Infrared Imaging of Cotton Fiber Bundles Using a Focal Plane Array Detector and a Single Reflectance Accessory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. FTIR Examination
2.3. Chemical Imaging
2.4. Fiber Analysis
3. Results
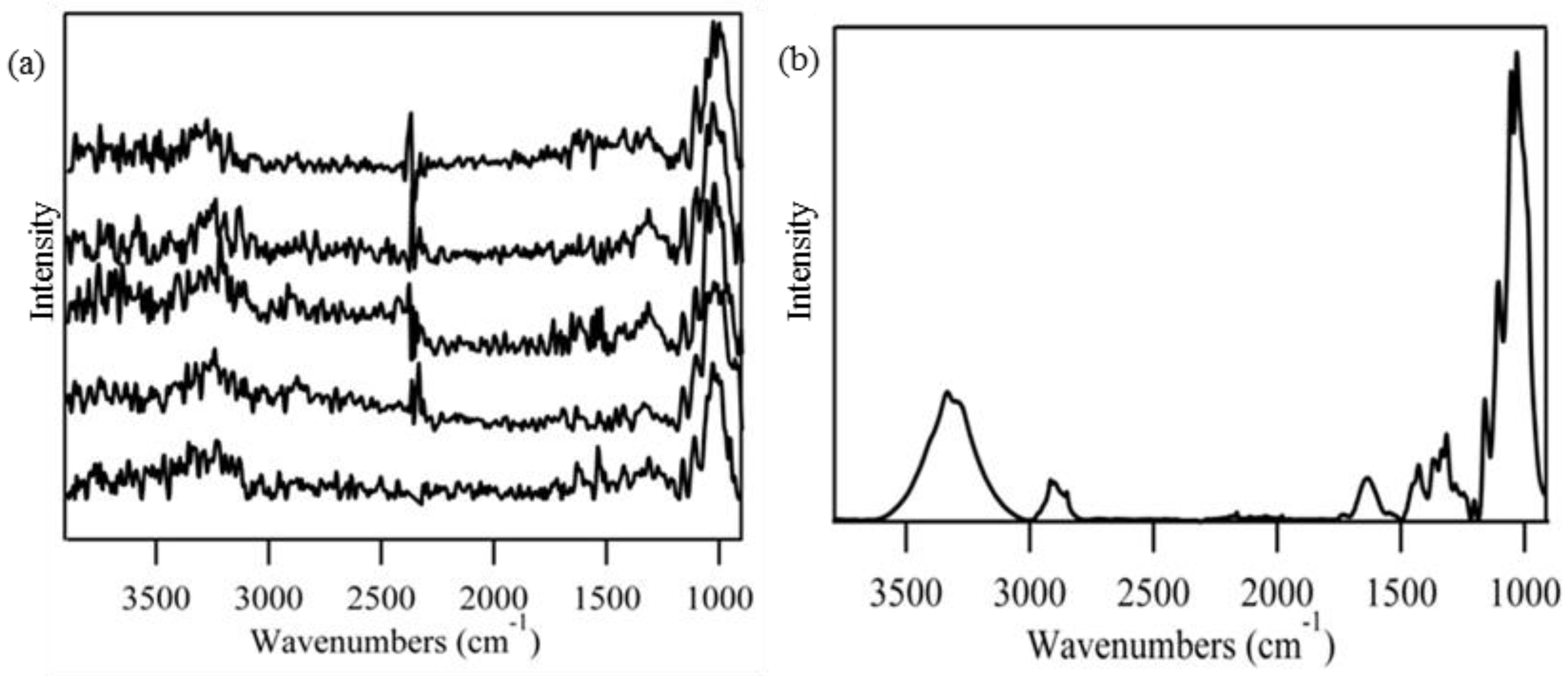
