The Effect of Nutritional Status in the Pathogenesis of Critical Illness Myopathy (CIM)
Abstract
:1. Introduction
1.1. Nutritional Guidelines
2. Experimental Section
2.1. Animals
| 100 mL vein infusion, low energy (11 kcal/day/kg bw) | Volumes |
|---|---|
| (0.6 mL/h) | |
| Lactated ringers | 32 mL |
| Oxacillin | 2.8 g |
| Glucose 50% | 12.8 mL |
| 100 mL vein infusion, high energy (41 kcal/day/kg bw) | Volumes |
|---|---|
| (0.6 mL/h) | |
| Lactated ringers | 32 mL |
| Oxacillin | 2.8 g |
| Glucose 50% | 31.6 mL |
| Vamin 114 mg/mL | 9.4 mL |
| Intralipid 200 mg/mL | 9.4 mL |
2.2. Nutritional Information
2.3. Experimental ICU Model
2.4. Passive Loading
2.5. Muscle Tissue Preparation and Fiber Membrane Permeabilization
2.6. Single Muscle Fiber Experimental Procedure and Specific Force Measurements
2.7. Myosin Heavy Chain (MyHC) Isoform Expression and Myosin:Actin Ratios
2.8. Statistical Analysis
3. Results and Discussion
3.1. Body and Muscle Weight
| Group | BW (g) | TA (mg) | EDL (mg) | GAST (mg) | SOL (mg) | |||||
| Pre | Post | Left | Right | Left | Right | Left | Right | Left | Right | |
| Controls (0 days) n = 4 | 297 ± 23 | 297 ± 23 | 592 ± 10 | 598 ± 12 | 148 ± 6 | 148 ± 6 | 1690 ± 39 | 1692 ± 40 | 132 ± 3 | 135 ± 3 |
| LC (10, 10 and –14 days) n = 3 | 295 ± 21 | 215 ± 20 | 330 ± 30 | 250 ± 20 | 95 ± 7 | 74 ± 6 | 780 ± 30 | 740 ± 20 | 100 ± 8 | 70 ± 3 |
| EC (10 days) n = 2 | 290 and 308 | 219 and 223 | 342 and 384 | 319 and 376 | 101 and 109 | 88 and 105 | 876 and 953 | 790 and 900 | 85 and 101 | 71 and 64 |
3.2. Myosin:Actin Ratios
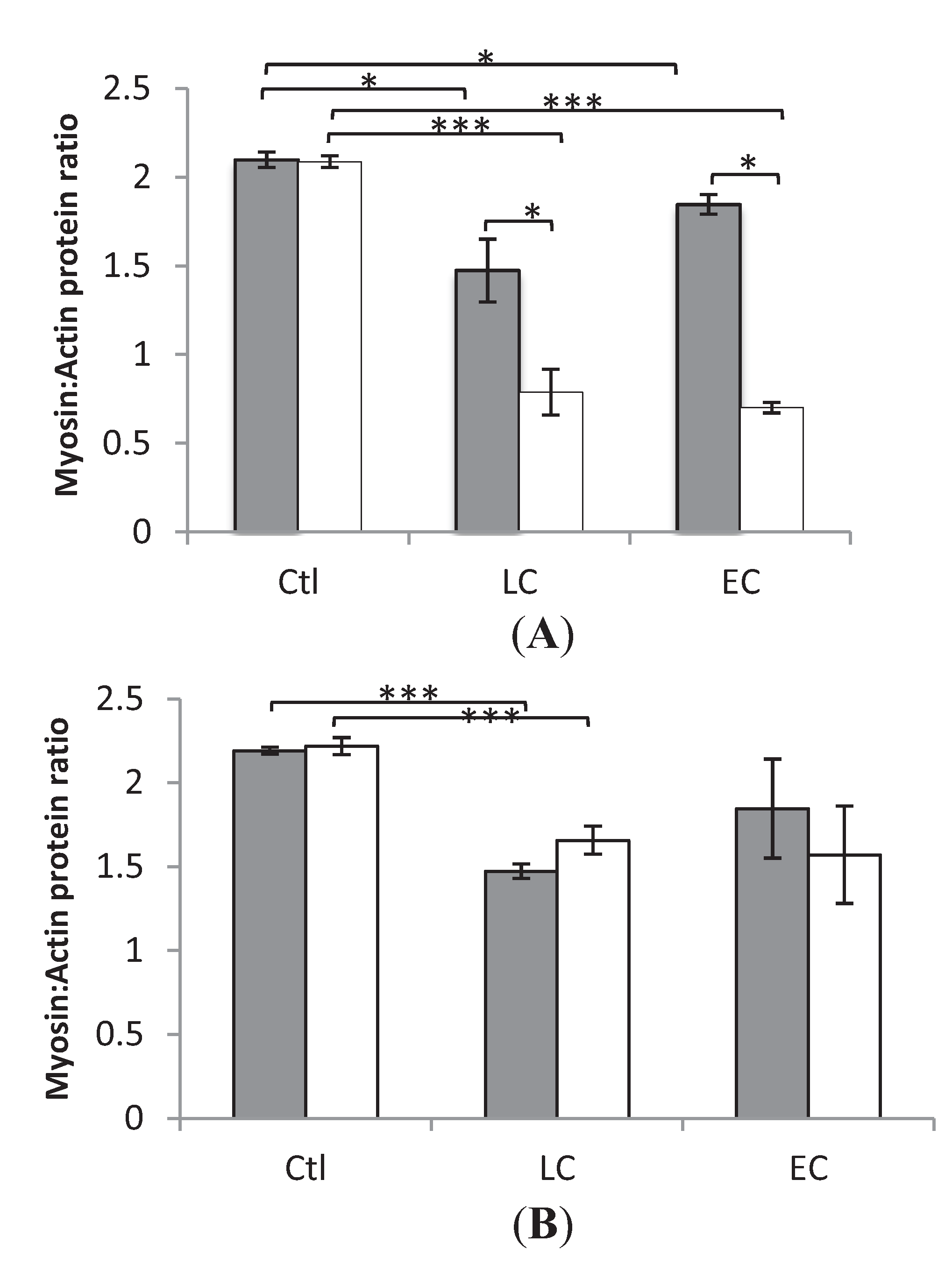
3.3. Muscle Fiber Size and Specific Force in Muscle Fibers Expressing Fast and Slow Myosin Heavy Chain (MyHC) Isoforms
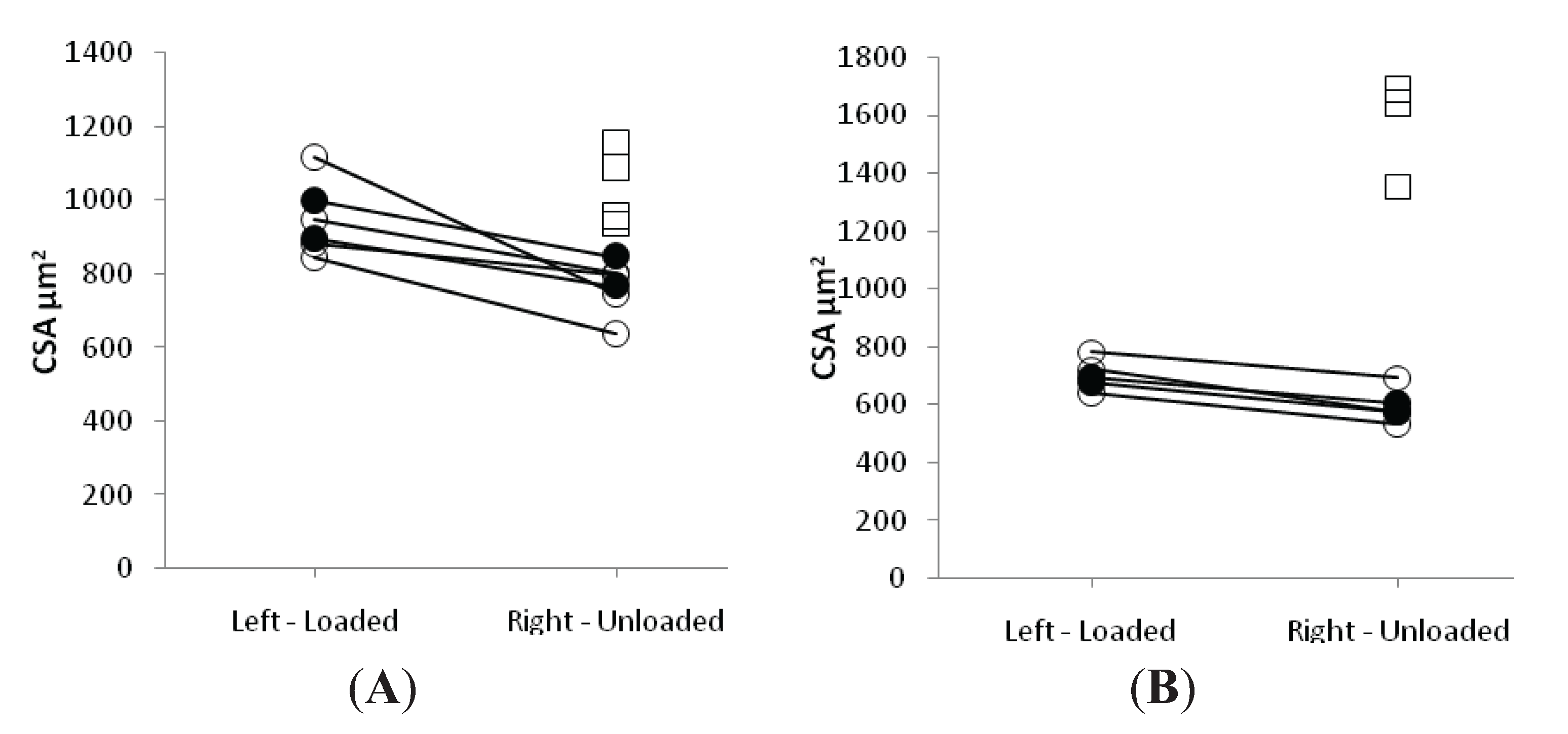
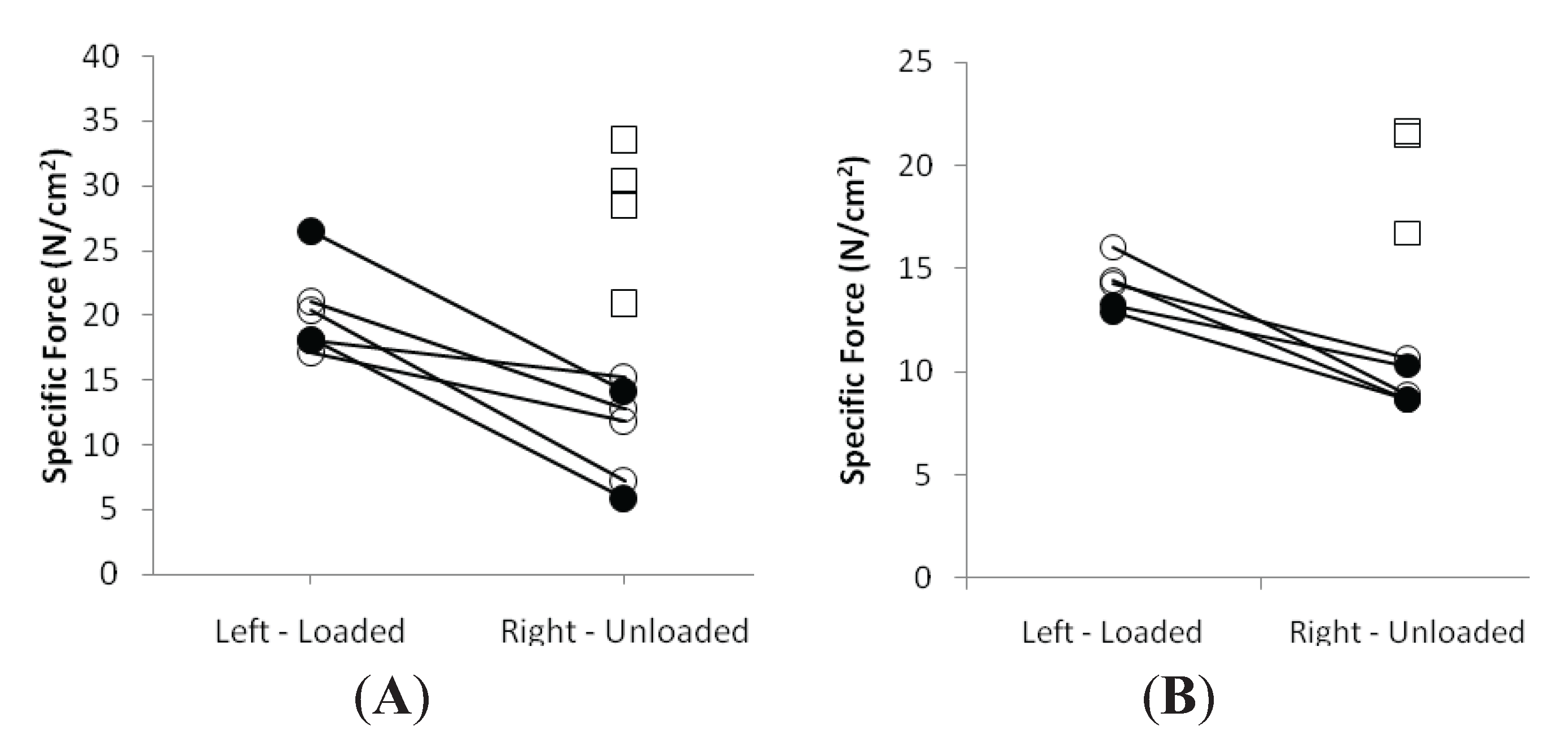
3.4. Discussion
