3.1. Amino Acid, Moisture, Oil, and Protein Evaluation
As shown in
Table 2 the protein, oils, and moisture content varied somewhat among the DDGS, LPC, and PPC matrices. The protein quality and quantity in various matrices is considered to be the major factor responsible for their adhesive properties [
26,
27,
28,
30,
31,
32]. For the DDGS matrix fractions, DDGS/E matrix exhibited the highest protein concentration (33.1%) while DDGS matrix had the lowest (26.6%). Since this study is interested in developing an adhesive product from these materials, we compared their protein contents to soybeans, which are the commercial standards for bio-adhesives. Protein adhesives (e.g., animal protein, caseins, and soy flours) have been employed to bond wood products for several years [
30]. The DDGS and press cake materials have considerably less protein than soybean materials (
Table 2). Prolia and SPI contain 52% and 89% protein, respectively. The final calculated amount of protein in the composite panels for the by-product matrices varied from 13.3 (DDGS) to 17.5% (PPC/E), while the composite panels employing SBM and SPI were calculated to contain 24.3% and 44.9%, respectively.
Moisture content in the ingredients is important, because excess water disrupts production speed and product quality [
30]. Similarly, we found that employment of ingredients high in moisture (25–30%) resulted in unsatisfactory panels that exhibited blistering and cracking. This was caused by excessive steam and water explusion during the molding process. Ingredients contained a relatively low moisture content of less than 9%. As shown in
Table 2, the moisture content of DDGS, LPC, and PPC varied. The amino acid profiles of the various materials studied herein are presented in
Table 3.
The percentages of amino acid varied somewhat among the press cakes, DDGS, and SBM. The functional groups of amino acids of the proteins interact with reinforcement material (wood) and contribute toward adhesion [
47]. Amino acids are often classified into general groups based on their polarity and charge [
47,
48]. DDGS were found to have a higher percentage of non-polar hydrophobic amino acids versus polar hydrophilic amino acids than PPC, LPC, or SBM. The individual amino acid profiles are given only for archival information, since we do not know how these amino acid functional groups are oriented in the protein types.
Essentially, there are four general protein types in plant seeds, and the specific type and quantity is distinct for each species (
Table 4) [
43,
44,
45,
46,
47]. Therefore, the bio-based adhesive composition is composed of a distinct protein profile (
Table 4). For example, SBM consists of 80% globulins (glycinin and conglycinin) [
30,
47], while for DDGS 50% of the proteins were prolamins (specifically zein) [
43]. The main protein types in PPC and LPC were albumins, globulins, and glutelins [
44,
45].
Very little is actually known as to how these plant proteins manifest adhesive properties, although models have been proposed [
30,
47,
48,
49,
50,
51,
52]. Seed proteins exist in quaternary structures that have little or no adhesive properties. Seed proteins are characterized as globular structures with a hydrophobic interior [
48]. Denaturation of these proteins causes them to unfold into tertiary and secondary structures, allowing them to express interior amino acids. In this study, seed proteins subjected to high temperatures and pressure were able denature and express adhesive properties. As the proteins denature or unfold, the hydrophilic and hydrophobic groups of the amino acids interact with wood carbohydrate portion through hydrogen bonding, Van der Waal forces, and cross linking to create adhesion [
47,
48].
We attribute the majority of the extractables to residual oils. The type of extraction method affecting extraction yields (i.e., weight of material removed from the original seed meal) for each seed meal is shown in
Table 5. The hexane extraction method (H) probably extracted primarily residual oils. When SC-CO
2 was employed, lesquerella exhibited the highest extraction yield, while DDGS exhibited the lowest yield. This suggests that lesquerella contains more non-polar material that is extracted by the non-polar SC-CO
2. However, when ETOH was employed, DDGS gave the highest yield, while pennycress gave the lowest yield. We can attribute this to the removal of the residual corn oil, proteins (zein), solubles, and carbohydrates from DDGS. Winkler et al. [
53] similarly reported that ETOH extraction gave higher yields for DDGS than using hexane or SC-CO
2 extraction. DDGS apparently contain a higher amount of polar compounds that are removed by the relatively polar ethanol.
