Sea Ice Microorganisms: Environmental Constraints and Extracellular Responses
Abstract
:1. Introduction

“It is a curious fact, and yet a well-known experience, to find that bacteria may live dormant in ice for prolonged periods, and that infection may be carried through ice, but it is not so generally recognized that some bacteria prefer to grow on ice. Microorganisms, as a rule, are capable of resisting a low temperature when their ordinary activities cease, and they tend, either as single units or in clusters, to throw out a mucilaginous protein substance for their protection.”
2. Sea Ice as a Microbial Environment
2.1. Incorporation into Sea Ice
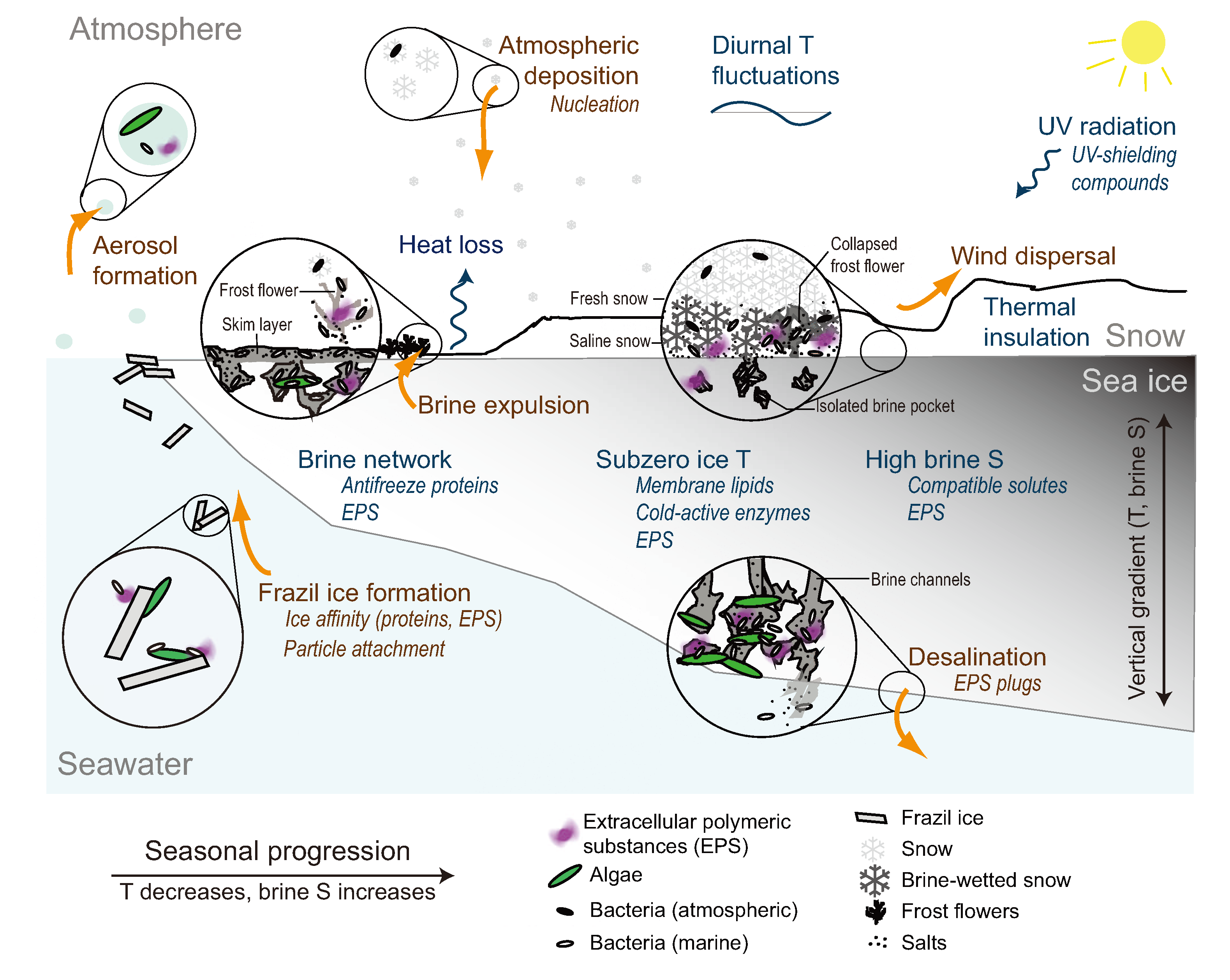
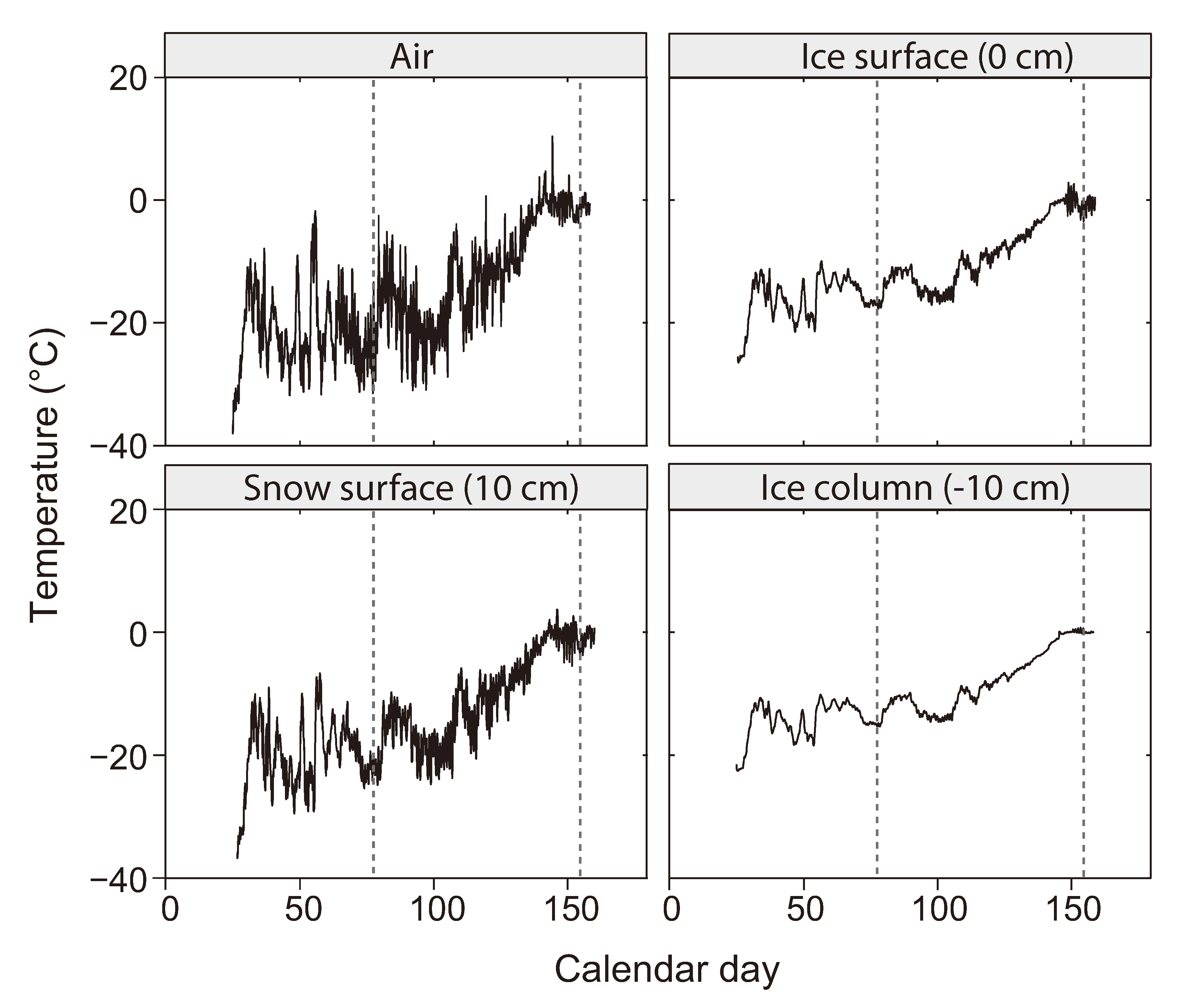
2.2. The Low Temperature Constraint
2.3. The Brine Channel Network
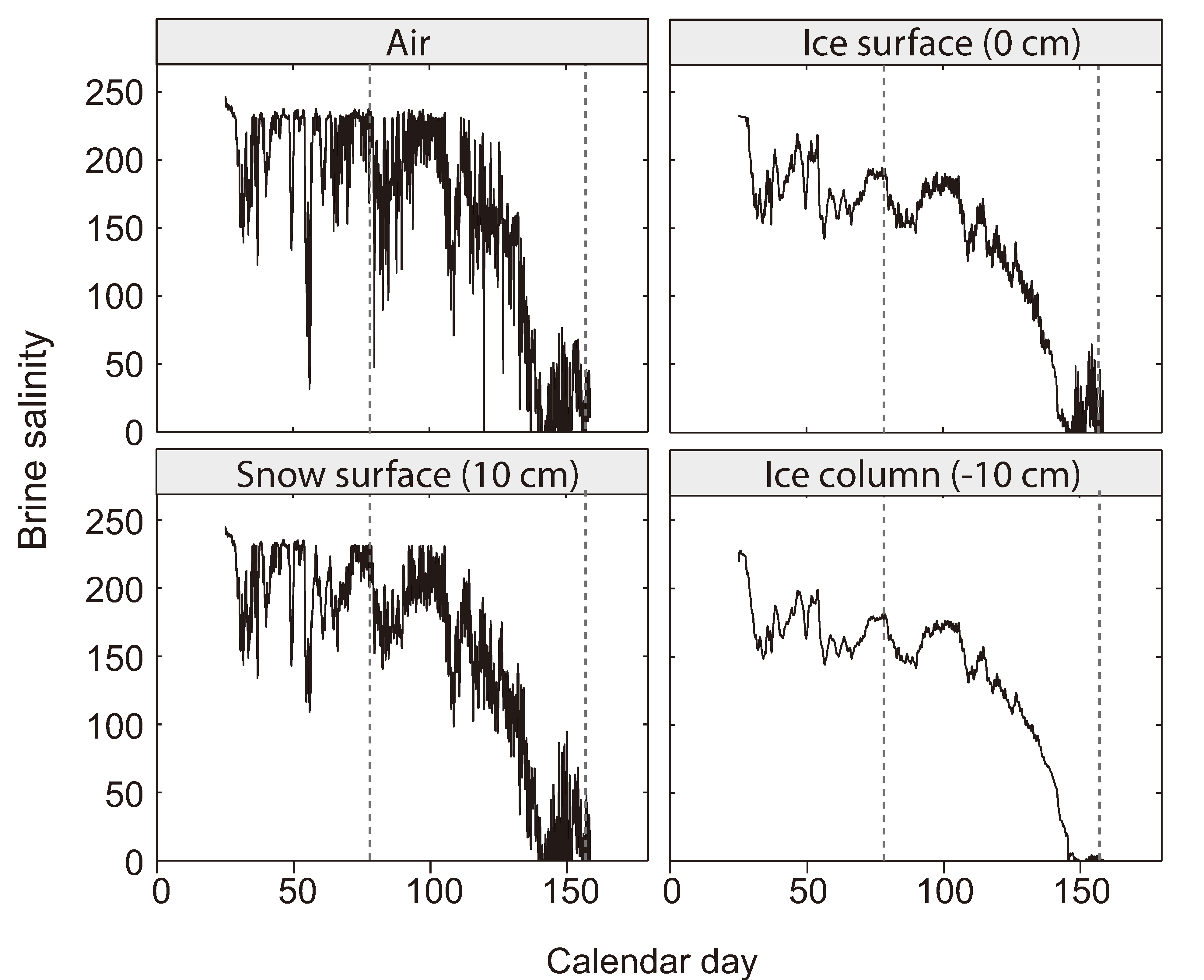
2.4. The Brine Salinity Constraint
| Organism | Membrane | Cytoplasmic | Environment |
|---|---|---|---|
| Extremely halophilic | |||
| Halobacterium salinarum | 3.88 | 16.8 | Highly saline lakes |
| Halophilic | |||
| Psychrobacter cryohalolentis | 2.22 | 3.27 | Cryopeg |
| Roseobacter denitrificans | 2.22 | 3.00 | Marine |
| Psychrobacter arcticus | 2.14 | 3.34 | Permafrost |
| Sphingopyxis alaskensis | 1.90 | 2.23 | Marine |
| Shewanella frigidimarina | 1.52 | 2.90 | Marine, sea ice |
| Colwellia psychrerythraea | 1.47 | 3.17 | Marine sediments, sea ice |
| Shewanella oneidensis | 1.47 | 3.26 | Anaerobic sediments |
| Marinobacter aquaeolei | 1.42 | 3.13 | Marine |
| Oceanobacillus iheyensis | 1.15 | 3.78 | Marine sediments |
| Psychromonas ingrahamii | 1.10 | 2.00 | Sea ice / water interface |
| Non-halophilic | |||
| Flavobacterium psychrophilum | 0.75 | 1.44 | Freshwater fish |
| Lactococcus lactis subsp. lactis | 0.74 | 4.03 | Gut flora |
| Sphingomonas wittichii | 0.69 | 2.28 | River |
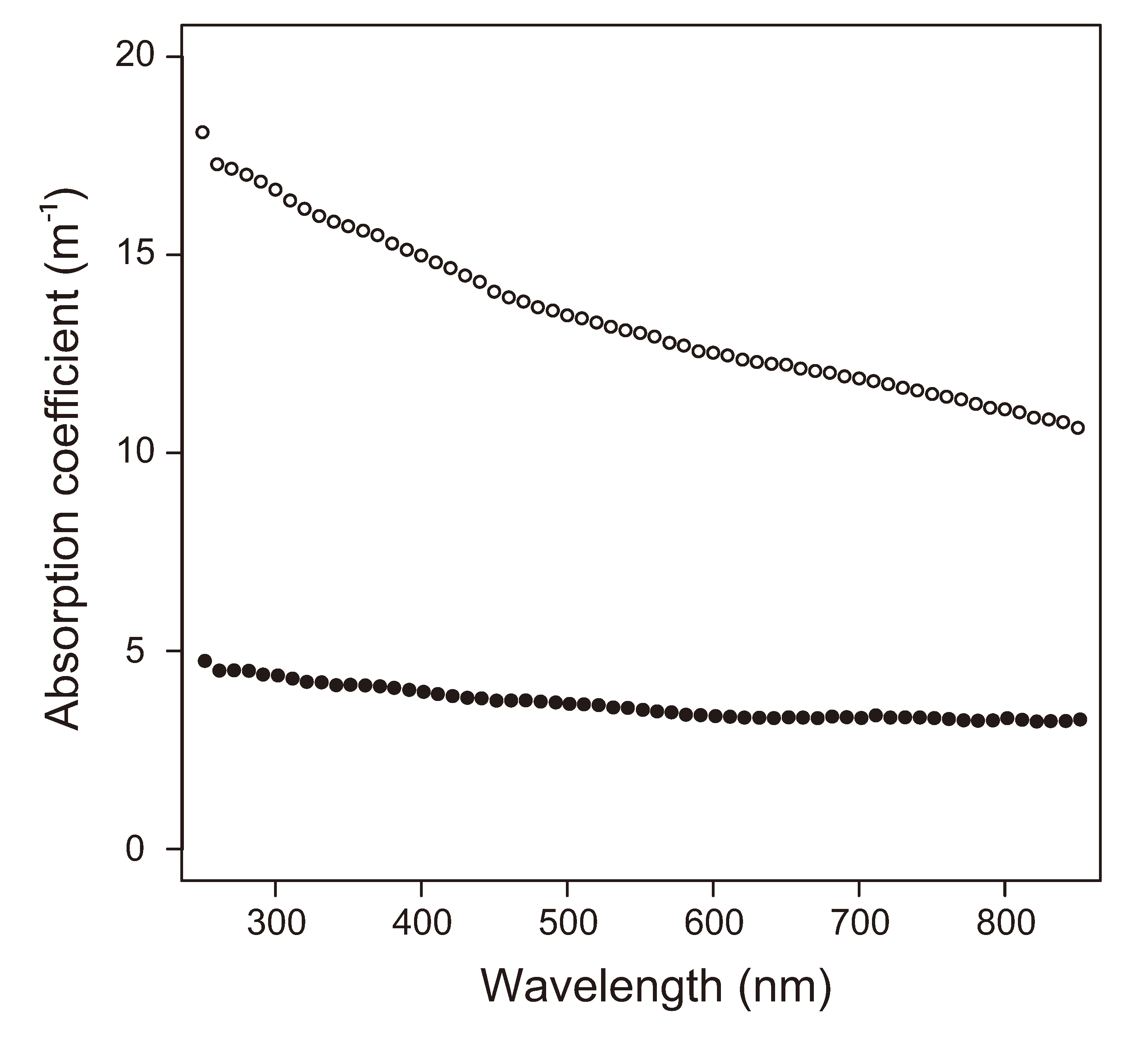
2.5. Insolation
3. Extracellular Responses to Sea-Ice Environmental Constraints
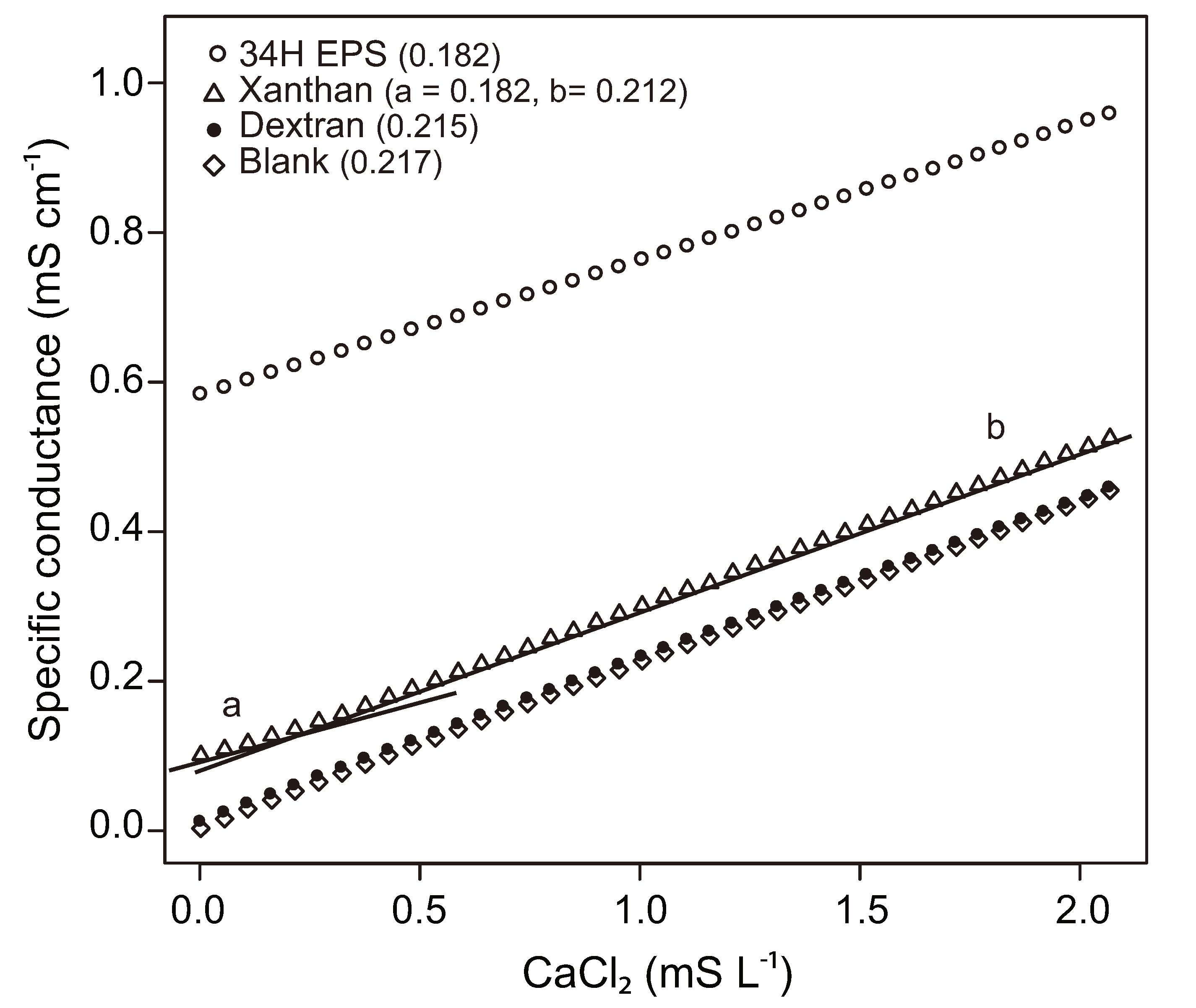
3.1. Extracellular Polymeric Substances
3.2. EPS in Sea Ice
3.3. Influence of EPS on Physical-Chemical Properties of Sea Ice
4. Prospectus for Future Research
Acknowledgements
References
- Junge, K.; Eicken, H.; Deming, J.W. Bacterial activity at –2 to –20 °C in Arctic wintertime sea ice. Appl. Environ. Microbiol. 2004, 70, 550–557. [Google Scholar] [CrossRef]
- Søgaard, D.H.; Kristensen, M.; Rysgaard, S.; Glud, R.N.; Hansen, P.J.; Hilligsøe, K.M. Autotrophic and heterotrophic activity in Arctic first-year sea ice: Seasonal study from Malene Bight, SW Greenland. Mar. Ecol. Prog. Ser. 2010, 419, 31–45. [Google Scholar] [CrossRef]
- Brown, M.V.; Bowman, J.P. A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microbiol. Ecol. 2001, 35, 267–275. [Google Scholar] [CrossRef]
- Brinkmeyer, R.; Knittel, K.; Jürgens, J.; Weyland, H.; Amann, R.; Helmke, E. Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl. Environ. Microbiol. 2003, 69, 6610–6619. [Google Scholar] [CrossRef]