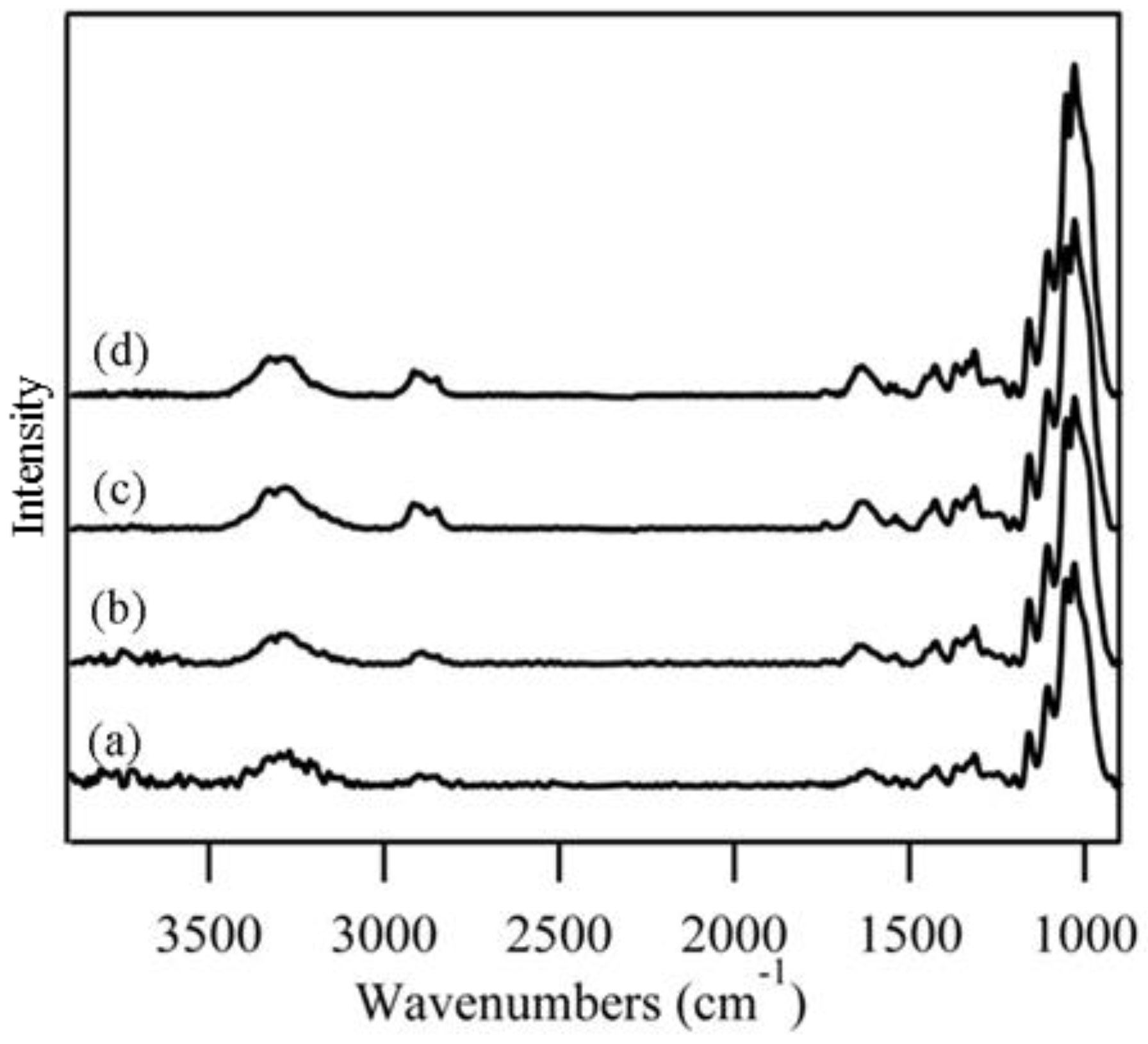
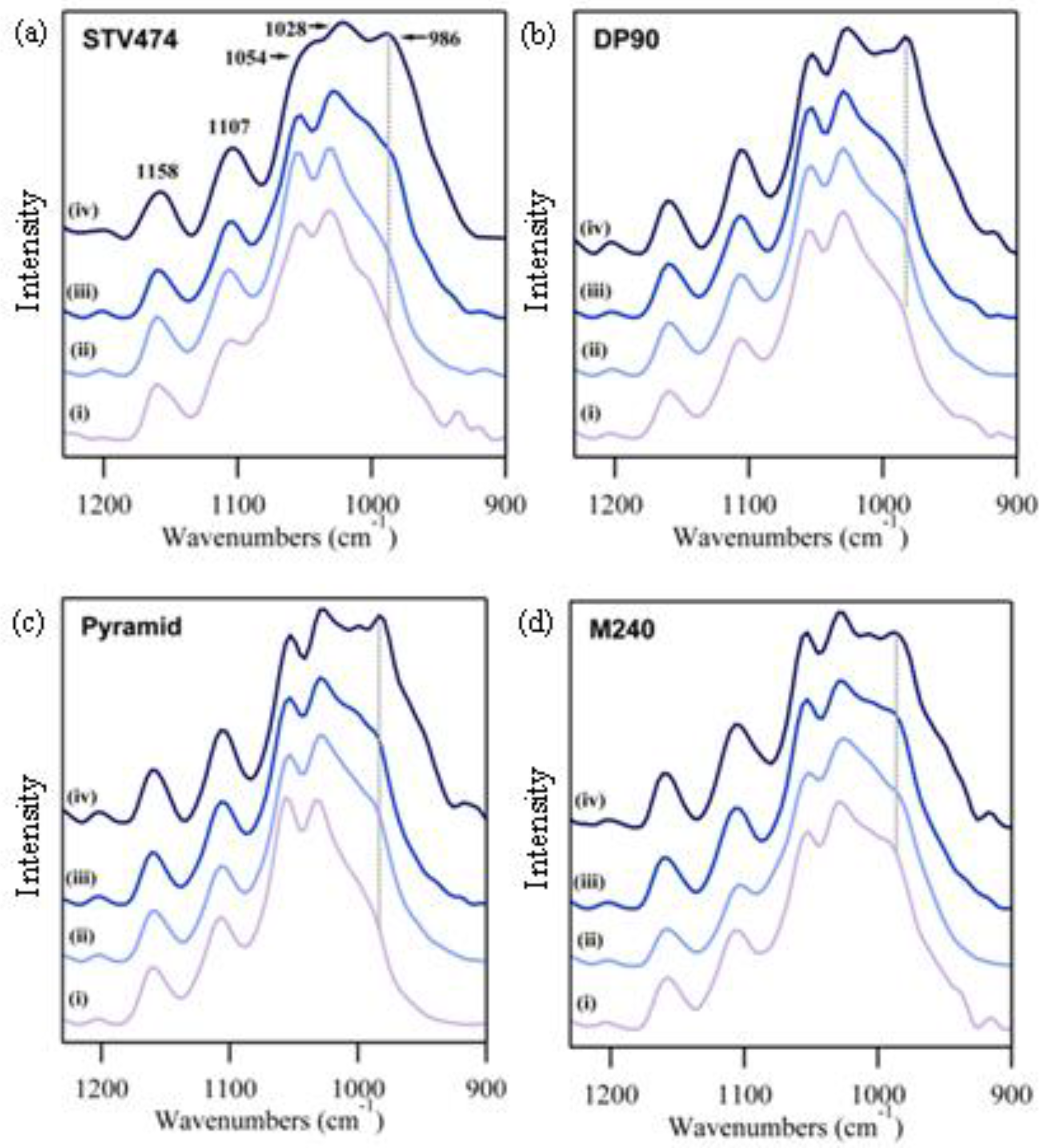
3.1. Grouping of Spectral Data
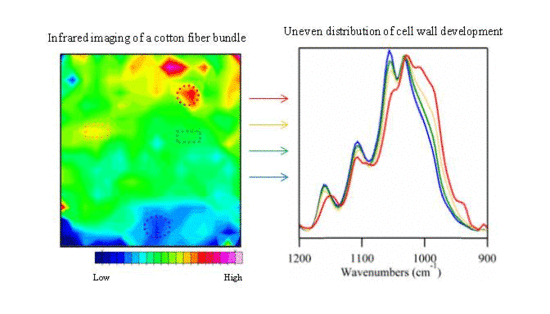