3.5. Study Limitations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Hirano, M.; Ott, B.R.; Raps, E.C.; Minetti, C.; Lennihan, L.; Libbey, N.P.; Bonilla, E.; Hays, A.P. Acute quadriplegic myopathy: A complication of treatment with steroids, nondepolarizing blocking agents, or both. Neurology 1992, 42, 2082–2087. [Google Scholar]
- Lacomis, D. Critical illness myopathy. Curr. Rheumatol. Rep. 2002, 4, 403–408. [Google Scholar] [CrossRef]
- MacFarlane, I.A.; Rosenthal, F.D. Severe myopathy after status asthmaticus. Lancet 1977, 2, 615. [Google Scholar] [CrossRef]
- Leijten, F.S.; Harinck-de Weerd, J.E.; Poortvliet, D.C.; de Weerd, A.W. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA 1995, 274, 1221–1225. [Google Scholar] [CrossRef]
- Lacomis, D.; Zochodne, D.W.; Bird, S.J. Critical illness myopathy. Muscle Nerve 2000, 23, 1785–1788. [Google Scholar] [CrossRef]
- Friedrich, O.; Fink, R.H.; Hund, E. Understanding critical illness myopathy: Approaching the pathomechanism. J. Nutr. 2005, 135, 1813S–1817S. [Google Scholar]
- Ochala, J.; Gustafson, A.M.; Diez, M.L.; Renaud, G.; Li, M.; Aare, S.; Qaisar, R.; Banduseela, V.C.; Hedstrom, Y.; Tang, X.; Dworkin, B.; Ford, G.C.; Nair, K.S.; Perera, S.; Gautel, M.; Larsson, L. Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: Underlying mechanisms. J. Physiol. 2011, 589, 2007–2026. [Google Scholar] [CrossRef]
- Akkad Hazem, C.R.; Corpeno, R.; Larsson, L. Masseter muscle myofibrillar protein synthesis and degradation in an experimental critical illness myopathy model. PLoS One 2014, 9, e92622. [Google Scholar]
- Larsson, L.; Li, X.; Edstrom, L.; Eriksson, L.I.; Zackrisson, H.; Argentini, C.; Schiaffino, S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: Mechanisms at the cellular and molecular levels [see comments]. Crit. Care Med. 2000, 28, 34–45. [Google Scholar] [CrossRef]