- Bowman, J.S.; Rasmussen, S.; Blom, N.; Deming, J.W.; Rysgaard, S.; Sicheritz-Ponten, T. Microbial community structure of Arctic multiyear sea ice and surface seawater by 454 sequencing of the 16S RNA gene. ISME J. 2012, 6, 11–20. [Google Scholar] [CrossRef]
- Maas, E.W.; Simpson, A.M.; Martin, A.; Thompson, S.; Koh, E.Y.; Davy, S.K.; Ryan, K.G.; O’Toole, R.F. Phylogenetic analyses of bacteria in sea ice at Cape Hallett, Antarctica. N. Z. J. Mar. Freshw. Res. 2012, 46, 3–12. [Google Scholar] [CrossRef]
- Horner, R.; Ackley, S.F.; Dieckmann, G.S.; Gulliksen, B.; Hoshiai, T.; Legendre, L.; Melnikov, I.A.; Reeburgh, W.S.; Spindler, M.; Sullivan, C.W. Ecology of sea ice biota. Polar Biol. 1992, 12, 417–427. [Google Scholar]
- Gradinger, R.; Ikävalko, J. Organism incorporation into newly forming Arctic sea ice in the Greenland Sea. J. Plankton Res. 1998, 20, 871–886. [Google Scholar] [CrossRef]
- Krembs, C.; Gradinger, R.; Spindler, M. Implications of brine channel geometry and surface area for the interaction of sympagic organisms in Arctic sea ice. J. Exp. Mar. Biol. Ecol. 2000, 243, 55–80. [Google Scholar] [CrossRef]
- Lizotte, M.P. The microbiology of sea ice. In Sea Ice: An Introduction to Its Physics, Chemistry, Biology and Geology; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell: Oxford, UK, 2003; pp. 184–210. [Google Scholar]
- Collins, R.E.; Rocap, G.; Deming, J.W. Persistence of bacterial and archaeal communities in sea ice through an Arctic winter. Environ. Microbiol. 2010, 12, 1828–1841. [Google Scholar] [CrossRef]
- Miller, L.A.; Papakyriakou, T.N.; Collins, R.E.; Deming, J.W.; Ehn, J.K.; Macdonald, R.W.; Mucci, A.; Owens, O.; Raudsepp, M.; Sutherland, N. Carbon dynamics in sea ice: A winter flux time series. J. Geophys. Res. 2011, 116, C02028. [Google Scholar] [CrossRef]
- Lee, S.H.; Stockwell, D.A.; Joo, H.M.; Son, Y.B.; Kang, C.K.; Whitledge, T.E. Phytoplankton production from melting ponds on Arctic sea ice. J. Geophys. Res. 2012, 117, C04030. [Google Scholar]
- Kaartokallio, H. Evidence for active microbial nitrogen transformations in sea ice (Gulf of Bothnia, Baltic Sea) in midwinter. Polar Biol. 2001, 24, 21–28. [Google Scholar] [CrossRef]
- Rysgaard, S.R.; Glud, R.N. Anaerobic N2 production in Arctic sea ice. Limnol. Oceanogr. 2004, 49, 86–94. [Google Scholar] [CrossRef]
- Delille, D.; Basseres, A.; Dessommes, A. Seasonal variation of bacteria in sea ice contaminated by diesel fuel and dispersed crude oil. Microb. Ecol. 1997, 33, 97–105. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Nonstad, I.; Faksness, L.G.; Brandvik, P.J. Responses of microbial communities in Arctic sea ice after contamination by crude petroleum oil. Microb. Ecol. 2008, 55, 540–552. [Google Scholar] [CrossRef]
- Barkay, T.; Poulain, A.J. Mercury (micro) biogeochemistry in polar environments. FEMS Microbiol. Ecol. 2006, 59, 232–241. [Google Scholar] [CrossRef]
- Møller, A.K.; Barkay, T.; Abu Al-Soud, W.; Sørensen, S.J.; Skov, H.; Kroer, N. Diversity and characterization of mercury-resistant bacteria in snow, freshwater and sea-ice brine from the High Arctic. FEMS Microbiol. Ecol. 2011, 75, 390–401. [Google Scholar] [CrossRef]
