Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
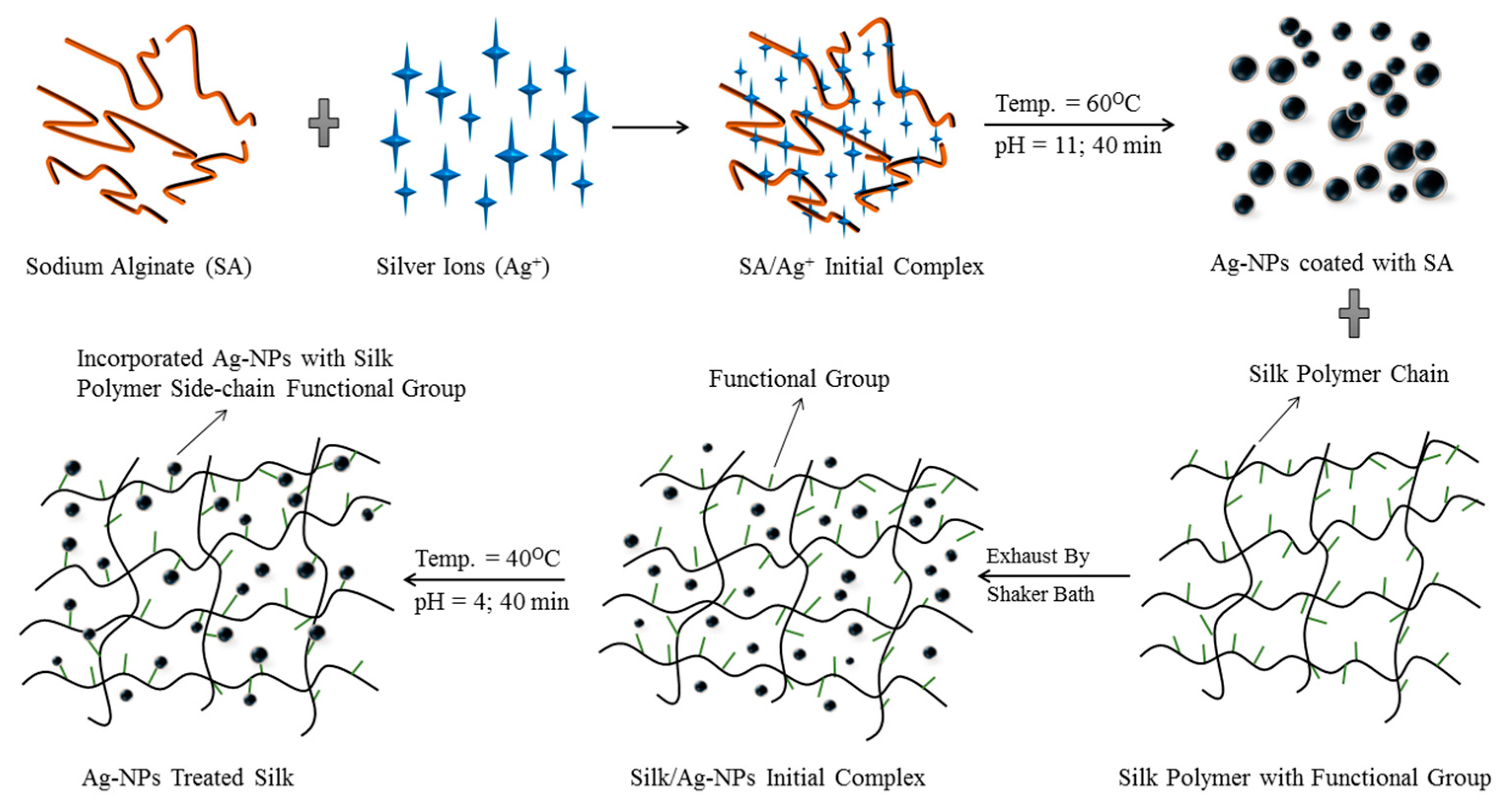
2.2. Green Synthesis of Silver Nanoparticles
2.3. Application of AgNPs to Rajshahi Silk
2.4. Measurements and Characterization
3. Results and Discussion
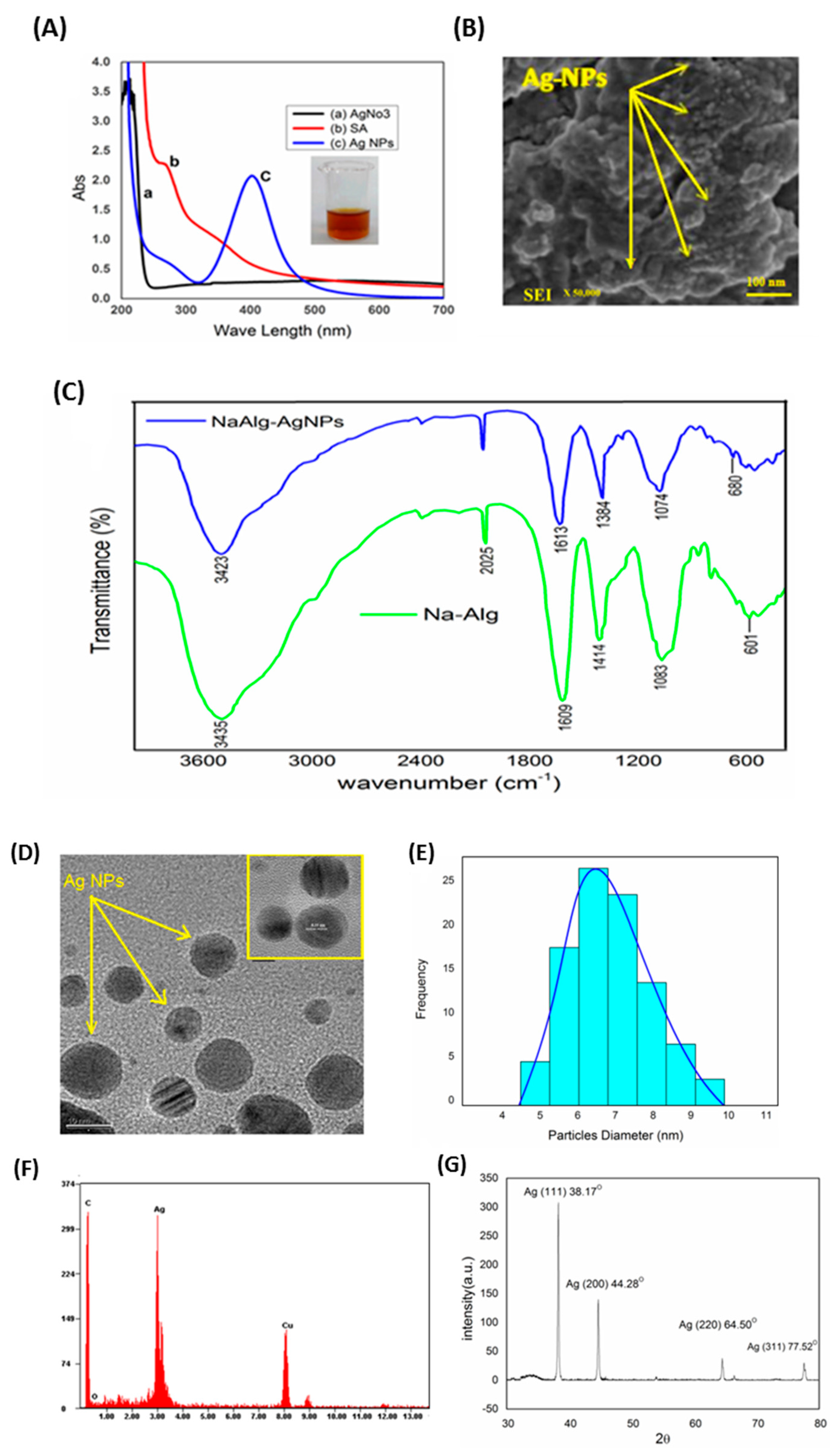
3.1. Characterization of AgNPs
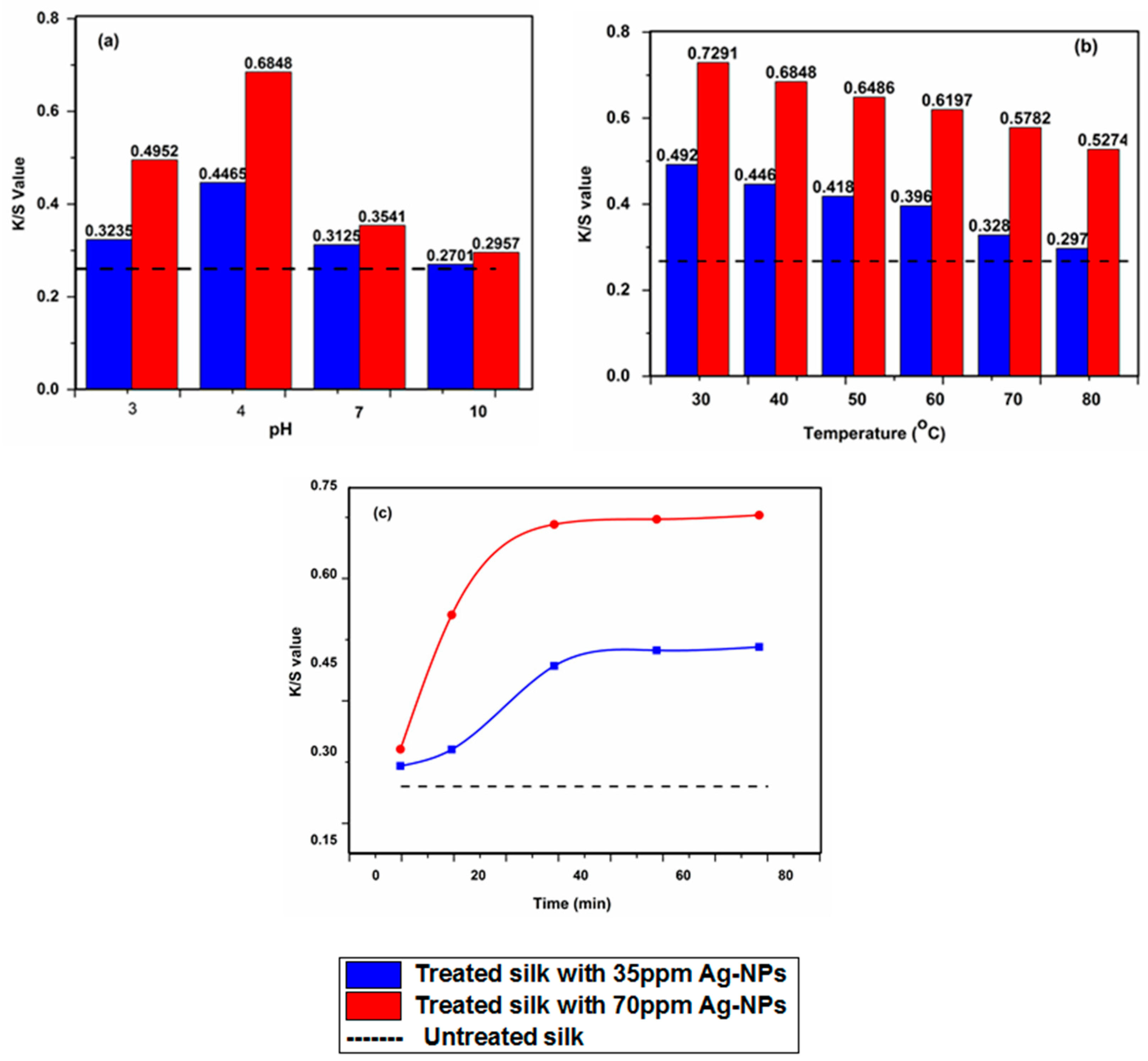
3.2. Optimization of Silk Treatment Conditions
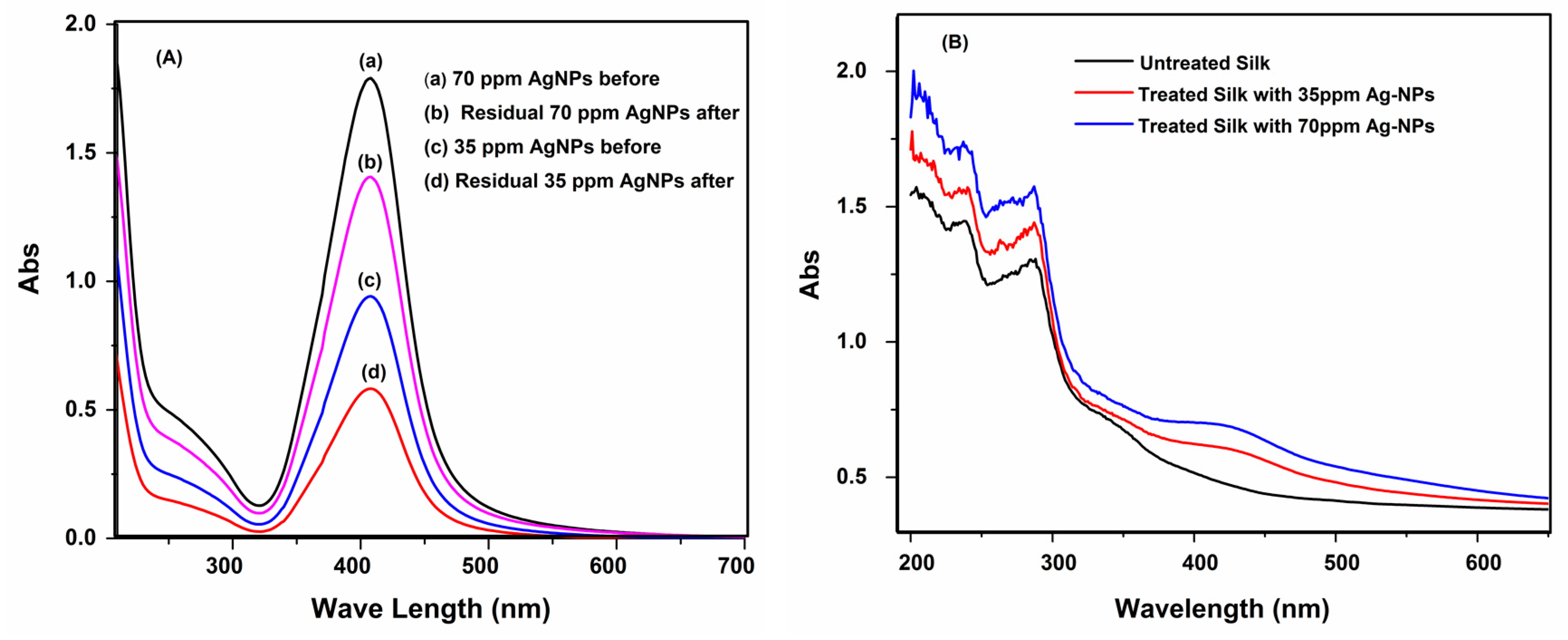
3.3. Absorption Characteristics
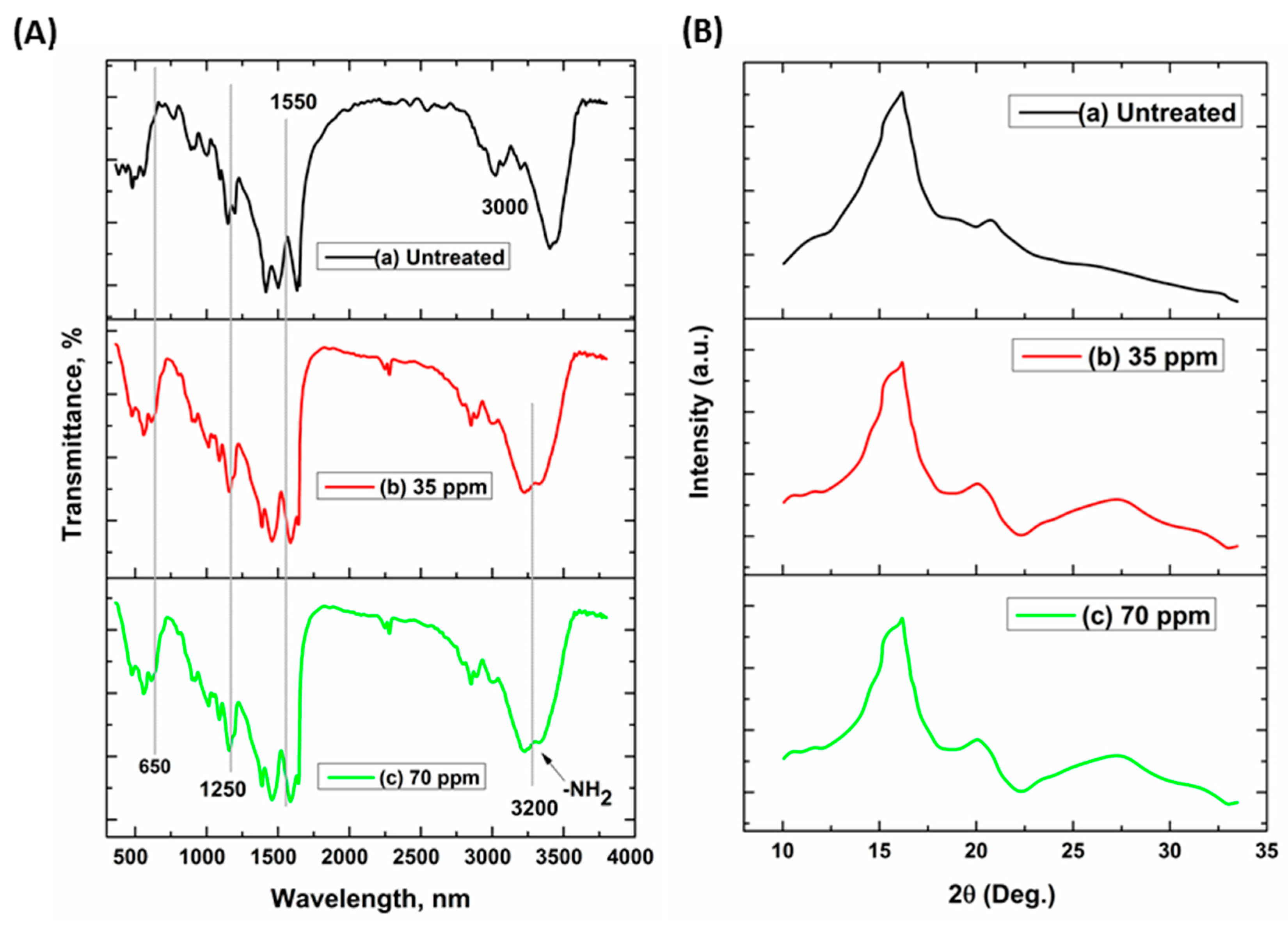
3.4. FT-IR and XRD Analysis
3.5. Thermal Properties
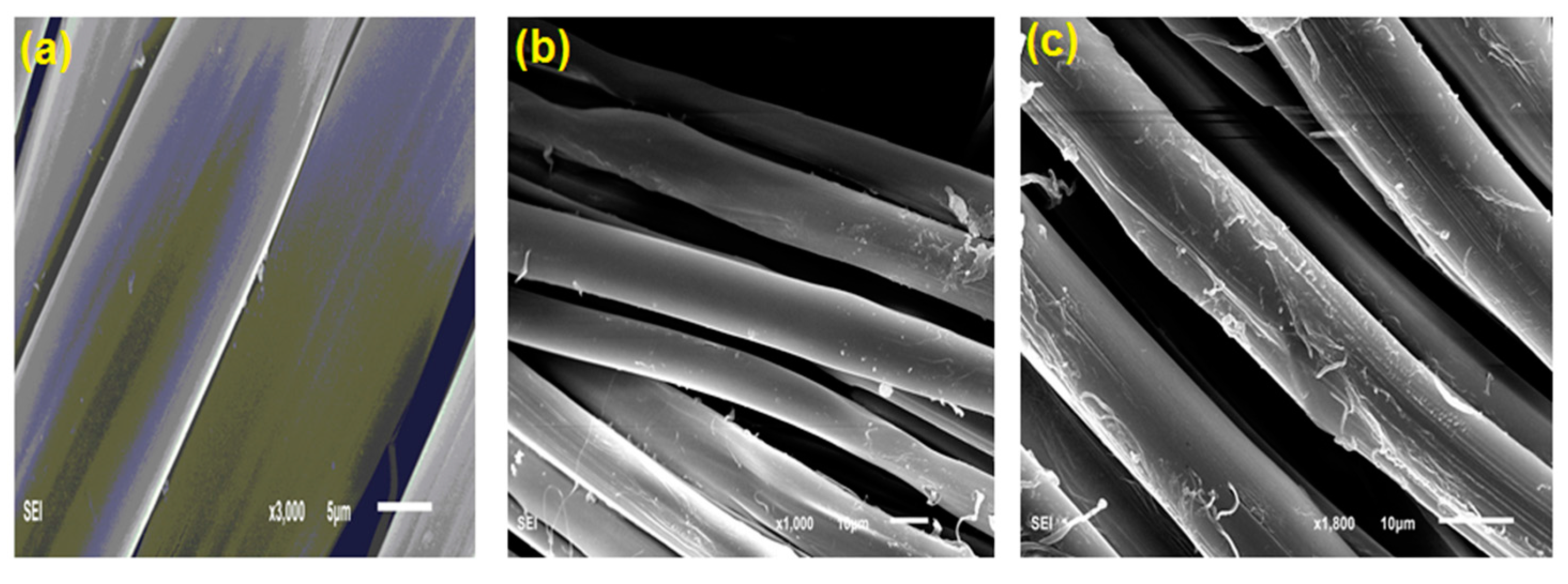
3.6. Scanning Electron Microscope (SEM)
3.7. Color Measurements and Fastness
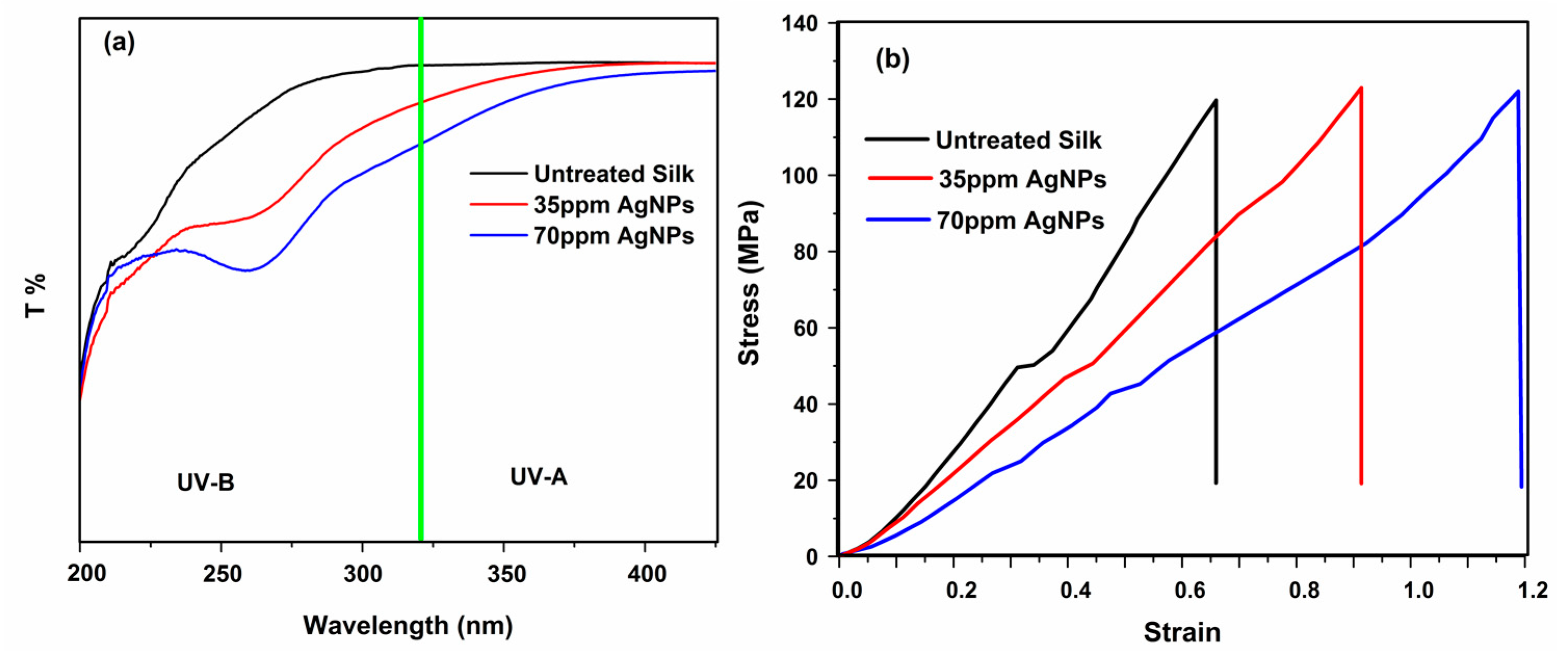
3.8. UV Protection
3.9. Antibacterial Activity
3.10. Surface Wettability
3.11. Mechanical Properties
3.12. Physical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dubey, S.P.; Lahtinen, M.; Särkkä, H.; Sillanpää, M. Bioprospective of sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B 2010, 80, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, J.; Song, N.; Li, Q.; Yang, B.; Dai, Y. Study on silver nanopowder prepared by chemical reduction with an organic reductant. Guijinshu (Pre. Met.) 2006, 27, 14–17. [Google Scholar]
- Choi, N.; Seo, D.; Lee, J. Preparation and stabilization of silver colloids protected by surfactant. Mater. Sci. For. 2005, 29, 394–396. [Google Scholar]
- Jiangmei, Y.; Huiwang, T.; Muling, Z.; Jun, T.; Zhang, S.; Zhiying, Y.; Wei, W.; Jiaqiang, W. PVP-capped silver nanoparticles as catalyst for oxidative coupling of thiols to disulfides. Chin. J. Catal. 2009, 30, 856–858. [Google Scholar]
