulation on soot deposition and combustion in diesel particulate filter. Proc. Combust. Inst. 2009, 32, 1965–1972. [Google Scholar] [CrossRef]
- Tsuneyoshi, K.; Takagi, O.; Yamamoto, K. Effects of washcoat on initial PM filtration efficiency and pressure drop in SiC DPF. SAE Tech. Pap. 2011, 1, 817. [Google Scholar] [CrossRef]
- Stein, H.J. Diesel oxidation catalysts for commercial vehicle engines: Strategies on their application for controlling particulate emissions. Appl. Catal. B: Environ. 1996, 10, 69–82. [Google Scholar] [CrossRef]
- Wang, S.; Haynes, B. Catalytic combustion of soot on metal oxides and their supported metal chlorides. Catal. Commun. 2003, 4, 591–596. [Google Scholar] [CrossRef]
- Tzamkiozis, T.; Ntziachristos, L.; Samaras, Z. Diesel passenger car PM emissions: From Euro 1 to Euro 4 with particle filter. Atmos. Environ. 2010, 44, 909–916. [Google Scholar] [CrossRef]
- Feng, Y.; Rao, P.M.; Kim, D.R.; Zheng, X. Methane oxidation over catalytic copper oxides nanowires. Proc. Combust. Inst. 2011, 33, 3169–3175. [Google Scholar] [CrossRef]
- Susu, A.A.; Ogogo, E.O.; Ngomo, H.M. The effect of sintering-redispersion on the selective aromatic yield on supported Platinum catalysts. Trans IChemE Part A 2006, 84, 664–676. [Google Scholar] [CrossRef]
- Kim, M.Y.; Choi, J.S.; Toops, T.J.; Jeong, E.S.; Han, S.W.; Schwartz, V.; Chen, J. Coating SiO2 support with TiO2 or ZrO2, and effects on structure and CO oxidation performance of Pt catalysts. Catalysts 2013, 3, 88–103. [Google Scholar] [CrossRef]
- Yamamoto, K.; Satake, S.; Yamashita, H.; Takada, N.; Misawa, M. Fluid simulation and X-ray CT images for soot deposition in a diesel filter. Eur. Phys. J. 2009, 171, 205–212. [Google Scholar]
- Yamamoto, K.; Fujikake, F.; Matsui, K. Non-catalytic after-treatment for diesel particulates using carbon-fiber filter and experimental validation. Proc. Combust. Inst. 2011, 34, 2865–2875. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takada, N.; Misawa, M. Combustion simulation with lattice Boltzmann method in a three-dimensional porous structure. Proc. Combust. Inst. 2005, 30, 1509–1515. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakamura, M.; Yane, H.; Yamashita, H. Simulation on catalytic reaction in diesel particulate filter. Catal. Today 2010, 153, 118–124. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamauchi, K.; Takada, N.; Misawa, M.; Furutani, H.; Shinozaki, O. Lattice Boltzmann simulation on continuously regenerating diesel filter. Philos. Trans. R. Soc. A 2011, 369, 2584–2591. [Google Scholar] [CrossRef]
- Wehner, B.; Uhrner, U.; Lowis, S.; Zallinger, M.; Widensohler, A. Aerosol number size distributions within the exhaust plume of a diesel and a gasoline passenger car under on-road conditions and determination of emission factors. Atmos. Environ. 2009, 43, 1235–1245. [Google Scholar] [CrossRef]
- Misawa, M.; Tiseanu, I.; Hirashima, R.; Koizumi, K.; Ikeda, Y. Oblique view cone beam tomography for inspection of flat-shape objects. Key Eng. Mater. 2004, 270, 1135–1142. [Google Scholar]
- Barhate, R.S.; Sundarrajan, S.; Pliszka, D.; Ramakrishna, S. Fine chemical processing: The potential of nanofibres in filtration. Filtr. Sep. 2008, 45, 32–35. [Google Scholar]
- Yamamoto, K.; He, X.; Doolen, G.D. Simulation of combustion field with lattice Boltzmann method. J. Stat. Phys. 2002, 107, 367–383. [Google Scholar] [CrossRef]
- Qian, Y.H.; D’Humières, D.; Lallemand, P. Lattice BGK models for the Navier-Stokes equation. Europhys. Lett. 1992, 17, 479–484. [Google Scholar] [CrossRef]
- Filippova, O.; Hanel, D. A novel lattice-BGK approach for low Mach number combustion. J. Comput. Phys. 2000, 158, 139–160. [Google Scholar] [CrossRef]
- Chen, S.; Doolen, G.D. Lattice Boltzmann method for fluid flows. Ann. Rev. Fluid Mech. 1998, 30, 329–364. [Google Scholar] [CrossRef]
- Chopard, B.; Masselot, A.; Dupuis, A. A lattice gas model for erosion and particles transport in a fluid. Comput. Phys. Commun. 2000, 129, 167–176. [Google Scholar] [CrossRef]
- He, X.; Luo, L.-S. Lattice Boltzmann model for the incompressible Navier-Stokes equation. J. Stat. Phys. 1997, 88, 927–944. [Google Scholar] [CrossRef]
- Lee, K.B.; Thring, M.W.; Beer, J.M. On the rate of combustion of soot in a laminar soot flame. Combust. Flame 1962, 6, 137–145. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.John Wiley and Sons: New York, NY, USA, 2002; pp. 177–196. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamamoto, K.; Matsui, K. Diesel Exhaust After-Treatment by Silicon Carbide Fiber Filter. Fibers 2014, 2, 128-141. https://doi.org/10.3390/fib2020128
Yamamoto K, Matsui K. Diesel Exhaust After-Treatment by Silicon Carbide Fiber Filter. Fibers. 2014; 2(2):128-141. https://doi.org/10.3390/fib2020128
Chicago/Turabian StyleYamamoto, Kazuhiro, and Kenta Matsui. 2014. "Diesel Exhaust After-Treatment by Silicon Carbide Fiber Filter" Fibers 2, no. 2: 128-141. https://doi.org/10.3390/fib2020128