- Assur, A. Composition of Sea Ice and Its Tensile Strength; University of California Libraries: San Diego, CA, USA, 1960; pp. 106–138. [Google Scholar]
- Cox, G.F.N.; Weeks, W.F. Equations for determining the gas and brine volumes in sea-ice samples. J. Glaciol. 1983, 29, 306–316. [Google Scholar]
- Leppäranta, M.; Manninen, T. The brine and gas content of sea ice with attention to low salinities and high temperatures. Finn. Inst. Mar. Res. Int. Rep. 1988, 2, 1–14. [Google Scholar]
- Thomas, D.N.; Papadimitriou, S.; Michel, C. Biogeochemistry of Sea Ice. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 425–467. [Google Scholar]
- Petri, R.; Imhoff, J.F. Genetic analysis of sea-ice bacterial communities of the Western Baltic Sea using an improved double gradient method. Polar Biol. 2001, 24, 252–257. [Google Scholar] [CrossRef]
- Meiners, K.; Krembs, C.; Gradinger, R. Exopolymer particles: Microbial hotspots of enhanced bacterial activity in Arctic fast ice (Chuckchi Sea). Aquat. Microb. Ecol. 2008, 52, 195–207. [Google Scholar] [CrossRef]
- Brinkmeyer, R.; Glockner, F.O.; Helmke, E.; Amann, R. Predominance of β-proteobacteria in summer melt pools on Arctic pack ice. Limnol. Oceanogr. 2004, 49, 1013–1021. [Google Scholar] [CrossRef]
- Gradinger, R.; Meiners, K.; Plumley, G.; Zhang, Q.; Bluhm, B.A. Abundance and composition of the sea-ice meiofauna in off-shore pack ice of the Beaufort Gyre in summer 2002 and 2003. Polar Biol. 2005, 28, 171–181. [Google Scholar] [CrossRef]
- Mundy, C.J.; Gosselin, M.; Ehn, J.K.; Belzile, C.; Poulin, M.; Alou, E.; Roy, S.; Hop, H.; Lessard, S.; Papakyriakou, T.N.; et al. Characteristics of two distinct high-light acclimated algal communities during advanced stages of sea ice melt. Polar Biol. 2011, 34, 1869–1886. [Google Scholar] [CrossRef]
- McLean, A.L. Bacteria of ice and snow in Antarctica. Nature 1918, 102, 35–39. [Google Scholar] [CrossRef]
- Legendre, L.; Ackley, S.F.; Dieckmann, G.S.; Gulliksen, B.; Horner, R.; Hoshiai, T.; Melnikov, I.A.; Reeburgh, W.S.; Spindler, M.; Sullivan, C.W. Ecology of sea ice biota. Polar Biol. 1992, 12, 429–444. [Google Scholar]
- Ackley, S.F.; Sullivan, C.W. Physical controls on the development and characteristics of Antarctic sea ice biological communities—A review and synthesis. Deep Sea Res. Part I 1994, 41, 1583–1604. [Google Scholar] [CrossRef]
- Staley, J.T.; Gosink, J.J. Poles apart: Biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 1999, 53, 189–215. [Google Scholar] [CrossRef]
- Thomas, D.N.; Dieckmann, G.S. Antarctic sea ice—A habitat for extremophiles. Science 2002, 295, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Mock, T.; Thomas, D.N. Recent advances in sea-ice microbiology. Environ. Microbiol. 2005, 7, 605–619. [Google Scholar] [CrossRef]
- Deming, J.W. Extremophiles: Cold Environments. In Encyclopedia of Microbiology; Shaechter, M., Ed.; Elsevier: Oxford, UK, 2009; pp. 147–158. [Google Scholar]