3.2. Examination of Cotton Fiber Development
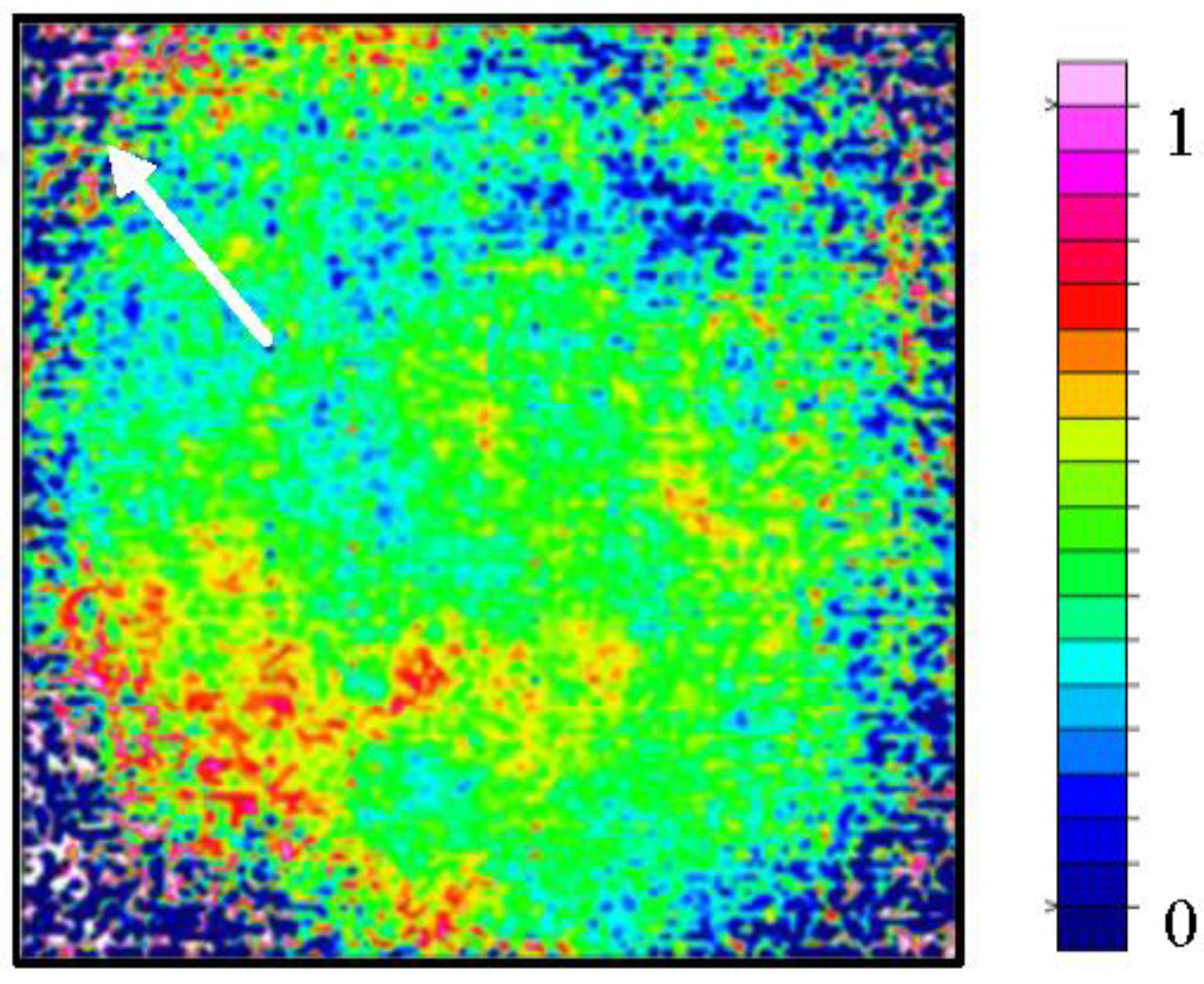
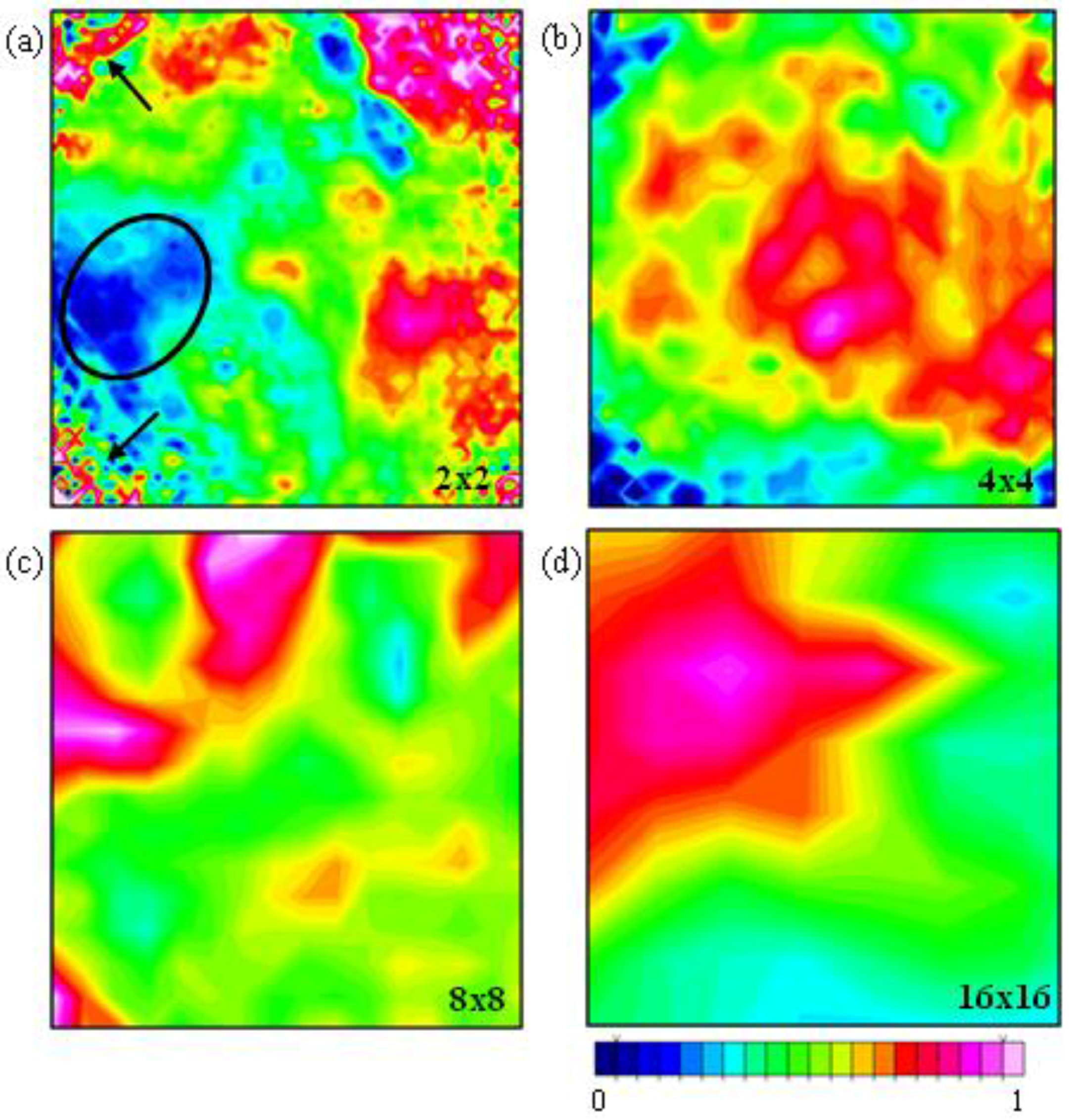