3.2. Adhesive Properties of Materials
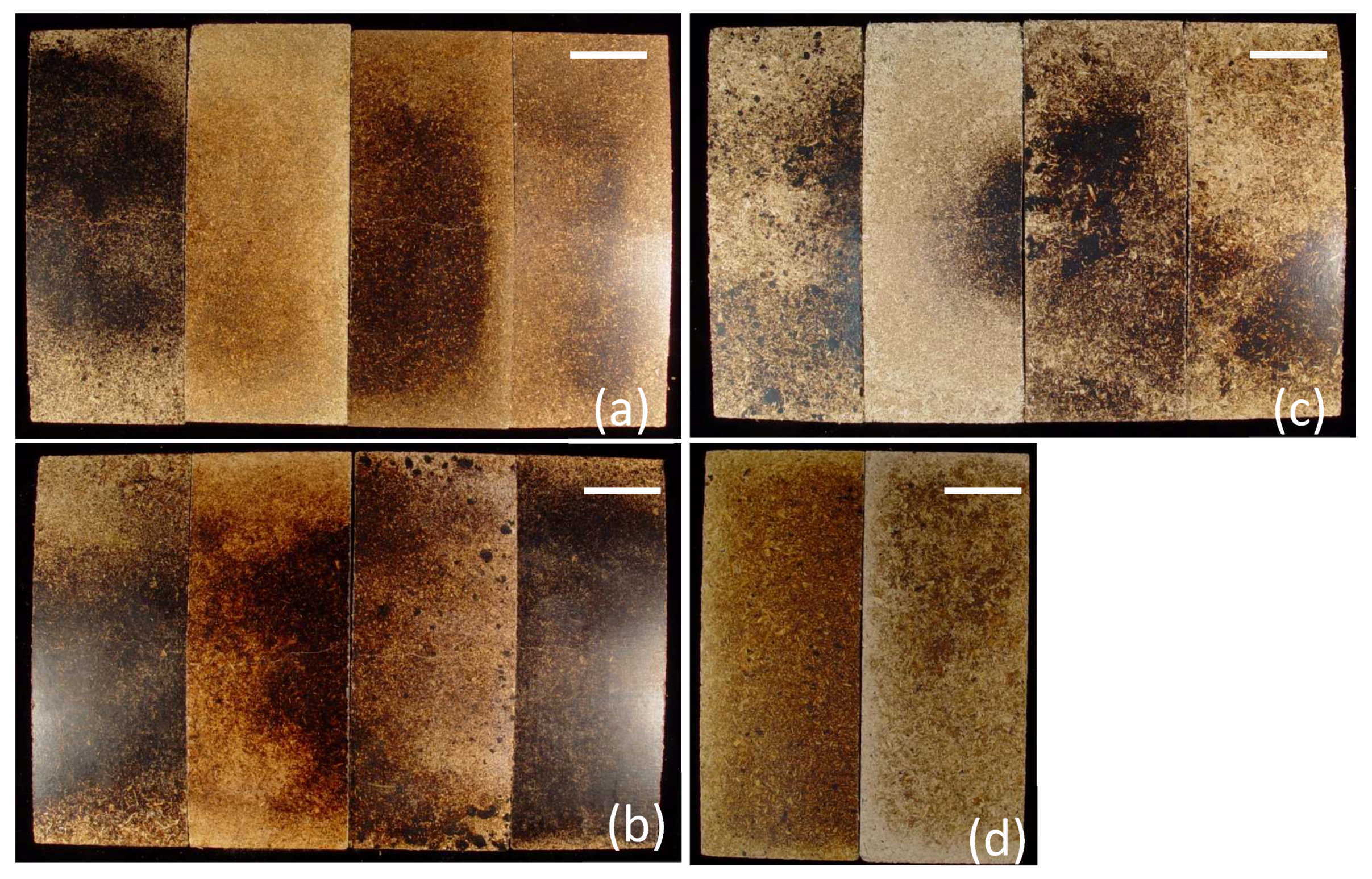
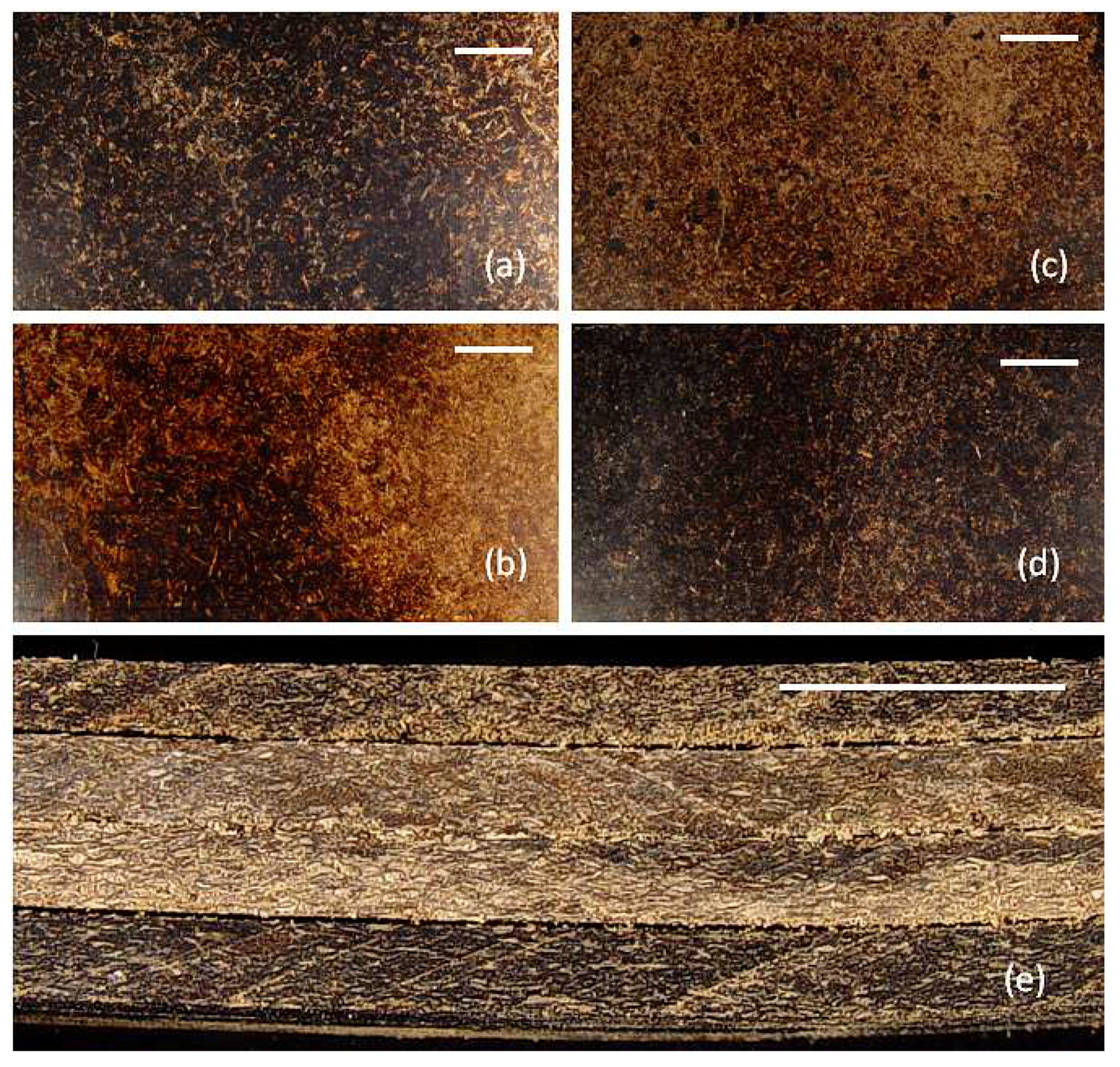
The optical images of the flexural panels provide information concerning how the matrix and reinforcement materials interact (
Figure 1 and
Figure 2). The surface portions of the panels had a smooth topography and did not exhibit any obvious imperfections. Visible differences between the composites were mainly those of color (
Figure 1 and
Figure 2). The dark portions of the panels probably represent over-concentration of the matrix materials. Generally, composites containing the original untreated matrices were usually darker in color than the composites containing the treated matrices. The cross sections revealed color changes for the matrices. For example, the LPC-PW and LPC/H-PW composites were considerably darker than the LPC/E-PW and LPC/CO
2-PW composites (
Figure 1 and
Figure 2). The sawn cross sections of composites also had a smooth topography (
Figure 2). Wood particles were immersed in the matrices in a random manner. Clumping of wood particles was not observed, which indicates adequate distribution of the matrices and wood particles within the composites.
