A Fiber Optic Fabry–Perot Cavity Sensor for the Probing of Oily Samples
Abstract
:1. Introduction
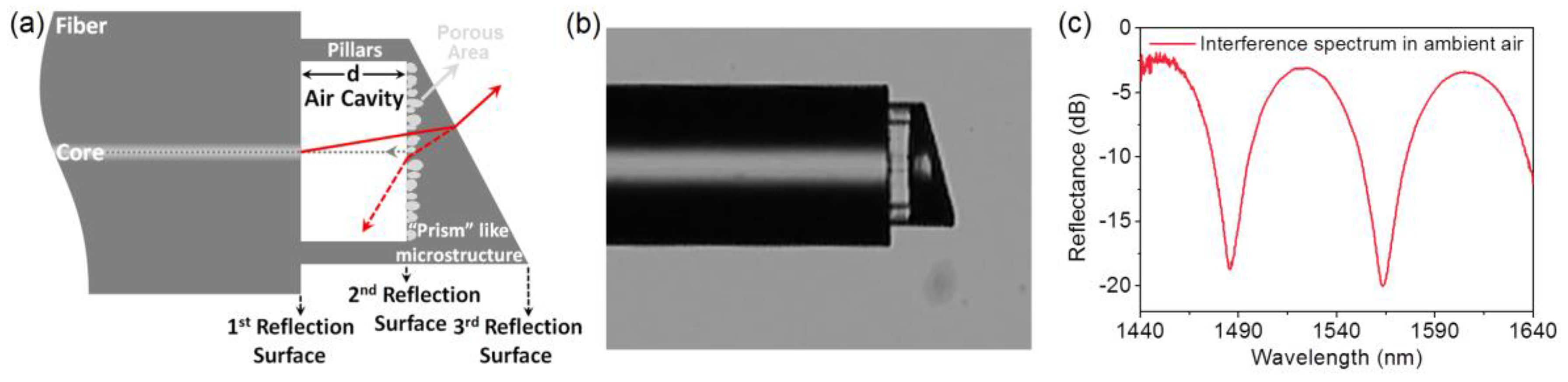
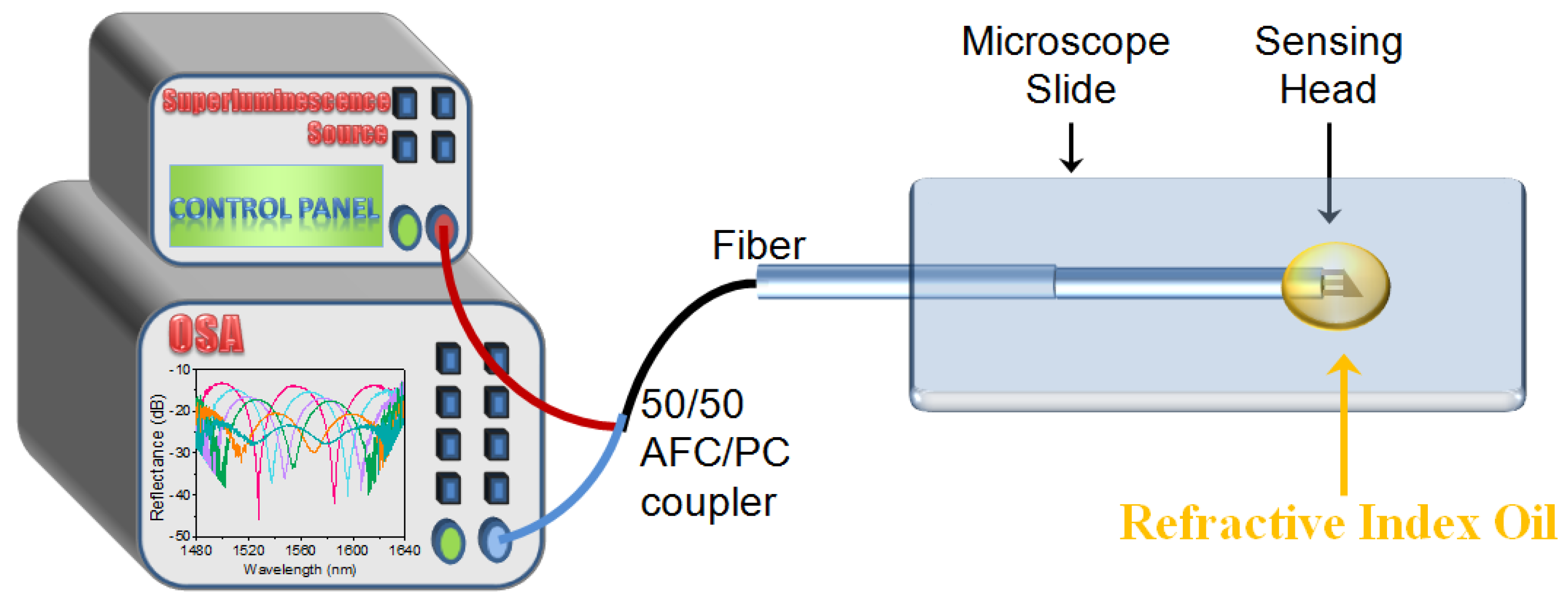
2. Materials and Methods
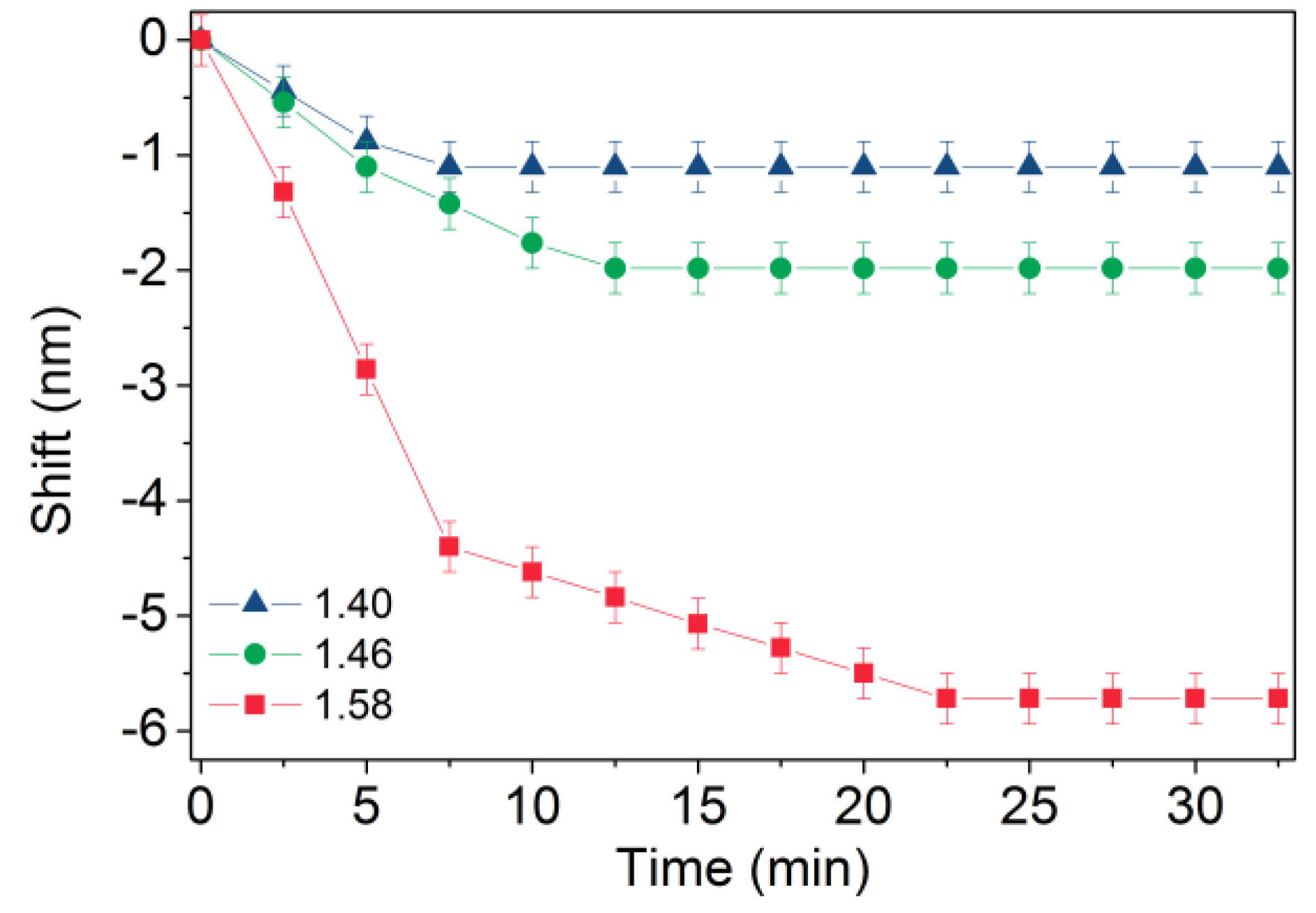
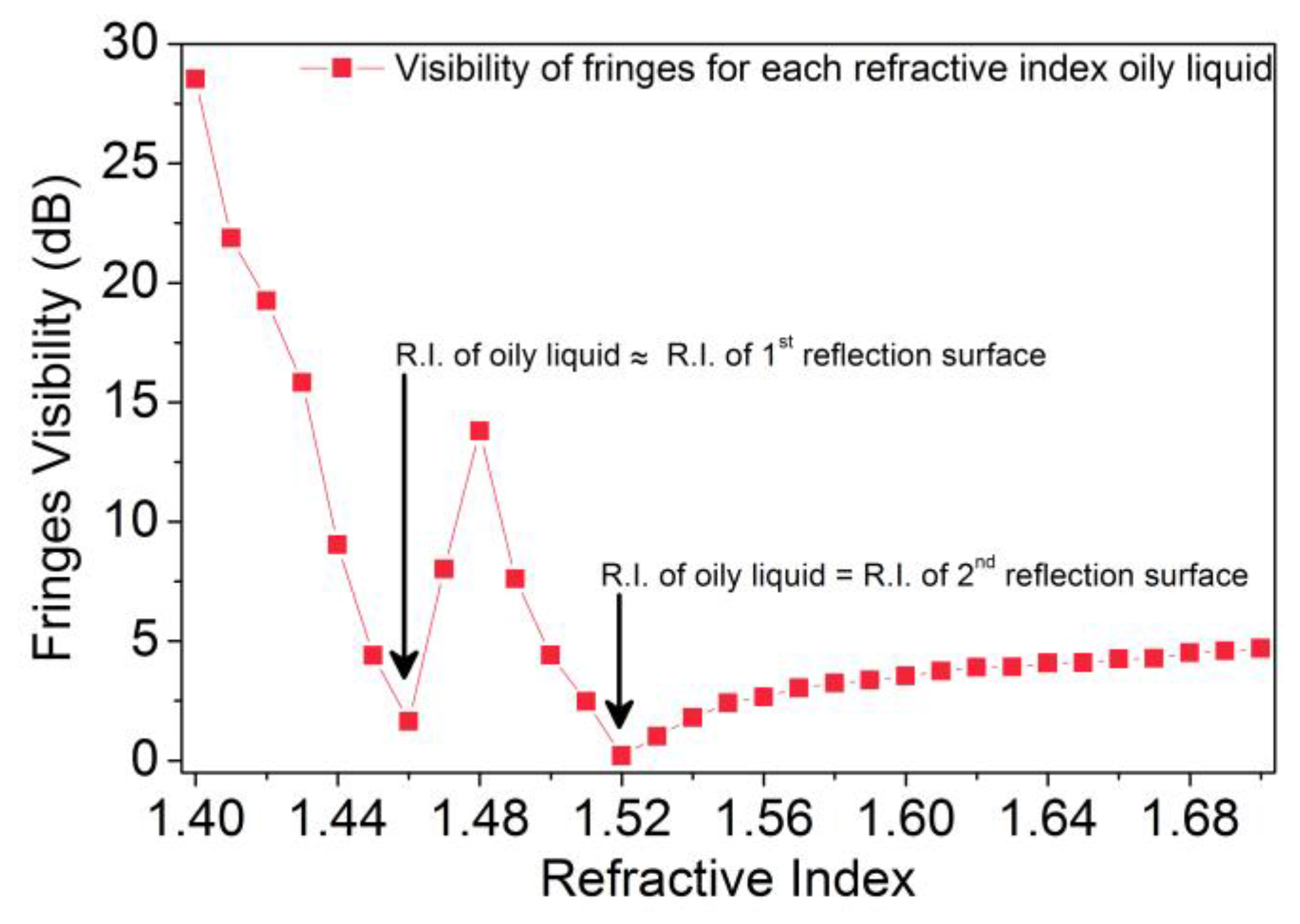
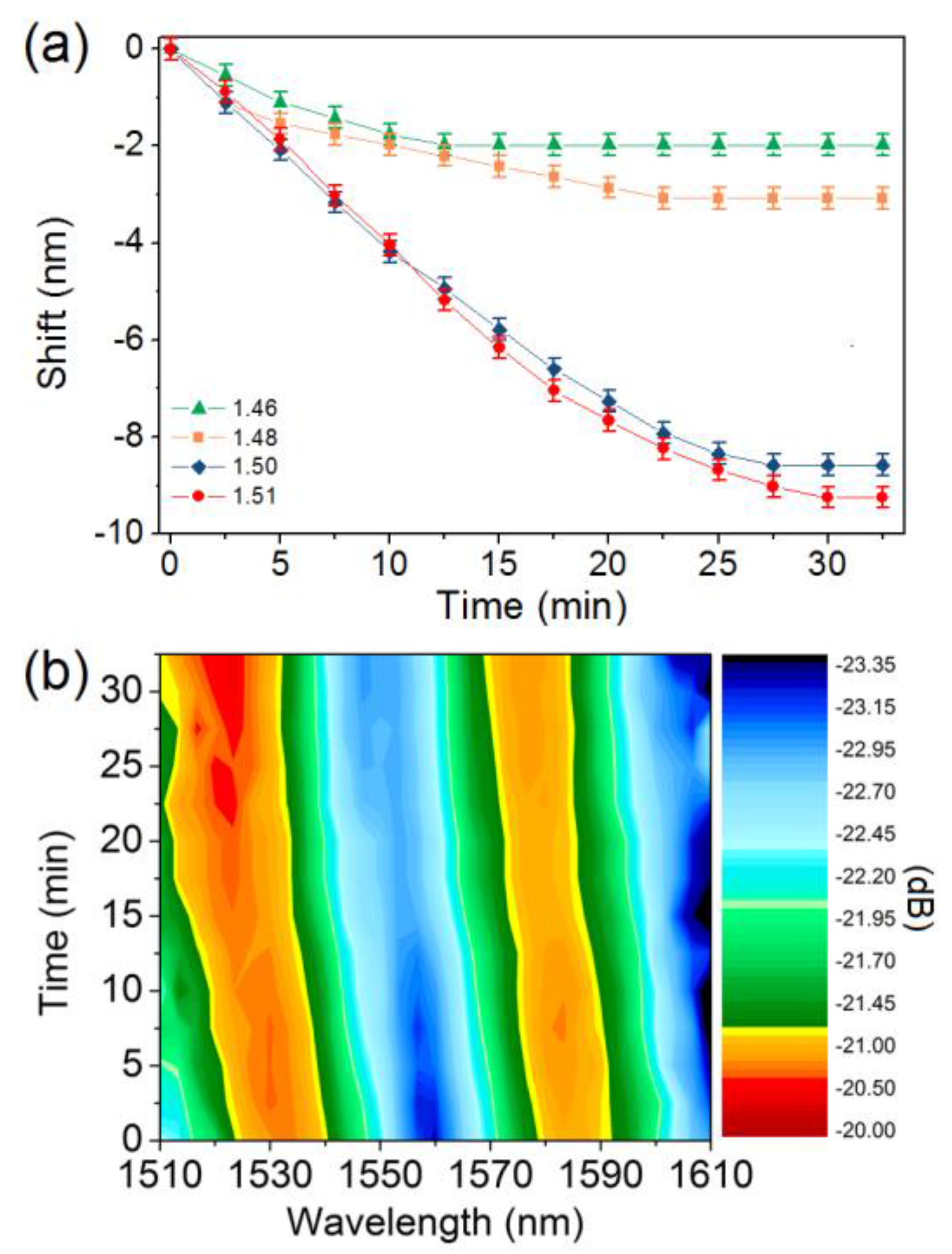
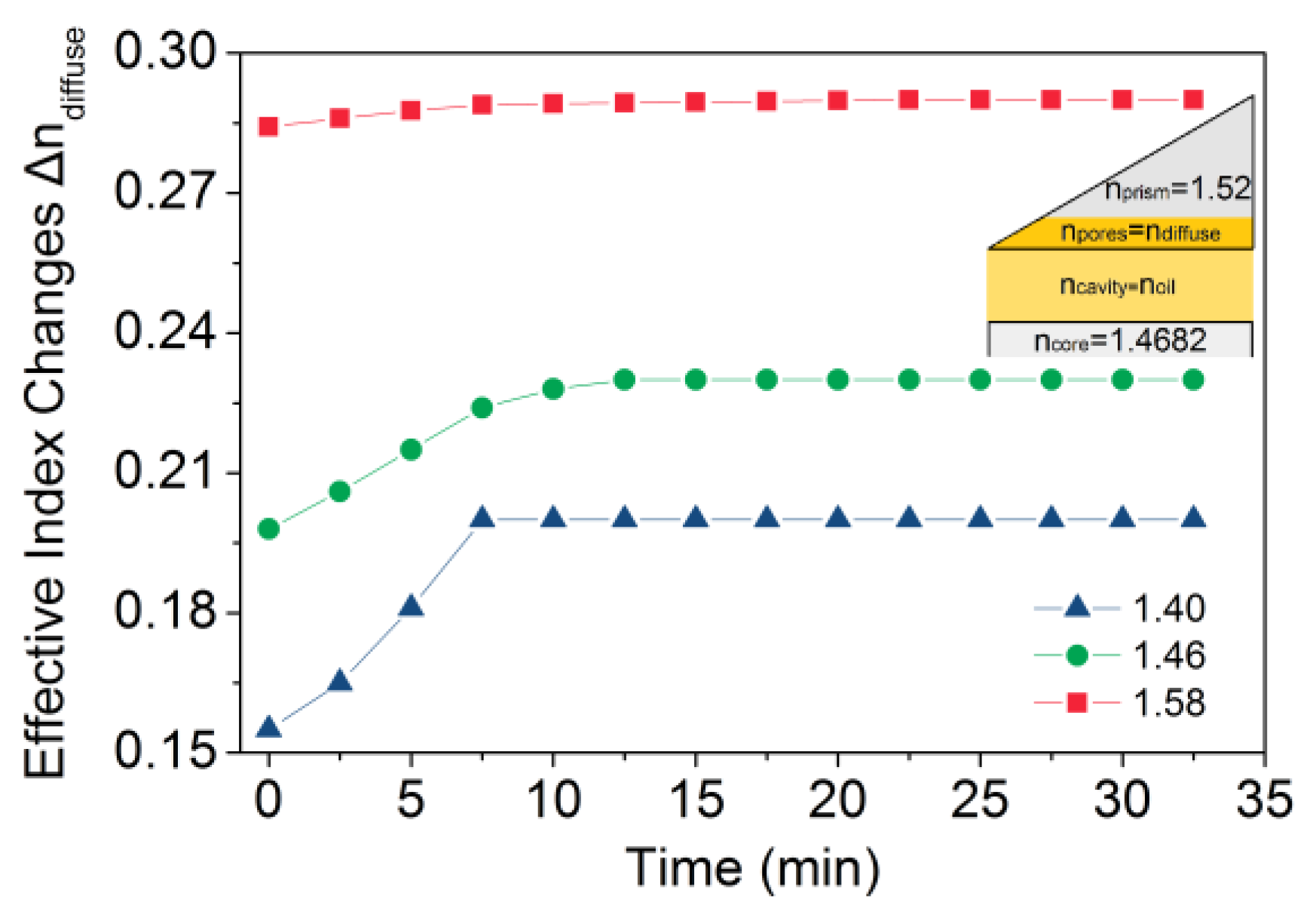
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdelhaseib, M.U.; Singh, A.K.; Bailey, M.; Singh, M.; El-Khateib, T.; Bhunia, A.K. Fiber optic and light scattering sensors: Complimentary approaches to rapid detection of salmonella enterica in food samples. Food Control 2016, 61, 135–145. [Google Scholar] [CrossRef]
- Candiani, A.; Sozzi, M.; Cucinotta, A.; Selleri, S.; Veneziano, R.; Corradini, R.; Marchelli, R.; Childs, P.; Pissadakis, S. Optical fiber ring cavity sensor for label-free DNA detection. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1176–1183. [Google Scholar] [CrossRef]
- Mignani, A.G.; Ciaccheri, L.; Cucci, C.; Mencaglia, A.A.; Cimato, A.; Attilio, C.; Ottevaere, H.; Thienpont, H.; Paolesse, R.; Mastroianni, M.; et al. Eat-by-light: Fiber-optic and micro-optic devices for food quality and safety assessment. IEEE Sens. J. 2008, 8, 1342–1354. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Hendriks, B.H.W.; van der Voort, M.; Nachabe, R.; Bierhoff, W.; Braun, G.; Babic, D.; Rathmell, J.P.; Holmin, S.; Soderman, M.; et al. Epidural needle with embedded optical fibers for spectroscopic differentiation of tissue: Ex vivo feasibility study. Biomed. Opt. Express 2011, 2, 1452–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gassino, R.; Braglia, A.; Vallan, A.; Perrone, G. Fibre probe for tumour laser thermotherapy with integrated temperature measuring capabilities. Electron. Lett. 2016, 52, 798–800. [Google Scholar] [CrossRef]
- Frosch, T.; Yan, D.; Popp, J. Ultrasensitive fiber enhanced UV resonance raman sensing of drugs. Anal. Chem. 2013, 85, 6264–6271. [Google Scholar] [CrossRef] [PubMed]
- Pevec, S.; Donlagic, D. Miniature fiber-optic sensor for simultaneous measurement of pressure and refractive index. Opt. Lett. 2014, 39, 6221–6224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2013–2015). Anal. Chem. 2016, 88, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Carrasquilla, C.; Xiao, Y.; Xu, C.Q.; Li, Y.F.; Brennan, J.D. Enhancing sensitivity and selectivity of long-period grating sensors using structure-switching aptamers bound to gold-doped macroporous silica coatings. Anal. Chem. 2011, 83, 7984–7991. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Han, Y.; Joo, K.I.; Lee, Y.W.; Kang, S.W.; Kim, H.R. Optical detection of volatile organic compounds using selective tensile effects of a polymer-coated fiber bragg grating. Opt. Express 2010, 18, 24753–24761. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Amao, Y. 1-pyrenedecanoic acid chemisorption film as a novel oxygen sensing material. Sens. Actuator B 2002, 85, 175–178. [Google Scholar] [CrossRef]
- Widera, J.; Bunker, C.E.; Pacey, G.E.; Katta, V.R.; Brown, M.S.; Elster, J.L.; Jones, M.E.; Gord, J.R.; Buckner, S.W. Kinetic behavior of polymer-coated long-period-grating fiber-optic sensors. Appl. Opt. 2005, 44, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Melissinaki, V.; Farsari, M.; Pissadakis, S. A fiber-endface, Fabry-Perot vapor microsensor fabricated by multiphoton polymerization. IEEE J. Sel. Top. Quantum Electron. 2015, 21, 10. [Google Scholar] [CrossRef]
- Melissinaki, V.; Konidakis, I.; Farsari, M.; Pissadakis, S. Fiber endface Fabry-Perot microsensor with distinct response to vapors of different chlorinated organic solvents. IEEE Sens. J. 2016, 16, 7094–7100. [Google Scholar] [CrossRef]