- Norman, H.; Zackrisson, H.; Hedström, Y.; Andersson, P.; Nordquist, J.; Eriksson, L.I.; Libelius, R.; Larsson, L. Myofibrillar protein and gene expression in acute quadriplegic myopathy. J. Neurol. Sci. 2009, 285, 28–38. [Google Scholar] [CrossRef]
- Renaud, G.; Llano-Diez, M.; Ravara, B.; Gorza, L.; Feng, H.Z.; Jin, J.P.; Cacciani, N.; Gustafson, A.M.; Ochala, J.; Corpeno, R.; Li, M.; Hedstrom, Y.; Ford, G.C.; Nair, K.S.; Larsson, L. Sparing of muscle mass and function by passive loading in an experimental intensive care unit model. J. Physiol. 2013, 591, 1385–1402. [Google Scholar]
- Llano-Diez, M.; Renaud, G.; Andersson, M.; Marrero, H.G.; Cacciani, N.; Engquist, H.; Corpeno, R.; Artemenko, K.; Bergquist, J.; Larsson, L. Mechanisms underlying icu muscle wasting and effects of passive mechanical loading. Crit. Care 2012, 16, R209. [Google Scholar] [CrossRef]
- Griffiths, R.D.; Jones, C.; Palmer, T.E. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 1997, 13, 295–302. [Google Scholar]
- Schetz, M.; Casaer, M.P.; Van den Berghe, G. Does artificial nutrition improve outcome of critical illness? Crit. Care 2013, 17, 302. [Google Scholar]
- Winkelman, C. Mechanisms for muscle health in the critically ill patient. Crit. Care Nurs. Q. 2013, 36, 5–16. [Google Scholar] [CrossRef]
- Beck, A.M.; Balknas, U.N.; Furst, P.; Hasunen, K.; Jones, L.; Keller, U.; Melchior, J.C.; Mikkelsen, B.E.; Schauder, P.; Sivonen, L.; Zinck, O.; Oien, H.; Ovesen, L. Food and nutritional care in hospitals: How to prevent undernutrition—Report and guidelines from the council of europe. Clin. Nutr. 2001, 20, 455–460. [Google Scholar] [CrossRef]
- Plank, L.D.; Hill, G.L. Similarity of changes in body composition in intensive care patients following severe sepsis or major blunt injury. Ann. New York Acad. Sci. 2000, 904, 592–602. [Google Scholar] [CrossRef]