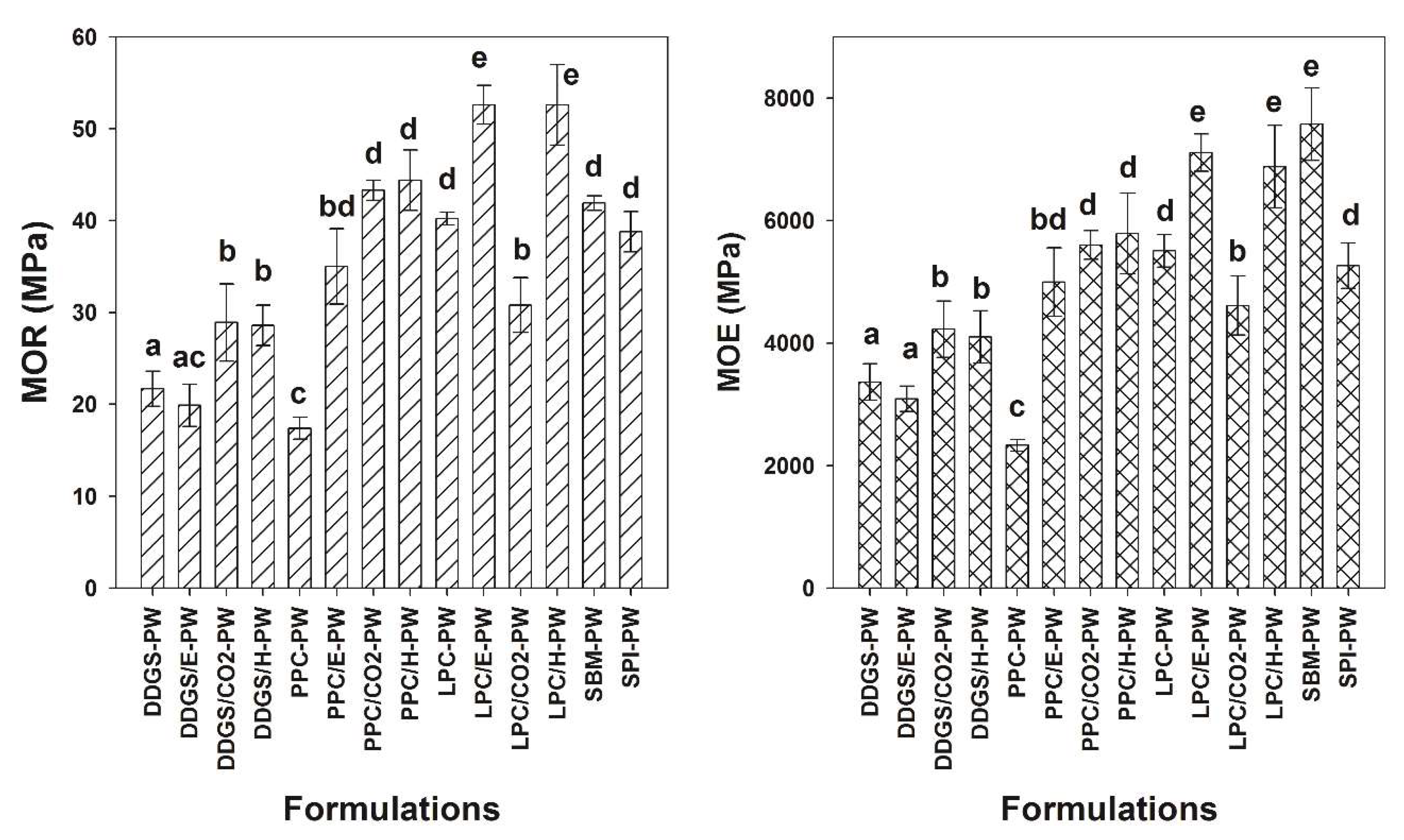
Figure 3 shows the flexural properties of panels fabricated in this study. The method of extraction employed on the matrix material had significant effects on the resulting panels’ flexural properties. Generally, superior flexural properties were obtained from composites when the matrix material was subjected to extraction methods versus the untreated original matrix materials. For example, DDGS-PW panels exhibited modulus of rupture (MOR) and modulus of elasticity (MOE) values of 21.7 ± 1.9 MPa and 3365 ± 300 MPa, respectively, while DDGS/H-PW composite panels exhibited MOR and MOE values of 28.9 ± 2.2 MPa and 4965 ± 460 MPa, respectively. This translates in into percent increase in MOR and MOE values of +32% and +22%, respectively. For DDGS composites, no difference in flexural properties occurred comparing DDGS-PW to DDGS/E-PW, while significantly higher flexural properties were obtained from DDGS/CO
2-PW and DDGS/H-PW composites (
Figure 3). In the case of DDGS/E-PW, the ethanol extraction appeared to be considerably less effective, probably due to the inadvertent removal of both carbohydrates and prolamin (zein) proteins. It is interesting to note that although this treatment (DDGS/E-PW) increased overall protein content, it still did not enhance the flexural properties over the untreated DDGS-PW composite. It is well documented that ethanol solvent is notable for removing prolamins in plant seeds [
43,
44,
45,
46,
47]. The H and SC-CO
2 extraction generated a DDGS matrix material that had greater interfacial adhesion to the wood materials. We attribute this result to the effective removal of residual oils by these treatments. This situation is somewhat similar to that observed in hexane extraction of the DDGS results in a superior plastic composite when compared to plastic composites containing untreated DDGS [
34].
For the pennycress composites, the untreated control (PPC-PW) exhibited the lowest flexural properties compared to the composites resulting from treated substrates (
Figure 3). For example, the MOR and MOE of PPC/E-PW, PPC/CO