- Villatoro, J.; Kreuzer, M.P.; Jha, R.; Minkovich, V.P.; Finazzi, V.; Badenes, G.; Pruneri, V. Photonic crystal fiber interferometer for chemical vapor detection with high sensitivity. Opt. Express 2009, 17, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Roriz, P.; Frazão, O. Refractive index measurement of liquids based on microstructured optical fibers. Photonics 2014, 1, 516–529. [Google Scholar] [CrossRef]
- Tian, J.J.; Lu, Y.J.; Zhang, Q.; Han, M. Microfluidic refractive index sensor based on an all-silica in-line Fabry-Perot interferometer fabricated with microstructured fibers. Opt. Express 2013, 21, 6633–6639. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.L.; Rao, Y.J.; Liu, W.J.; Liao, X.; Chiang, K.S. Laser-micromachined Fabry-Perot optical fiber tip sensor for high-resolution temperature-independent measurement of refractive index. Opt. Express 2008, 16, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Han, Y.K.; Li, Y.J.; Tsai, H.L.; Xiao, H. Temperature-insensitive miniaturized fiber inline Fabry-Perot interferometer for highly sensitive refractive index measurement. Opt. Express 2008, 16, 5764–5769. [Google Scholar] [CrossRef] [PubMed]
- Some, S.; Xu, Y.; Kim, Y.; Yoon, Y.; Qin, H.; Kulkarni, A.; Kim, T.; Lee, H. Highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes. Sci. Rep. 2013, 3, 1868. [Google Scholar] [CrossRef] [PubMed]