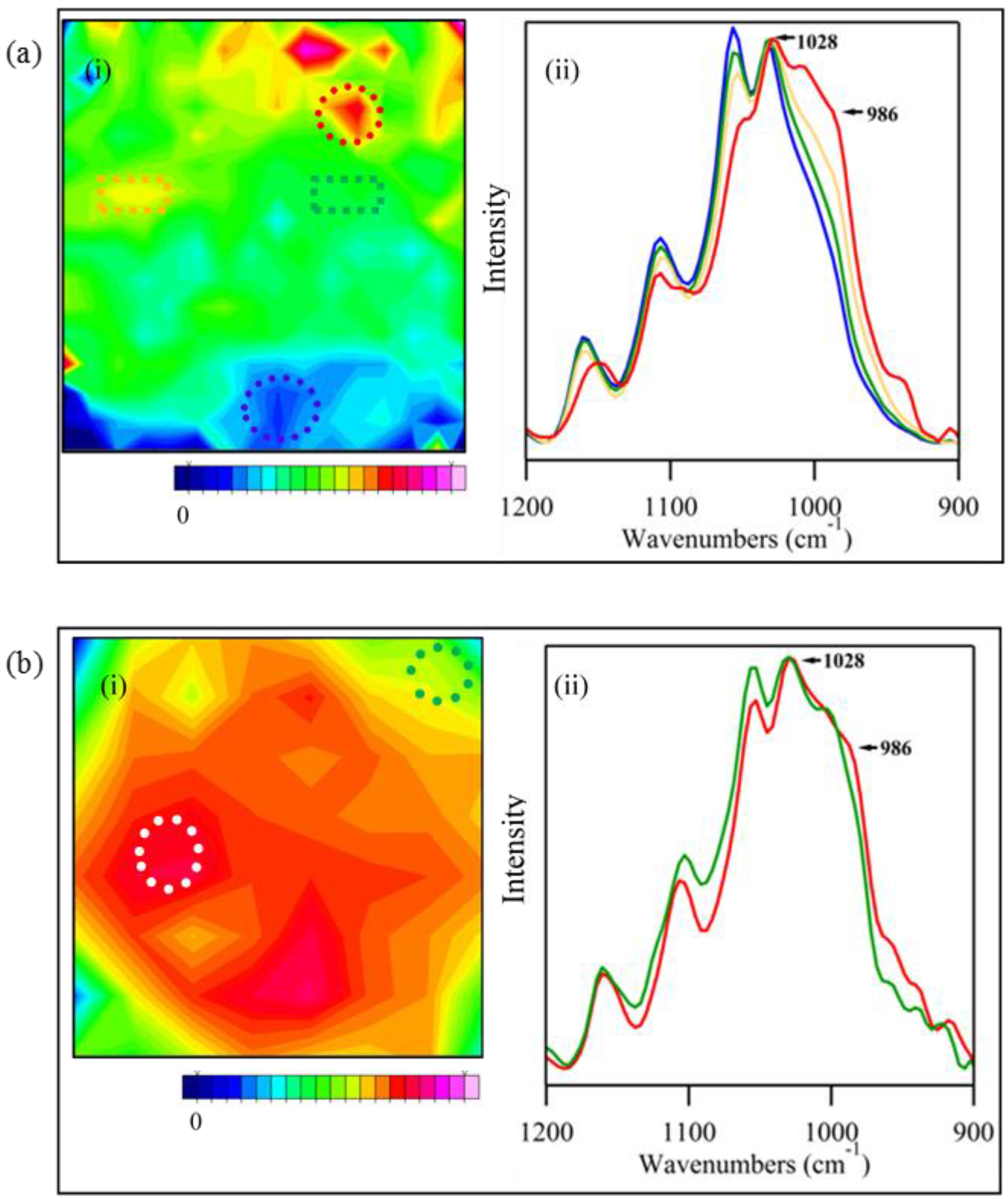
3.3. Examination of Sample Distribution
4. Conclusions
Disclaimer
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agricultural-Marketing-Service. The Classification of Cotton; United States Department of Agriculture: Washington, DC, USA, 1995.