- Friedlander, A.L.; Braun, B.; Pollack, M.; MacDonald, J.R.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Henderson, G.C.; Horning, M.A.; Brooks, G.A.; Hoffman, A.R.; Cymerman, A. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am. J. Physiol. Endocrinol. Metab. 2005, 289, 3. [Google Scholar]
- Casaer, M.P.; Hermans, G.; Wilmer, A.; Van den Berghe, G. Impact of early parenteral nutrition completing enteral nutrition in adult critically ill patients (epanic trial): A study protocol and statistical analysis plan for a randomized controlled trial. Trials 2011, 12, 21. [Google Scholar] [CrossRef]
- Hiesmayr, M. Nutrition risk assessment in the icu. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 174–180. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; Velloso, C.; Seymour, J.; Agley, C.C.; Selby, A.; Limb, M.; Edwards, L.M.; Smith, K.; Rowlerson, A.; Rennie, M.J.; Moxham, J.; Harridge, S.D.; Hart, N.; Montgomery, H.E. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Derde, S.; Vanhorebeek, I.; Ververs, E.J.; Vanhees, I.; Darras, V.M.; Van Herck, E.; Larsson, L.; Van den Berghe, G. Increasing intravenous glucose load in the presence of normoglycemia: Effect on outcome and metabolism in critically ill rabbits. Crit. Care Med. 2010, 38, 602–611. [Google Scholar] [CrossRef]
- O'Leary-Kelley, C.M.; Puntillo, K.A.; Barr, J.; Stotts, N.; Douglas, M.K. Nutritional adequacy in patients receiving mechanical ventilation who are fed enterally. Am. J. Crit. Care 2005, 14, 222–231. [Google Scholar]
- Kim, H.; Shin, J.A.; Shin, J.Y.; Cho, O.M. Adequacy of nutritional support and reasons for underfeeding in neurosurgical intensive care unit patients. Asian Nurs. Res. 2010, 4, 102–110. [Google Scholar]