- Konstantaki, M.; Klini, A.; Anglos, D.; Pissadakis, S. An ethanol vapor detection probe based on a zno nanorod coated optical fiber long period grating. Opt. Express 2012, 20, 8472–8484. [Google Scholar] [CrossRef] [PubMed]
- Hlubina, P.; Kadulova, M.; Ciprian, D.; Sobota, J. Reflection-based fibre-optic refractive index sensor using surface plasmon resonance. J. Eur. Opt. Soc. Rapid Publ. 2014, 9, 14033. [Google Scholar] [CrossRef]
- Villar, I.D.; Matias, I.R.; Arregui, F.J.; Claus, R.O. ESA-based in-fiber nanocavity for hydrogen-peroxide detection. IEEE Trans. Nanotechnol. 2005, 4, 187–193. [Google Scholar]
- Coelho, L.; Viegas, D.; Santos, J.L.; de Almeida, J. Detection of extra virgin olive oil thermal deterioration using a long period fibre grating sensor coated with titanium dioxide. Food Bioprocess Technol. 2015, 8, 1211–1217. [Google Scholar] [CrossRef]
- Biswas, P.; Basumallick, N.; Dasgupta, K.; Ghosh, A.; Bandyopadhyay, S. Application of strongly overcoupled resonant modes of long-period fiber gratings to measure the adulteration of olive oil. Appl. Opt. 2016, 55, 5118–5126. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; Konstantinidou, V.; Fitó, M. Olive oil and cardiovascular health. J. Cardiovasc. Pharmacol. 