- Petrich, C.; Eicken, H. Growth, Structure and Properties of Sea Ice. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 247–282. [Google Scholar]
- Junge, K.; Krembs, C.; Deming, J.W.; Stierle, A.; Eicken, H. A microscopic approach to investigate bacteria under in-situ conditions in sea-ice samples. Ann. Glaciol. 2001, 33, 304–310. [Google Scholar] [CrossRef]
- Weissenberger, J.; Grossmann, S. Experimental formation of sea ice: Importance of water circulation and wave action for incorporation of phytoplankton and bacteria. Polar Biol. 1998, 20, 178–188. [Google Scholar] [CrossRef]
- Garrison, D.L.; Ackley, S.F.; Buck, K.R. A physical mechanism for establishing algal populations in frazil ice. Nature 1983, 306, 363–365. [Google Scholar] [CrossRef]
- Garrison, D.L.; Close, A.R.; Reimnitz, E. Algae concentrated by frazil ice: Evidence from laboratory experiments and field measurements. Antarct. Sci. 1989, 1, 313–316. [Google Scholar]
- Knopf, D.A.; Alpert, P.A.; Wang, B.; Aller, J.Y. Stimulation of ice nucleation by marine diatoms. Nature Geosci. 2011, 4, 88–90. [Google Scholar] [CrossRef]
- Grossmann, S.; Dieckmann, G.S. Bacterial standing stock, activity, and carbon production during formation and growth of sea ice in the Weddell Sea, Antarctia. Appl. Environ. Microbiol. 1994, 60, 2746–2753. [Google Scholar]
- Riedel, A.; Michel, C.; Gosselin, M.; LeBlanc, B. Enrichment of nutrients, exopolymeric substances and microorganisms in newly formed sea ice on the Mackenzie shelf. Mar. Ecol. Prog. Ser. 2007, 342, 55–67. [Google Scholar] [CrossRef]
- Meiners, K.; Gradinger, R.; Fehling, J.; Civitarese, G.; Spindler, M. Vertical distribution of exopolymer particles in sea ice of the Fram Strait (Arctic) during autumn. Mar. Ecol. Prog. Ser. 2003, 248, 1–13. [Google Scholar] [CrossRef]
- Deming, J.W. Sea Ice Bacteria and Viruses. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 247–282. [Google Scholar]
- Ewert, M.; Deming, J.W. Selective retention in saline ice of extracellular polysaccharides produced by the cold-adapted marine bacterium Colwellia psychrerythraea strain 34H. Ann. Glaciol. 2011, 52, 111–117. [Google Scholar] [CrossRef]
- Bowman, J.S.; Deming, J.W. Elevated bacterial abundance and exopolymers in saline frost flowers and implications for atmospheric chemistry and microbial dispersal. Geophys. Res. Lett. 2010, 37, L13501. [Google Scholar] [CrossRef]
- Ewert, M.; Carpenter, S.D.; Colangelo-Lillis, J.; Deming, J.W. Bacterial and extracellular polysaccharide content of brine-wetted snow over Arctic winter first-year sea ice. J. Geophys. Res. 2013. [Google Scholar] [CrossRef]
- Collins, R.E.; Carpenter, S.D.; Deming, J.W. Spatial heterogeneity and temporal dynamics of particles, bacteria, and pEPS in Arctic winter sea ice. J. Mar. Syst. 2008, 74, 902–917. [Google Scholar] [CrossRef]
- Druckenmiller, M.L.; Eicken, H.; Johnson, M.; Pringle, D.; Williams, C. Towards an integrated coastal sea-ice observatory: System components and a case study at Barrow, Alaska. Cold Reg. Sci. Technol. 2009, 56, 61–72. [Google Scholar] [CrossRef]