2-PW, and PPC/H-PW composites were +101% and +114%, +149% and +140%, and +155% and +148% greater, respectively, than the MOR and MOE values of PPC-PW composite. However, the PPC/E-PW composite exhibited lower flexural values compared to the flexural properties exhibited by PPC/CO
2-PW and PPC/H-PW composites. We attribute this situation to the type of extraction method employed. Reifschneider et al. [
21] reported superior mechanical performance of PPC-plastic composites if the oils were removed by hexane compared to composites prepared with PPC containing oils. Clearly, removal of oils from the agriculture by-products improves the resultant composite’s performance (
Figure 3).
The highest MOE and MOR values were obtained from the LPC compared to the DDGS and PPC composites. For example, MOR and MOE values of LCP-PW were +85% and +163% greater than MOR and MOE values of DDGS-PW. This suggests that LCP may have some additional adhesive properties over those of the other two materials tested that not attributed to the protein content. This observed characteristic of the LPC matrix can be rationalized from earlier reported composition of the seed polysaccharide, namely, the seed surface gum, an arabinogalactan comprising some 15% galacturonic acid residues that enhance viscosity [
45,
54,
55,
56,
57,
58,
59]. It has been suggested that the polysaccharide gums found in lesquerella could be employed as an adhesive [
22,
23]. Apparently, these results confirm these earlier observations. The combination of the proteins (30–36%) with polysaccharide gums (15–20%) results in superior adhesive properties exhibited by LPC when compared to DDGS or PPC. Extraction of LPC by ethanol and hexane extraction resulted in composites (LPC/E-PW and LPC/H-PW) that had the highest flexural properties obtained in this study (
Figure 1). The MOR and MOE of LPC/E and LPC/H were +31% and +29% and +31 and +25% greater than the MOR and MOE values of LPC-PW composite. Interestingly, the supercritical fluid extraction method resulted in a composite (LPC/CO
2-PW) that had inferior flexural properties to all other LPC composites. Further research would be necessary to clarify why this particular method failed to improve the adhesive properties of LPC.