2009, 54, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I. Olive oil and the cardiovascular system. Pharmacol. Res. 2007, 55, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Fragaki, G.; Spyros, A.; Siragakis, G.; Salivaras, E.; Dais, P. Detection of extra virgin olive oil adulteration with lampante olive oil and refined olive oil using nuclear magnetic resonance spectroscopy and multivariate statistical analysis. J. Agric. Food Chem. 2005, 53, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Dourtoglou, V.G.; Dourtoglou, T.; Antonopoulos, A.; Stefanou, E.; Lalas, S.; Poulos, C. Detection of olive oil adulteration using principal component analysis applied on total and regio fa content. J. Am. Oil Chem. Soc. 2003, 80, 203–208. [Google Scholar] [CrossRef]
- Bi, X.; Pan, X.; Yuan, S.; Wang, Q. Plasticizer contamination in edible vegetable oil in a U.S. Retail market. J. Agric. Food Chem. 2013, 61, 9502–9509. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Watson, R.R. Olives and Olive Oil in Health and Disease Prevention; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Abro, R.; Chen, X.; Harijan, K.; Dhakan, Z.A.; Ammar, M. A comparative study of recycling of used engine oil using extraction by composite solvent, single solvent, and acid treatment methods. ISRN Chem. Eng. 2013, 2013, 5. [Google Scholar] [CrossRef]