- Setua, P.; Chakraborty, A.; Seth, D.; Bhatta, M.U.; Satyam, P.; Sarkar, N. Synthesis, optical properties, and surface enhanced raman scattering of silver nanoparticles in nonaqueous methanol reverse micelles. J. Phys. Chem. C 2007, 111, 3901–3907. [Google Scholar] [CrossRef]
- Bonilla, J.J.A.; Guerrero, D.J.P.; Sáez, R.G.T.; Ishida, K.; Fonseca, B.B.; Rozental, S.; López, C.C.O. Green synthesis of silver nanoparticles using maltose and cysteine and their effect on cell wall envelope shapes and microbial growth of Candida spp. J. Nanosci. Nanotechnol. 2017, 17, 1729–1739. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, Y.; Weyers, A.; Kim, Y.; Linhardt, R. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, S.; Fang, Y. Viscosity study of interactions between sodium alginate and ctab in dilute solutions at different ph values. Carbohydr. Polym. 2009, 75, 333–337. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, M.; Ahmad, M.B.; Abdullah, A.H.; Ibrahim, N.A.; Shameli, K. Effect of accelerator in green synthesis of silver nanoparticles. Int. J. Mol. Sci. 2010, 11, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xiao, J.; Wang, Y.; Meng, M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 2015, 452, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.R.K.; Farouqui, A.N.; Yahya, R.; Hassan, A. Effect of acid modification on dyeing properties of rajshahi silk fabric with different dye classes. Fibers Polym. 2011, 12, 642–647. [Google Scholar] [CrossRef]