- Georlette, D.; Blaise, V.; Collins, T.; D’Amico, S.; Gratia, E.; Hoyoux, A.; Marx, J.C.; Sonan, G.; Feller, G.; Gerday, C. Some like it cold: Biocatalysis at low temperatures. FEMS Microbiol. Rev. 2004, 28, 25–42. [Google Scholar] [CrossRef]
- Bakermans, C.; Tollaksen, S.L.; Giometti, C.S.; Wilkerson, C.; Tiedje, J.M.; Thomashow, M.F. Proteomic analysis of Psychrobacter cryohalolentis K5 during growth at subzero temperatures. Extremophiles 2007, 11, 343–354. [Google Scholar] [CrossRef]
- Shivaji, S.; Prakash, J.S.S. How do bacteria sense and respond to low temperature? Arch. Microbiol. 2010, 192, 85–95. [Google Scholar]
- Bowman, J.P.; McCammon, S.A.; Brown, M.V.; Nichols, D.S.; McMeekin, T.A. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 1997, 63, 3068–3078. [Google Scholar]
- Huston, A.L.; Krieger-Brockett, B.B.; Deming, J.W. Remarkably low temperature optima for extracellular enzyme activity from Arctic bacteria and sea ice. Environ. Microbiol. 2001, 2, 383–388. [Google Scholar]
- Deming, J.W. Life in Ice Formations at Very Cold Temperatures. In Physiology and Biochemistry of Extremophiles; ASM Press: Washington, DC, USA, 2007; pp. 133–144. [Google Scholar]
- Weissenberger, J.; Dieckmann, G.; Gradinger, R.; Spindler, M. Sea ice: A cast technique to examine and analyze brine pockets and channel structure. Limnol. Oceanogr. 1992, 37, 179–183. [Google Scholar] [CrossRef]
- Golden, K.M.; Eicken, H.; Heaton, A.L.; Miner, J.; Pringle, D.J.; Zhu, J. Thermal evolution of permeability and microstructure in sea ice. Geophys. Res. Lett. 2007, 34, L16501. [Google Scholar]
- Golden, K.M.; Ackley, S.F.; Lytle, V.I. The percolation phase transition in sea ice. Science 1998, 282, 2238–2241. [Google Scholar] [CrossRef]
- Rysgaard, S.R.; Bendtsen, J.; Delille, B.; Dieckmann, G.S.; Glud, R.N.; Kennedy, H.; Mortensen, J.; Papadimitriou, S.; Thomas, D.N.; Tison, J.L. Sea ice contribution to the air-sea CO2 exchange in the Arctic and Southern Oceans. Tellus B 2011, 63, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Collins, R.E.; Deming, J.W. Abundant dissolved genetic material in Arctic sea ice Part II: Viral dynamics during autumn freeze-up. Polar Biol. 2011, 34, 1831–1841. [Google Scholar] [CrossRef]
- Collins, R.E.; Deming, J.W. Abundant dissolved genetic material in Arctic sea ice Part I: Extracellular DNA. Polar Biol. 2011, 34, 1819–1830. [Google Scholar] [CrossRef]
- Wells, L.E.; Deming, J.W. Modelled and measured dynamics of viruses in Arctic winter sea-ice brines. Environ. Microbiol. 2006, 8, 1115–1121. [Google Scholar] [CrossRef]
- Collins, R.E. Microbial evolution in sea ice: Communities to genes. PhD thesis, University of Washington, Seattle, WA, USA, 2009. [Google Scholar]
- Gradinger, R.; Friedrich, C.; Spindler, M. Abundance, biomass and composition of the sea ice biota of the Greenland Sea pack ice. Deep Sea Res. Part II 1999, 46, 1457–1472. [Google Scholar] [CrossRef]
- Krembs, C.; Eicken, H.; Deming, J.W. Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic. Proc. Natl. Acad. Sci. USA 2011, 108, 3653–3658. [Google Scholar] [CrossRef]