- Singer, P.; Berger, M.M.; Van den Berghe, G.; Biolo, G.; Calder, P.; Forbes, A.; Griffiths, R.; Kreyman, G.; Leverve, X.; Pichard, C. Espen guidelines on parenteral nutrition: Intensive care. Clin. Nutr. 2009, 28, 387–400. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Vanek, V.W.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of critical care medicine (sccm) and american society for parenteral and enteral nutrition (a.S.P.E.N.). JPEN 2009, 33, 277–316. [Google Scholar] [CrossRef]
- Hermans, G.; Casaer, M.P.; Clerckx, B.; Guiza, F.; Vanhullebusch, T.; Derde, S.; Meersseman, P.; Derese, I.; Mesotten, D.; Wouters, P.J.; Van Cromphaut, S.; Debaveye, Y.; Gosselink, R.; Gunst, J.; Wilmer, A.; Van den Berghe, G.; Vanhorebeek, I. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: A subanalysis of the epanic trial. Lancet Respir. Med. 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; Vlasselaers, D.; Debaveye, Y.; Desmet, L.; Dubois, J.; Van Assche, A.; Vanderheyden, S.; Wilmer, A.; Van den Berghe, G. Early versus late parenteral nutrition in critically ill adults. New Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef]
- Dworkin, B.R.; Dworkin, S. Baroreflexes of the rat. Iii. Open-loop gain and electroencephalographic arousal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R597–605. [Google Scholar] [CrossRef]
- Dworkin, B.R.; Dworkin, S. Learning of physiological responses: I. Habituation, sensitization, and classical conditioning. Behav. Neurosci. 1990, 104, 298–319. [Google Scholar] [CrossRef]
- Krinke, G.J. The laboratory rat. In The Handbook of Experimental Animals; Bullock, G., Bunton, T.E., Eds.; Academic Press: Waltham, MA, USA, 2000. [Google Scholar]
- Norman, H.; Nordquist, J.; Andersson, P.; Ansved, T.; Tang, X.; Dworkin, B.; Larsson, L. Impact of post-synaptic block of neuromuscular transmission, muscle unloading and mechanical ventilation on skeletal muscle protein and mrna expression. Pflugers Arch. 2006, 453, 53–66. [Google Scholar] [CrossRef]
- Frontera, W.R.; Larsson, L. Contractile studies of single human skeletal muscle fibers: A comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve 1997, 20, 948–952. [Google Scholar] [CrossRef]