- Zhang, D.; Toh, G.W.; Lin, H.; Chen, Y. In situ synthesis of silver nanoparticles on silk fabric with PNP for antibacterial finishing. J. Mater. Sci. 2012, 47, 5721–5728. [Google Scholar] [CrossRef]
- Naik, R.; Wen, G.; Hureau, S.; Uedono, A.; Wang, X.; Liu, X.; Cookson, P.G.; Smith, S.V. Metal ion binding properties of novel wool powders. J. Appl. Polym. Sci. 2010, 115, 1642–1650. [Google Scholar] [CrossRef]
- Yin, H.; Ai, S.; Shi, W.; Zhu, L. A novel hydrogen peroxide biosensor based on horseradish peroxidase immobilized on gold nanoparticles–silk fibroin modified glassy carbon electrode and direct electrochemistry of horseradish peroxidase. Sens. Actuators B 2009, 137, 747–753. [Google Scholar] [CrossRef]
- Gulrajani, M.; Gupta, D.; Periyasamy, S.; Muthu, S. Preparation and application of silver nanoparticles on silk for imparting antimicrobial properties. J. Appl. Polym. Sci. 2008, 108, 614–623. [Google Scholar] [CrossRef]
- Pervez, M.N.; Rahman, M.A.; Yu, L.; Cai, Y. A novel study on uv protection and antibacterial properties of washed denim garment. MATEC Web Conf. 2017, 108, 03004. [Google Scholar] [CrossRef]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.; Ávalos-Borja, M.; Castillón-Barraza, F.; Posada-Amarillas, A. Classical theoretical characterization of the surface plasmon absorption band for silver spherical nanoparticles suspended in water and ethylene glycol. Phys. E Low-Dimen. Syst. Nanostruct. 2005, 27, 104–112. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C. Light emitting diode irradiation can control the morphology and optical properties of silver nanoparticles. J. Am. Chem. Soc. 2010, 132, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.; Al-Deyab, S.S. Utilization of hydroxypropyl cellulose for green and efficient synthesis of silver nanoparticles. Carbohydr. Polym. 2011, 86, 1615–1622. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, S.; Dilbaghi, N.; Bhanjana, G.; Chopra, M.; Kaur, H.; Kumar, R.; Manuja, B.K.; Singh, S.K.; Yadav, S.C. Quinapyramine sulfate-loaded sodium alginate nanoparticles show enhanced trypanocidal activity. Nanomedicine 2014, 9, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Jiron, G.; Leal, D.; Matsuhiro, B.; Osorio-Roman, I. Vibrational spectroscopy and density functional theory calculations of poly-d-mannuronate and heteropolymeric fractions from sodium alginate. J. Raman Spectrosc. 2011, 42, 870–878. [Google Scholar] [CrossRef]
- Sartori, C.; Finch, D.S.; Ralph, B.; Gilding, K. Determination of the cation content of alginate thin films by FTi.r. Spectroscopy. Polymer 1997, 38, 43–51. [Google Scholar] [CrossRef]
- Mandal, A.; Sekar, S.; Chandrasekaran, N.; Mukherjee, A.; Sastry, T.P. Vibrational spectroscopic investigation on interaction of sago starch capped silver nanoparticles with collagen: A comparative physicochemical study using ft-ir and ft-raman techniques. RSC Adv. 2015, 5, 15763–15771. [Google Scholar] [CrossRef]