- Jentzsch, P.; Ramos, L.; Ciobotă, V. Handheld Raman spectroscopy for the distinction of essential oils used in the cosmetics industry. Cosmetics 2015, 2, 162–176. [Google Scholar] [CrossRef]
- Sakellari, I.; Kabouraki, E.; Gray, D.; Purlys, V.; Fotakis, C.; Pikulin, A.; Bityurin, N.; Vamvakaki, M.; Farsari, M. Diffusion-assisted high-resolution direct femtosecond laser writing. Acs Nano 2012, 6, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, M.; Zukauskas, A.; Purlys, V.; Belazaras, K.; Momot, A.; Paipulas, D.; Gadonas, R.; Piskarskas, A.; Gilbergs, H.; Gaidukeviciute, A.; et al. Femtosecond laser polymerization of hybrid/integrated micro-optical elements and their characterization. J. Opt. 2010, 12, 124010. [Google Scholar] [CrossRef]
- Balluffi, R.W.; Allen, S.M.; Carter, W.C. The diffusion equation. In Kinetics of Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 77–97. [Google Scholar]
- Doremus, R.H. Diffusion of water in silica glass. J. Mater. Res. 1995, 10, 2379–2389. [Google Scholar] [CrossRef]
- Shamiryan, D.; Baklanov, M.R.; Lyons, P.; Beckx, S.; Boullart, W.; Maex, K. Diffusion of solvents in thin porous films. Colloids Surf. A 2007, 300, 111–116. [Google Scholar] [CrossRef]