The highest MOE and MOR values were obtained from the LPC compared to the DDGS and PPC composites. For example, MOR and MOE values of LCP-PW were +85% and +163% greater than MOR and MOE values of DDGS-PW. This suggests that LCP may have some additional adhesive properties over those of the other two materials tested that not attributed to the protein content. This observed characteristic of the LPC matrix can be rationalized from earlier reported composition of the seed polysaccharide, namely, the seed surface gum, an arabinogalactan comprising some 15% galacturonic acid residues that enhance viscosity [
45,
54,
55,
56,
57,
58,
59]. It has been suggested that the polysaccharide gums found in lesquerella could be employed as an adhesive [
22,
23]. Apparently, these results confirm these earlier observations. The combination of the proteins (30–36%) with polysaccharide gums (15–20%) results in superior adhesive properties exhibited by LPC when compared to DDGS or PPC. Extraction of LPC by ethanol and hexane extraction resulted in composites (LPC/E-PW and LPC/H-PW) that had the highest flexural properties obtained in this study (
Figure 1). The MOR and MOE of LPC/E and LPC/H were +31% and +29% and +31 and +25% greater than the MOR and MOE values of LPC-PW composite. Interestingly, the supercritical fluid extraction method resulted in a composite (LPC/CO
2-PW) that had inferior flexural properties to all other LPC composites. Further research would be necessary to clarify why this particular method failed to improve the adhesive properties of LPC.
The extraction method employed to remove oils was found to profoundly affect the adhesive properties of DDGS and press cakes in the composites (
Figure 3). For DDGS, supercritical fluid extraction and hexane extraction produced superior composites, compared composites employing matrices obtained from no extraction, or ethanol extraction. In the case of Pennycress, supercritical fluid extraction and hexane again gave rise to superior composites compared to unextracted matrix or ethanol extraction. However, PPC/E-PW composites obtained from ethanol extraction were clearly superior to untreated controls (PPC-PW). Superior mechanical properties were obtained in Lesquerella composites that were derived from press cakes subjected to ethanol and hexane extraction compared to untreated control composites. Extraction of LPC by supercritical fluid extraction resulted in an inferior composite (LPC/CO
2-PW) compared to composites derived from the other treatments. These results suggest hexane extraction overall appears to be the most reliable method to treat DDGS and press cakes, while CO
2 and ethanol extraction may result in an inferior composite depending on the starting material employed.
A comparison between MOR and MOE values of composites produced from a matrix of DDGS, PPC, or LPC and composites of a matrix using SBM or SPI is shown in
Figure 3. The flexural properties of the SBM-PW composite were similar to several composites (e.g., PPC/CO
2-PW, PPC/H-PW, and LPC-PW). SBM and SPI matrix composites were superior to all DDGS composites (
Figure 3). For example, MOR and MOE MPa values of SBM-PW were 41.8 ± 0.8 and 7.575 ± 578, respectively, compared to MOR and MOE MPa values of DDGS/H-PW, which exhibited 28.6 ± 2.2 and 4.228 ± 427, respectively. It should be noted that SBM contains ~50% protein, while the hexane extracted DDGS, PPC, and LPC contained 29%, 31%, and 36% protein, respectively (
Table 2). This suggests that protein content may not be the only factor involved in the adhesive properties of these matrix materials. The SPI-PW composite contained ~45% proteins but did not exhibit greater flexural properties than the SBM-PW composite, which contained 24% protein or several other press cake composites (
Figure 3). Several PPC and LPC composites (i.e., PPC/CO
2-PW and LPC/E-PW) were found to exceed the SPI-PW composite in terms of flexural properties. Several investigators have demonstrated the adhesive properties of various commercially available soy flours and products [
30,
32,
48]. It is the contention of the authors that alternative agriculture by-products—namely, DDGS, PPC, and LPC—also have adhesive properties that may compete with soy flours.