- Devaraj, P.; Kumari, P.; Aarti, C.; Renganathan, A. Synthesis and characterization of silver nanoparticles using cannonball leaves and their cytotoxic activity against mcf-7 cell line. J. Nanotechnol. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- El-Rafie, M.; El-Naggar, M.; Ramadan, M.; Fouda, M.M.; Al-Deyab, S.S.; Hebeish, A. Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization. Carbohydr. Polym. 2011, 86, 630–635. [Google Scholar] [CrossRef]
- Mohammed Fayaz, A.; Balaji, K.; Girilal, M.; Kalaichelvan, P.; Venkatesan, R. Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. J. Agric. Food Chem. 2009, 57, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using cocos nucifera coir extract and its larvicidal activity. Ind. Crop. Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Colonna, G.; Scotti, E.; Boschi, A.; Murakami, R.; Tsukada, M. Absorption of metal cations by modified B. mori silk and preparation of fabrics with antimicrobial activity. J. Appl. Polym. Sci. 2001, 80, 297–303. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Li, T.; Wang, J. Surface modification and functionalization of silk fibroin fibers/fabric toward high performance applications. Mater. Sci. Eng. C 2012, 32, 627–636. [Google Scholar] [CrossRef]
- Sur, I.; Altunbek, M.; Kahraman, M.; Culha, M. The influence of the surface chemistry of silver nanoparticles on cell death. Nanotech 2012, 23, 375102. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Freddi, G.; Innocenti, R.; Kaplan, D.; Tsukada, M. Acylation of silk and wool with acid anhydrides and preparation of water-repellent fibers. J. Appl. Polym. Sci. 2001, 82, 2832–2841. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Zhao, C. Preparation of chitosan–nylon-6 blended membranes containing silver ions as antibacterial materials. Carbohydr. Res. 2008, 343, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Li, M.; Zhao, C. Structure and properties of regenerated antheraea pernyi silk fibroin in aqueous solution. Int. J. Biol. Macromol. 2007, 40, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Kang, B.; Dai, Y.; Chen, D. Synthesis of antimicrobial silver nanoparticles on silk fibers via γ-radiation. J. Appl. Polym. Sci. 2009, 112, 2511–2515. [Google Scholar] [CrossRef]
- Zhang, H.; Magoshi, J.; Becker, M.; Chen, J.Y.; Matsunaga, R. Thermal properties of bombyx mori silk fibers. J. Appl. Polym. Sci. 2002, 86, 1817–1820. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, D.; Park, W.H.; Lee, S.G.; Han, S.O.; Drzal, L.T. Novel silk/poly (butylene succinate) biocomposites: The effect of short fibre content on their mechanical and thermal properties. Compos. Sci. Technol. 2005, 65, 647–657. [Google Scholar] [CrossRef]
- Asakura, T. Structural characteristics of silk fibroin and the application as enzyme-immobilized material. Bioindustry 1987, 4, 878–886. [Google Scholar]
- Shin, H.S.; Yang, H.J.; Kim, S.B.; Lee, M.S. Mechanism of growth of colloidal silver nanoparticles stabilized by polyvinyl pyrrolidone in γ-irradiated silver nitrate solution. J. Colloid Interface Sci. 2004, 274, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Mowafi, S.; Rehan, M.; Mashaly, H.M.; Abou El-Kheir, A.; Emam, H.E. Influence of silver nanoparticles on the fabrics functions prepared by in-situ technique. J. Text. Inst. 2017, 108, 1–12. [Google Scholar] [CrossRef]