- Riegel, B.; Kiefer, W.; Hofacker, S.; Schottner, G. Investigations on the organic crosslinking in ormocers by means of Raman spectroscopy. Ber. Bunsen Ges. Phys. Chem. Chem. Phys. 1998, 102, 1573–1576. [Google Scholar] [CrossRef]
| Refractive Index Liquid Series | First Basic Substance | Second Basic Substance | Refractive Indices Range (nD) |
|---|---|---|---|
| SERIES AA | Mixture of Aliphatic/Alicyclic Hydrocarbons | ― | 1.400–1.458 |
| SERIES A1 | Mixture of Aliphatic/Alicyclic Hydrocarbons | Hydrogenated Terphenyl | 1.460–1.570 |
| SERIES A2 | Hydrogenated Terphenyl | 1-Bromonaphthalene | 1.572–1.640 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melissinaki, V.; Farsari, M.; Pissadakis, S. A Fiber Optic Fabry–Perot Cavity Sensor for the Probing of Oily Samples. Fibers 2017, 5, 1. https://doi.org/10.3390/fib5010001
Melissinaki V, Farsari M, Pissadakis S. A Fiber Optic Fabry–Perot Cavity Sensor for the Probing of Oily Samples. Fibers. 2017; 5(1):1. https://doi.org/10.3390/fib5010001
Chicago/Turabian StyleMelissinaki, Vasileia, Maria Farsari, and Stavros Pissadakis. 2017. "A Fiber Optic Fabry–Perot Cavity Sensor for the Probing of Oily Samples" Fibers 5, no. 1: 1. https://doi.org/10.3390/fib5010001