- Janech, M.G.; Krell, A.; Mock, T.; Kang, J.; Raymond, J.A. Ice-binding proteins from sea ice diatoms (Bacillarophyceae). J. Phycol. 2006, 42, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Raymond, J.A.; Fritsen, C.; Shen, K. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol. Ecol. 2007, 61, 214–221. [Google Scholar] [CrossRef]
- Raymond, J.A. Algal ice-binding proteins change the structure of sea ice. Proc. Natl. Acad. Sci. USA 2011, 108, E198. [Google Scholar] [CrossRef]
- Bayer-Giraldi, M.; Weikusat, I.; Besir, H.; Dieckmann, G. Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology 2011, 63, 210–219. [Google Scholar] [CrossRef]
- Dinnbier, U.; Limpinsel, E.; Schmid, R.; Bakker, E.P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 1988, 150, 348–357. [Google Scholar] [CrossRef]
- Wood, J.M. Osmosensing by bacteria: Signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 1999, 63, 230–262. [Google Scholar]
- Roberts, M. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Olman, V.; Stuart, R.; Paulsen, I.T.; Palenik, B.; Xu, Y. Computational prediction of the osmoregulation network in Synechococcus sp. WH8102. BMC Genomics 2010, 11, 291. [Google Scholar] [CrossRef]
- Kennedy, S.P.; Ng, W.V.; Salzberg, S.L.; Hood, L.; DasSarma, S. Understanding the adaptation of Halobacterium species NRC-1 to its extreme environment through computational analysis of its genome sequence. Genome Res. 2001, 11, 1641–1650. [Google Scholar] [CrossRef]
- Saum, S.H.; Pfeiffer, F.; Palm, P.; Rampp, M.; Schuster, S.C.; Müller, V.; Oesterhelt, D. Chloride and organic osmolytes: A hybrid strategy to cope with elevated salinities by the moderately halophilic, chloride-dependent bacterium Halobacillus halophilus. Environ. Microbiol. 2012. [Google Scholar] [CrossRef]
- Aslam, S.; Cresswell-Maynard, T.; Thomas, D.N.; Underwood, G.J.C. Production and characterization of the intra-and extracellular carbohydrates and polymeric substances (EPS) of three sea-ice diatom species, and evidence for a cryoprotective role for EPS. J. Phycol. 2012, 48, 1494–1509. [Google Scholar] [CrossRef]
- Liu, S.B.; Chen, X.L.; He, H.L.; Zhang, X.Y.; Xie, B.B.; Yu, Y.; Chen, B.; Zhou, B.C.; Zhang, Y.Z. Structure and ecological roles of a novel exopolysaccharide from the Arctic sea ice bacterium Pseudoalteromonas sp. Strain SM20310. Appl. Environ. Microbiol. 2013, 79, 224–230. [Google Scholar]
- Ghosh, D.; Roy, K.; Williamson, K.E.; Srinivasiah, S.; Wommack, K.E.; Radosevich, M. Acyl-homoserine lactones can induce virus production in lysogenic bacteria: An alternative paradigm for prophage induction. Appl. Environ. Microbiol. 2009, 75, 7142–7152. [Google Scholar] [CrossRef]
- Shkilnyj, P.; Koudelka, G.B. Effect of salt shock on stability of γimm433 lysogens. J. Bacteriol. 2007, 189, 3115–3123. [Google Scholar] [CrossRef]
- ExPASy. SIB Bioinformatics Resource Portal, Compute pI/Mw. Available online: http://web.expasy.org/compute_pi/ (accessed on 1 November 2012).