- Wakelyn, P.J.; Bertoniere, N.R.; French, A.D.; Thibodeaux, D.P.; Triplett, B.A.; Rouselle, M.-A.; Goynes, W.R.; Edwards, J.V.; Hunter, L.; McAllister, D.D.; et al. Cotton Fiber Chemistry and Technology; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- McCreight, D.J.; Feil, R.W.; Booterbaugh, J.H.; Backe, E.E. Test instruments and quality assurance methods. In Short Staple Yarn Manufacturing; Carolina Academic Press: Durham, NC, USA, 1997; pp. 439–480. [Google Scholar]
- Naylor, G.R.; Delhom, C.D.; Cui, X.; Gourlot, J.-P.; Rodgers, J. Understanding the influence of fiber length on the high volume instrument™ measurement of cotton fiber strength. Text. Res. J. 2014, 84, 979–988. [Google Scholar] [CrossRef]
- Turhan, Y.; Toprakci, O. Comparison of high-volume instrument and advanced fiber information systems based on prediction performance of yarn properties using a radial basis function neural network. Text. Res. J. 2012, 83, 130–147. [Google Scholar] [CrossRef]
- Drieling, A.; Gourlot, J.-P. Cotton/worldwide harmonisation. In Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; Müssig, J., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 353–370. [Google Scholar]
- Liu, Y.; Thibodeaux, D.; Gamble, G. Development of fourier transform infrared spectroscopy in direct, non-destructive, and rapid determination of cotton fiber maturity. Text. Res. J. 2011, 81, 1559–1567. [Google Scholar]
- Montalvo, J.; VonHoven, T. Analysis of cotton. In Near-Infrared Spectroscopy in Agriculture; Roberts, C.A., Workman, J., Reeves, J.B., Eds.; American Society of Agronomy, Agronomy Monograph: Madison, WI, USA, 2004; Volume N0. 44, pp. 671–728. [Google Scholar]
- Rodgers, J.; Montalvo, J.; Davidonis, G.; VonHoven, T. Near infrared measurement of cotton fiber micronaire, maturity and fineness-A comparative investigation. Text. Res. J. 2010, 80, 780–793. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Fourier transform infrared spectroscopic approach to the study of the secondary cell wall development in cotton fiber. Cellulose 2010, 17, 309–320. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Cabrales, L. Applications of fourier transform infrared spectroscopy to study cotton fibers. In Fourier Transforms-New Analytical Approaches and Ftir Strategies; Nikolic, G., Ed.; InTech: Cairo, Egypt, 2011. [Google Scholar]
- Abidi, N.; Hequet, E.; Cabrales, L.; Gannaway, J.; Wilkins, T.; Wells, L.W. Evaluating cell wall structure and composition of developing cotton fibers using fourier transform infrared spectroscopy and thermogravimetric analysis. J. Appl. Polym. Sci. 2008, 107, 476–486. [Google Scholar] [CrossRef]
- Fahrenfort, J. Attenuated total reflection. Spectrochim. Acta Mol. Biomol. Spectrosc. 1989, 45, 251–263. [Google Scholar] [CrossRef]
- Oberg, K.A.; Fink, A.L. A new attenuated total reflectance fourier transform infrared spectroscopy method for the study of proteins in solution. Anal. Biochem. 1998, 256, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Hind, A.R.; Bhargava, S.K.; McKinnon, A. At the solid/liquid interface: FTIR/ATR-The tool of choice. Adv. Colloid Interface Sci. 2001, 93, 91–114. [Google Scholar] [CrossRef]
- Offermann, V.; Grosse, P.; Feuerbacher, M.; Dittmar, G. Experimental aspects of attenuated total reflectance spectroscopy in the infrared. Vib. Spectrosc. 1995, 8, 135–140. [Google Scholar] [CrossRef]
- Fortier, C.A.; Rodgers, J.E.; Cintrón, M.S.; Cui, X.; Foulk, J.A. Identification of cotton and cotton trash components by fourier transform near-infrared spectroscopy. Text. Res. J. 2011, 81, 230–238. [Google Scholar] [CrossRef]
- Mustafic, A.; Jiang, Y.; Li, C. Cotton contamination detection and classification using hyperspectral fluorescence imaging. Text. Res. J. 2016, 86, 1574–1584. [Google Scholar] [CrossRef]
- Lang, P.L.; Katon, J.E.; O’Keefe, J.F.; Schiering, D.W. The identification of fibers by infrared and raman microspectroscopy. Microchem. J. 1986, 34, 319–331. [Google Scholar] [CrossRef]