- Moss, R.L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J. Physiol. 1979, 292, 177–192. [Google Scholar]
- Larsson, L.; Moss, R.L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J. Physiol. 1993, 472, 595–614. [Google Scholar]
- Fabiato, A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988, 157, 378–417. [Google Scholar] [CrossRef]
- Giulian, G.G.; Moss, R.L.; Greaser, M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal. Biochem. 1983, 129, 277–287. [Google Scholar] [CrossRef]
- Larsson, L.; Muller, U.; Li, X.; Schiaffino, S. Thyroid hormone regulation of myosin heavy chain isoform composition in young and old rats, with special reference to iix myosin. Acta Physiol. Scand. 1995, 153, 109–116. [Google Scholar] [CrossRef]
- Allison, S.P. The uses and limitations of nutritional support the arvid wretlind lecture given at the 14th espen congress in vienna, 1992. Clin. Nutr. 1992, 11, 319–330. [Google Scholar] [CrossRef]
- Bohe, J.; Low, J.F.; Wolfe, R.R.; Rennie, M.J. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 2001, 532, 575–579. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Haddad, S.H.; Tamim, H.M.; Rishu, A.H.; Sakkijha, M.H.; Kahoul, S.H.; Britts, R.J. Near-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. JPEN 2010, 34, 280–288. [Google Scholar] [CrossRef]
- Krishnan, J.A.; Parce, P.B.; Martinez, A.; Diette, G.B.; Brower, R.G. Caloric intake in medical icu patients: Consistency of care with guidelines and relationship to clinical outcomes. Chest 2003, 124, 297–305. [Google Scholar] [CrossRef]
- Rubinsky, M.D.; Clark, A.P. Early enteral nutrition in critically ill patients. Dimens. Crit. Care Nurs. 2012, 31, 267–274. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Goodenough, R.D.; Burke, J.F.; Wolfe, M.H. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann. Surg. 1983, 197, 163–171. [Google Scholar] [CrossRef]