- Universal Protein Resource (UniProt). Available online: http://www.uniprot.org/uniprot/ (accessed on 1 November 2012).
- Thomas, V.K.; Kuehn, K.A.; Francoeur, S.N. Effects of UV radiation on wetland periphyton: Algae, bacteria, and extracellular polysaccharides. J. Freshw. Ecol. 2009, 24, 315–326. [Google Scholar] [CrossRef]
- Underwood, G.J.C.; Nilsson, C.; Sundbäck, K.; Wulff, A. Short-term effects of UVB radiation on chlorophyll fluorescence, biomass, pigments, and carbohydrate fractions in a benthic diatom mat. J. Phycol. 1999, 35, 656–666. [Google Scholar]
- Jeffrey, S.W.; MacTavish, H.S.; Dunlap, W.C.; Vesk, M.; Groenewoud, K. Occurrence of UVA-and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar. Ecol. Prog. Ser. 1999, 189, 35–51. [Google Scholar] [CrossRef]
- Uusikivi, J.; Vähätalo, A.V.; Granskog, M.A.; Sommaruga, R. Contribution of mycosporine-like amino acids and colored dissolved and particulate matter to sea ice optical properties and ultraviolet attenuation. Limnol. Oceanogr. 2010, 55, 703–713. [Google Scholar]
- Horner, R.; Schrader, G.C. Relative contributions of ice algae, phytoplankton, and benthic microalgae to primary production in nearshore regions of the Beaufort Sea. Arctic 1982, 4, 485–503. [Google Scholar]
- Norman, L.; Thomas, D.N.; Stedmon, C.A.; Granskog, M.A.; Papadimitriou, S.; Krapp, R.H.; Meiners, K.M.; Lannuzel, D.; van der Merwe, P.; Dieckmann, G.S. The characteristics of dissolved organic matter (DOM) and chromophoric dissolved organic matter (CDOM) in Antarctic sea ice. Deep Sea Res. Part II 2011, 58, 1075–1091. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Retuerta, E.; Passow, U.; Duarte, C.M.; Reche, I. Effects of ultraviolet B radiation on (not so) transparent exopolymer particles. Biogeosci. Discuss. 2009, 6, 7599–7625. [Google Scholar] [CrossRef]
- Cockell, C.; Rettberg, P.; Horneck, G.; Scherer, K.; Stokes, D.M. Measurements of microbial protection from ultraviolet radiation in polar terrestrial microhabitats. Polar Biol. 2003, 26, 62–69. [Google Scholar]
- Sturm, M.; Massom, R.A. Snow and Sea Ice. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 153–204. [Google Scholar]
- Ruas-Madiedo, P.; de los Reyes-Gavilán, C.G. Methods for the screening, isolation and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef]
- Mancuso Nichols, C.; Garon Lardière, S.; Bowman, J.P.; Nichols, P.D.; Gibson, J.A.E.; Guézennec, J. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb. Ecol. 2005, 49, 578–589. [Google Scholar] [CrossRef]
- Lemoine, J.; Chirat, F.; Wieruszeski, J.M.; Strecker, G.; Favre, N.; Neeser, J.R. Structural characterization of the exocellular polysaccharides produced by Streptococcus thermophilus SFi39 and SFi12. Appl. Environ. Microbiol. 1997, 63, 3512–3518. [Google Scholar]
- Schiano Moriello, V.; Lama, L.; Poli, A.; Gugliandolo, C.; Maugeri, T.L.; Gambacorta, A.; Nicolaus, B. Production of exopolysaccharides from a thermophilic microorganism isolated from a marine hot spring in flegrean areas. J. Ind. Microbiol. Biotechnol. 2003, 30, 95–101. [Google Scholar]
- Vyrides, I.; Stuckey, D. Adaptation of anaerobic biomass to saline conditions: Role of compatible solutes and extracellular polysaccharides. Enzyme Microb. Technol. 2009, 44, 46–51. [Google Scholar] [CrossRef]
- Mancuso Nichols, C.; Bowman, J.P.; Guezennec, J. Effects of incubation temperature on growth and production of exopolysaccharides by an Antarctic sea ice bacterium grown in batch culture. Appl. Environ. Microbiol. 2005, 71, 3519–3523. [Google Scholar] [CrossRef]
- Guibaud, G.; Comte, S.; Bordas, F.; Dupuy, S.; Baudu, M. Comparison of the complexation potential of extracellular polymeric substances (EPS), extracted from activated sludge and produced by pure bacterial strains, for cadmium, lead and nickel. Chemosphere 2005, 59, 629–638. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial Exopolymer Secretions in Ocean Environments: Their Role(s) in Food Webs and Marine Processes. In Oceanography and Marine Biology, an Annual Review; Barnes, H., Barnes, M., Eds.; Aberdeen University Press: Aberdeen, UK, 1990; Volume 28, pp. 73–153. [Google Scholar]
- Ding, Y.X.; Chin, W.C.; Rodriguez, A.; Hung, C.C.; Santschi, P.H.; Verdugo, P. Amphiphilic exopolymers from Sagittula stellata induce DOM self-assembly and formation of marine microgels. Mar. Chem. 2008, 112, 11–19. [Google Scholar] [CrossRef]
- Krembs, C.; Eicken, H.; Junge, K.; Deming, J.W. High concentrations of exopolymeric substances in Arctic winter sea ice: Implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res. Part I 2002, 49, 2163–2181. [Google Scholar]
- Aslam, S.; Underwood, G.J.C.; Kaartokallio, H.; Norman, L.; Autio, R.; Fischer, M.; Kuosa, H.; Dieckmann, G.S.; Thomas, D.N. Dissolved extracellular polymeric substances (dEPS) dynamics and bacterial growth during sea ice formation in an ice tank study. Polar Biol. 2012, 35, 661–676. [Google Scholar] [CrossRef]
- Underwood, G.J.C.; Fietz, S.; Papadimitriou, S.; Thomas, D.N.; Dieckmann, G. Distribution and composition of dissolved extracellular polymeric substances (EPS) in Antarctic sea ice. Mar. Ecol. Prog. Ser. 2010, 404, 1–19. [Google Scholar] [CrossRef]