- Himmelsbach, D.S.; Khalili, S.; Akin, D.E. The use of FT-IR microspectroscopic mapping to study the effects of enzymatic retting of flax (Linum usitatissimum L) stems. J. Sci. Food. Agric. 2002, 82, 685–696. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Tungol, M.W.; Bartick, E.G.; Montaser, A. The development of a spectral data base for the identification of fibers by infrared microscopy. Appl. Spectrosc. 1990, 44, 543–549. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, X.; Gao, W.; Chen, J. Characterization of bioscoured cotton fabrics using FT-IR ATR spectroscopy and microscopy techniques. Carbohydr. Res. 2006, 341, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Tungol, M.W.; Bartick, E.G.; Montaser, A. Forensic examination of synthetic textile fibers by microscopic infrared spectrometry. In Practical Guide to Infrared Microspectroscopy; Humecki, H.J., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1995; pp. 245–286. [Google Scholar]
- Lewis, E.N.; Treado, P.J.; Reeder, R.C.; Story, G.M.; Dowrey, A.E.; Marcott, C.; Levin, I.W. Fourier transform spectroscopic imaging using an infrared focal-plane array detector. Anal. Chem. 1995, 67, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- Cintrón, M.S.; Fortier, C.; Hinchliffe, D.J.; Rodgers, J.E. Chemical imaging of secondary cell wall development in cotton fibers using a mid-infrared focal-plane array detector. Text. Res. J. 2016. [Google Scholar] [CrossRef]
- Fortier, C.; Cintròn, M.S.; Rodgers, J. Fourier transform infrared macro-imaging of botanical cotton trash. AATCC J. Res. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- De-Juan, A.; Piqueras, S.; Maeder, M.; Hancewicz, T.; Duponchel, L.; Tauler, R. Chemometric tools for image analysis. In Infrared and Raman Spectroscopic Imaging, 2nd ed.; Salzer, R., Siesler, H.W., Eds.; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Cintrón, M.S.; Hinchliffe, D. FT-IR examination of the development of secondary cell wall in cotton fibers. Fibers 2015, 3. [Google Scholar] [CrossRef]
- Hoven, T.V.; Montalvo, J.; Santiago, M.C.; Dowd, M.; Armijo, C.B.; Byler, R.K. Fundamental research on spiking, recovery and understanding seed coat nep counts in AFIS analysis of pre-opened cotton. Text. Res. J. 2016. [Google Scholar] [CrossRef]
- Kim, H.J.; Rodgers, J.; Delhom, C.; Cui, X. Comparisons of methods measuring fiber maturity and fineness of upland cotton fibers containing different degrees of fiber cell wall development. Text. Res. J. 2014, 84, 1622–1633. [Google Scholar] [CrossRef]
| STV474 | DP90 | M240 | Pyramid | |||||
|---|---|---|---|---|---|---|---|---|
| 22 DPA * | Mature | 22 DPA * | Mature | 22 DPA * | Mature | 22 DPA * | Mature | |
| Mean | 0.798 | 0.942 | 0.812 | 0.958 | 0.840 | 0.968 | 0.846 | 0.974 |
| STDEV | 0.015 | 0.013 | 0.008 | 0.008 | 0.014 | 0.015 | 0.015 | 0.021 |
| CV | 1.859 | 1.384 | 1.030 | 0.873 | 1.684 | 1.532 | 1.793 | 2.129 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago Cintrón, M.; Montalvo, J.G.; Von Hoven, T.; Rodgers, J.E.; Hinchliffe, D.J.; Madison, C.; Thyssen, G.N.; Zeng, L. Infrared Imaging of Cotton Fiber Bundles Using a Focal Plane Array Detector and a Single Reflectance Accessory. Fibers 2016, 4, 27. https://doi.org/10.3390/fib4040027
Santiago Cintrón M, Montalvo JG, Von Hoven T, Rodgers JE, Hinchliffe DJ, Madison C, Thyssen GN, Zeng L. Infrared Imaging of Cotton Fiber Bundles Using a Focal Plane Array Detector and a Single Reflectance Accessory. Fibers. 2016; 4(4):27. https://doi.org/10.3390/fib4040027
Chicago/Turabian StyleSantiago Cintrón, Michael, Joseph G. Montalvo, Terri Von Hoven, James E. Rodgers, Doug J. Hinchliffe, Crista Madison, Gregory N. Thyssen, and Linghe Zeng. 2016. "Infrared Imaging of Cotton Fiber Bundles Using a Focal Plane Array Detector and a Single Reflectance Accessory" Fibers 4, no. 4: 27. https://doi.org/10.3390/fib4040027