- Shaw, J.H.; Wildbore, M.; Wolfe, R.R. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann. Surg. 1987, 205, 288–294. [Google Scholar] [CrossRef]
- Ishibashi, N.; Plank, L.D.; Sando, K.; Hill, G.L. Optimal protein requirements during the first 2 weeks after the onset of critical illness. Crit. Care Med. 1998, 26, 1529–1535. [Google Scholar] [CrossRef]
- Streat, S.J.; Beddoe, A.H.; Hill, G.L. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J. Trauma 1987, 27, 262–266. [Google Scholar] [CrossRef]
- Bailey, P.; Thomsen, G.E.; Spuhler, V.J.; Blair, R.; Jewkes, J.; Bezdjian, L.; Veale, K.; Rodriquez, L.; Hopkins, R.O. Early activity is feasible and safe in respiratory failure patients. Crit. Care Med. 2007, 35, 139–145. [Google Scholar]
- Ochala, J.; Ahlbeck, K.; Radell, P.J.; Eriksson, L.I.; Larsson, L. Factors underlying the early limb muscle weakness in acute quadriplegic myopathy using an experimental icu porcine model. PLoS One 2011, 6, e20876. [Google Scholar]
- Heyland, D.K. Critical care nutrition support research: Lessons learned from recent trials. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 176–181. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogilvie, H.; Larsson, L. The Effect of Nutritional Status in the Pathogenesis of Critical Illness Myopathy (CIM). Biology 2014, 3, 368-382. https://doi.org/10.3390/biology3020368
Ogilvie H, Larsson L. The Effect of Nutritional Status in the Pathogenesis of Critical Illness Myopathy (CIM). Biology. 2014; 3(2):368-382. https://doi.org/10.3390/biology3020368
Chicago/Turabian StyleOgilvie, Hannah, and Lars Larsson. 2014. "The Effect of Nutritional Status in the Pathogenesis of Critical Illness Myopathy (CIM)" Biology 3, no. 2: 368-382. https://doi.org/10.3390/biology3020368