- Emam, H.E.; Bechtold, T. Cotton fabrics with uv blocking properties through metal salts deposition. Appl. Surf. Sci. 2015, 357, 1878–1889. [Google Scholar] [CrossRef]
- Gorenšek, M.; Recelj, P. Nanosilver functionalized cotton fabric. Text. Res. J. 2007, 77, 138–141. [Google Scholar] [CrossRef]
- Xue, C.-H.; Chen, J.; Yin, W.; Jia, S.-T.; Ma, J.-Z. Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl. Surf. Sci. 2012, 258, 2468–2472. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Saito, M. Antibacterial, deodorizing, and uv absorbing materials obtained with zinc oxide (ZnO) coated fabrics. J. Coat. Fabr. 1993, 23, 150–164. [Google Scholar] [CrossRef]
- Drelich, J.; Marmur, A. Physics and applications of superhydrophobic and superhydrophilic surfaces and coatings. Surf. Innov. 2014, 2, 211–227. [Google Scholar] [CrossRef]
- Munakata, M.; Liu, S.Q.; Konaka, H.; Kuroda-Sowa, T.; Suenaga, Y.; Maekawa, M.; Nakagawa, H.; Yamazaki, Y. Syntheses, structures, and properties of intercalation compounds of silver (I) complex with [2.2] paracyclophane. Inorg. Chem. 2004, 43, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of nanoparticle incorporation and surface coating on mechanical properties of bone scaffolds: A brief review. J. Funct. Biomater. 2016, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.; Kan, C. Influence of low temperature plasma treatment on the properties of ink-jet printed cotton fabric. Fibers Polym. 2007, 8, 168–173. [Google Scholar] [CrossRef]