It should be noted that those composite panels that exhibited higher densities and were thinner also exhibited higher mechanical properties compared to composite panels that had lower densities and were thicker (
Figure 3,
Table 6). All composite materials contained the same amount of materials and were fabricated in the same manner. The matrix material and method of extraction were responsible for the physical properties of the composite panel, which in turn influenced its mechanical properties. The panels employed in this study were unique in that they contained a high proportion of matrix material versus wood, i.e., 50:50 mixture. The matrix itself also contains a considerable amount of lignocellulosic material (~43–52%); thus, the the composite is composed of 73–79% lignocellulose and 13.3–17.5% protein (
Table 2). Our lignocellulosic panels had a density between 986 to 1.256 kg/m
3. Commercial panels usually contain 85–90% wood, with the remaining components being adhesives and additives. High density fiber boards (HDF) panels have a density (900–1000 kg/m
3), and medium density fiberboards (MDF) have a density of (600–1000 kg/m
3). HDF and MDF are generally marketed in thickness varying from 3 to 12 mm in thickness. The panels employed in this study roughly correspond to HDF boards.
3.3. Dimensional Stability
It is important to ascertain the dimensional stability of our engineered panels and compare them to panels employing recognized adhesives [
60,
61]. The water absorption (WA) and thickness swelling (TS) of the various composites are shown in
Table 6. Following 24 h of soaking composites, TS increased from 29 to 83%, while WA increased from 28 to 84% depending on the composite composition. Generally, there was a close association between the thickness and density of the TS and WA values (
Table 6 and
Table 7). However, the extraction method and matrix type also were contributing factors (
Table 6). For example, comparing some DDGS composites, DDGS/H-PW had a thickness of 3.55 ± 0.10 mm, and a density of 1.188 ± 47 kg/m
3 exhibited TS of 42 ± 4% and WA of 37 ± 3%, while DDGS/E-PW had a thickness of 4.28 ± 0.28 mm, and a density of 986 ± 280 kg/m
3 exhibited 83 ± 4% TS and 84 ± 12% WA. Interestingly, the soy flour composites, SPI-PW and SBM-PW, exhibited relatively high TS and WA comparable to other composites tested. These results suggest that high protein content in these composites imbibes water, which adversely affects the dimensional stability. The employment of hexane extraction was found to be the most effective treatment for the various composites to improve the mechanical, physical, and dimensional stability properties (
Figure 3,
Table 6). Soy flour adhesives are noted to be effective in terms of their strength when compared to synthetic adhesives but exhibit poor durability, since they are susceptible to water damage [
30,
32,
48,
49].
Pearson correlation coefficients comparing the physical properties (thickness and density) of composites with their response to water (TS and WA) are presented in
Table 7. There were high correlations between composite panel thickness and TS (0.775) and thickness and WA (0.898). The thicker the panel, the greater the TS and WA values. High negative correlations occur between panel density and TS (−0.753) and density and WA (−0.849). These correlations indicate a close relationship between the thickness and density of the panel and its dimensional stability.
Frihart et al. [
48] noted that for a new adhesive to be accepted commercially, it must satisfy several requirements: (1) it must be abundant and have consistent properties; (2) it should be used with the typical industrial processing conditions and equipment; (3) it must have a similar viscosity compatible with existing equipment for specific processes; (4) it must not alter with the moisture content of the wood product during processing; and (5) it must provide a finished product of adequate strength and stiffness as defined by industry standards. In the wood industry, three main classes of fiberboard material classification are: interior non-structural, exterior non-structural, and structural [
62,
63,
64]. In our case, the lignocellulosic panels produced from various agricultural by-products satisfy some of these commercial requirements, especially in terms of their flexural properties, and as such are comparable to soybean flours. However, the dimensional stability of the composite panels presented in this study precludes their use in exterior applications. According to the European standards for the nominal properties of PB, MDF, and HDF, TS values are 35% and less (typically 10–25%). Only two composites (PPC/CO
2-PW and PPC/H-PW) were able to achieve TS values that were of these dimensional stability standards. European standards for HDF nominal MOR and MOE properties vary from 30–44 MPa to 2500–4500 MPa, respectively [
62]. Several of the LC panels fabricated in this study satisfy these property requirements (
Figure 3). In addition, these agricultural by-product adhesives are abundant (or could be if commercialized), can be employed as a powder (as soy flour often is), and confer strength/stiffness in composite panels. The dimensional stability results suggest that these agricultural adhesives should be restricted to interior non-structural uses. More research is necessary to enhance the use of these agricultural by-products to make them commercially acceptable. However, these results are a promising beginning.