- Wolfaardt, G.M.; Lawrence, J.R.; Korber, D.R. Function of EPS. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer Verlag: Berlin, Germany, 1999; pp. 171–200. [Google Scholar]
- Mora, P.; Rosconi, F.; Franco Fraguas, L.; Castro-Sowinski, S. Azospirillum brasilense Sp7 produces an outer-membrane lectin that specifically binds to surface-exposed extracellular polysaccharide produced by the bacterium. Arch. Microbiol. 2008, 189, 519–524. [Google Scholar] [CrossRef]
- Lind, J.L.; Heimann, K.; Miller, E.A.; van Vliet, C.; Hoogenraad, N.J.; Wetherbee, R. Substratum adhesion and gliding in a diatom are mediated by extracellular proteoglycans. Planta 1997, 203, 213–221. [Google Scholar] [CrossRef]
- Baker, M.G.; Lalonde, S.V.; Konhauser, K.O.; Foght, J.M. Role of extracellular polymeric substances in the surface chemical reactivity of Hymenobacter aerophilus, a psychrotolerant bacterium. Appl. Environ. Microbiol. 2010, 76, 102–109. [Google Scholar] [CrossRef]
- Bitton, G.; Freihofer, V. Influence of extracellular polysaccharides on the toxicity of copper and cadmium toward Klebsiella aerogenes. Microb. Ecol. 1977, 4, 119–125. [Google Scholar] [CrossRef]
- Knowes, E.J.; Castenholz, R.W. Effect of exogenous extracellular polysaccharides on the dessication and freezing tolerance of rock-inhabiting phototrophic microorganisms. FEMS Microbiol. Ecol. 2008, 66, 261–270. [Google Scholar]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. R. 2010, 17, 595–602. [Google Scholar] [CrossRef]
- Marx, J.G.; Carpenter, S.D.; Deming, J.W. Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can. J. Microbiol. 2009, 55, 63–72. [Google Scholar] [CrossRef]
- Krembs, C.; Deming, J.W. The role of exopolymers in microbial adaptation to sea ice. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerda, C., Eds.; Springer-Verlag: Berlin, Germany, 2008; pp. 247–264. [Google Scholar]
- Mancuso Nichols, C.; Guezennec, J.; Bowman, J.P. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef]
- Verdugo, P. Marine microgels. Annu. Rev. Mar. Sci. 2012, 4, 375–400. [Google Scholar] [CrossRef]
- Rysgaard, S.R.; Søgaard, D.S.; Cooper, M.; Pucko, M.; Lennert, K.; Papakyriakou, T.; Wang, F.; Geilfus, N.; Glud, R.N.; Ehn, J.; et al. Ikaite crystal distribution in Arctic winter sea ice and implications for CO2 system dynamics. Cryosphere 2013, in press. [Google Scholar]
- Fischer, M.; Thomas, D.N.; Krell, A.; Nehrke, G.; Göttlicher, J.; Norma, L.; Riaux-Gobin, C.; Dieckmann, G. Quantification of ikaite in Antarctic sea ice. Cryosphere Discuss. 2012, 6, 505–530. [Google Scholar]
- Chave, K.E.; Suess, E. Calcium carbonate saturation in seawater: Effects of dissolved organic matter. Limnol. Oceanogr. 1970, 15, 633–637. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Bergmann, D.; Furth, G.; Mayer, C. Binding of bivalent cations by xanthan in aqueous solution. Int. J. Biol. Macromol. 2008, 43, 245–251. [Google Scholar] [CrossRef]
- Farris, S.; Mora, L.; Capretti, G.; Piergiovanni, L. Charge density quantification of polyelectrolyte polysaccharides by conductometric titration: An analytical chemistry experiment. J. Chem. Educ. 2011, 89, 121–124. [Google Scholar]
- Hardikar, V.V.; Matijević, E. Influence of ionic and nonionic dextrans on the formation of calcium hydroxide and calcium carbonate particles. Colloid. Surf. A 2001, 186, 23–31. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard Mater. 2008, 151, 185–193. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, D.J.; Pan, X. Desorption of Hg (II) and Sb (V) on extracellular polymeric substances: Effects of pH, EDTA, Ca (II) and temperature shocks. Bioresour. Technol. 2013, 128, 711–715. [Google Scholar] [CrossRef]
- Bhaskar, P.V.; Bhosle, N.B. Bacterial extracellular polymeric substance (EPS): A carrier of heavy metals in the marine food-chain. Environ. Int. 2006, 32, 191–198. [Google Scholar] [CrossRef]
- Schlekat, C.E.; Decho, A.W.; Chandler, G.T. Bioavailability of particle-associated silver, cadmium, and zinc to the estuarine amphipod Leptocheirus plumulosus through dietary ingestion. Limnol. Oceanogr. 2000, 45, 11–21. [Google Scholar] [CrossRef]
- Campbell, L.M.; Norstrom, R.J.; Hobson, K.A.; Muir, D.C.G.; Backus, S.; Fisk, A.T. Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci. Total Environ. 2005, 351, 247–263. [Google Scholar]
- Turgeon, S.L.; Schmitt, C.; Sanchez, C. Protein–polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- Huston, A.L.; Methe, B.; Deming, J.W. Purification, characterization, and sequencing of an extracellular cold-active aminopeptidase produced by marine psychrophile Colwellia psychrerythraea strain 34H. Appl. Environ. Microbiol. 2004, 70, 3321–3328. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ewert, M.; Deming, J.W. Sea Ice Microorganisms: Environmental Constraints and Extracellular Responses. Biology 2013, 2, 603-628. https://doi.org/10.3390/biology2020603
Ewert M, Deming JW. Sea Ice Microorganisms: Environmental Constraints and Extracellular Responses. Biology. 2013; 2(2):603-628. https://doi.org/10.3390/biology2020603
Chicago/Turabian StyleEwert, Marcela, and Jody W. Deming. 2013. "Sea Ice Microorganisms: Environmental Constraints and Extracellular Responses" Biology 2, no. 2: 603-628. https://doi.org/10.3390/biology2020603