| Sample | L* | A* | B* | K/S | CFL 1 | CFW 2 |
|---|---|---|---|---|---|---|
| Untreated silk | 89.97 | −0.07 | 8.54 | 0.2603 | - | - |
| 35 ppm AgNPs | 83.68 | 1.72 | 15.43 | 0.4465 | 6 | 4–5 |
| 70 ppm AgNPs | 81.93 | 0.77 | 22.23 | 0.6848 | 6 | 4–5 |
| Samples | Bacterial Reduction (%) | Zone of Inhibition (mm) | ||
|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | |
| Commercial | 95 ± 2.2 | 97 ± 4.2 | 24 ± 1.04 | 29 ± 0.52 |
| 35 ppm AgNPs | 88 ± 1.10 | 91 ± 2.08 | 22 ± 0.44 | 24 ± 0.66 |
| 70 ppm AgNPs | 90 ± 2.12 | 94 ± 4.06 | 23 ± 0.38 | 26 ± 0.46 |
| Samples | Ag Content (mg/kg) | CRA (deg) | Bending Length (cm) | Yellowness Index |
|---|---|---|---|---|
| Untreated Silk | - | 124 | 2.30 | 22 |
| 35 ppm AgNPs-treated Silk | 7125 ± 15 | 132 (±6.02) | 2.46 (±6.00) | 65 |
| 70 ppm AgNPs-treated Silk | 8362 ± 23 | 135 (±5.55) | 2.53 (±5.02) | 82 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, S.; Sultana, M.Z.; Pervez, M.N.; Habib, M.A.; Liu, H.-H. Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles. Fibers 2017, 5, 35. https://doi.org/10.3390/fib5030035
Mahmud S, Sultana MZ, Pervez MN, Habib MA, Liu H-H. Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles. Fibers. 2017; 5(3):35. https://doi.org/10.3390/fib5030035
Chicago/Turabian StyleMahmud, Sakil, Mst. Zakia Sultana, Md. Nahid Pervez, Md. Ahsan Habib, and Hui-Hong Liu. 2017. "Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles" Fibers 5, no. 3: 35. https://doi.org/10.3390/fib5030035







