Interacting Memory Systems—Does EEG Alpha Activity Respond to Semantic Long-Term Memory Access in a Working Memory Task?
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Stimulus Presentation
2.3. Experimental Task and Trial Setup

2.4. EEG Acquisition
2.5. EEG Analysis
2.5.1. Scalp Level EEG Analysis
2.5.2. Source Level EEG Analysis
2.6. Statistical Analysis
2.6.1. Behavioural Analysis
2.6.2. EEG Data Analysis
3. Results and Discussion
3.1. Behavioural Data
3.2. EEG Data
3.2.1. Frontal Midline Theta and Distributed Theta Activity (4–7 Hz)

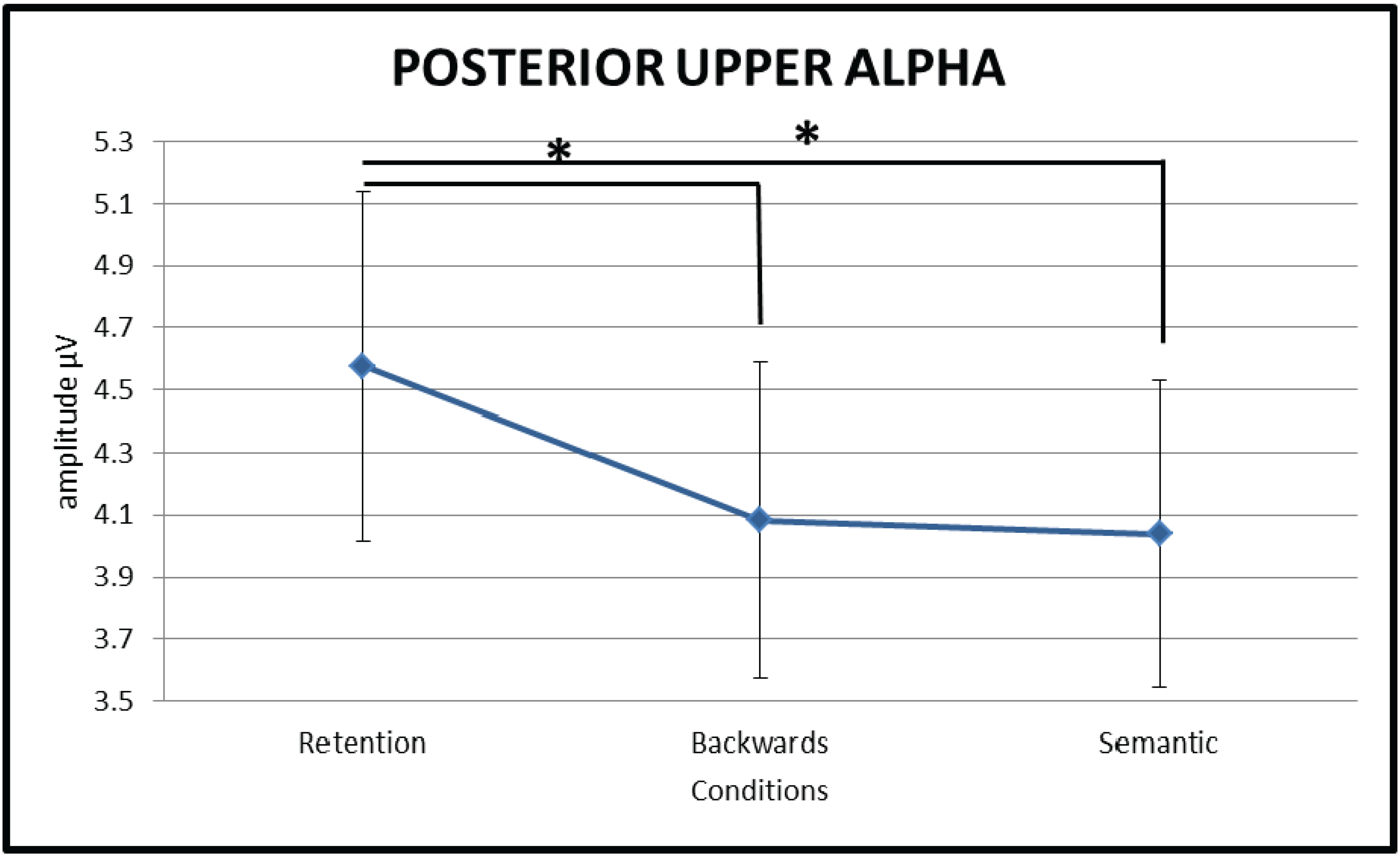
3.2.2. Upper Alpha (10–13 Hz)
3.2.3. Lower Beta (13–20 Hz)

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Baddeley, A. Working memory: Looking back and looking forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory. Sci. New Ser. 1992, 255, 556–559. [Google Scholar]
- Cowan, N. Attention and Memory: An Integrated Framework; Oxford Psychology Series, No. 26; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–185. [Google Scholar] [CrossRef] [PubMed]
- Ruchkin, D.S.; Grafman, J.; Cameron, K.; Berndt, R.S. Working memory retention systems: A state of activated long-term memory. Behav. Brain Sci. 2003, 26, 709–777. [Google Scholar] [PubMed]
- Fuster, J.M. More than working memory rides on long-term memory. Behav. Brain Sci. 2003, 26. [Google Scholar] [CrossRef]
- Fuster, J.M. Cortex and memory: Emergence of a new paradigm. J. Cogn. Neurosci. 2009, 21, 2047–2072. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M.; Bressler, S.L. Cognit activation: A mechanism enabling temporal integration in working memory. Trends Cogn. Sci. 2012, 16, 207–218. [Google Scholar] [CrossRef]
- D’Esposito, M. From cognitive to neural models of working memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Postle, B.R. Working memory as an emergent property of the mind and brain. Neuroscience 2006, 139, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control mechanisms in working memory: A possible function of EEG theta oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Freundberger, R.; Sauseng, P.; Gruber, W. A short review of slow phase synchronization and memory: Evidence for control processes in different memory systems? Brain Res. 2008, 1235, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Womelsdorf, T.; Vinck, M.; Stan Leung, L.; Everling, S. Selecive theta-synchronization of choice-relevant information subserves goal-directed behavior. Front. Hum. Neurosci. 2010, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Hoppe, J.; Klimesch, W.; Gerloff, C.; Hummel, F.C. Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. Eur. J. Neurosci. 2007, 25, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Griesmayr, B.; Berger, B.; Stelzig-Schoeler, R.; Aichhorn, W.; Bergmann, J.; Sauseng, P. EEG theta phase coupling during executive control of visual working memory investigated in individuals with schizophrenia and in healthy controls. Cogn. Affect. Behav. Neurosci. 2014. [Google Scholar] [CrossRef]
- Payne, L.; Kounios, J. Coherent oscillatory networks supporting short-term memory retention. Brain Res. 2009, 1247, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Schimke, H.; Schwaiger, J.; Doppelmayer, M.; Ripper, B.; Pfurtscheller, G. Event-related desynchronization (ERD) and the Dm-effect: Does alpha desynchronization during encoding predict alter recall performance? Int. J. Psychophysiol. 1996, 24, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Schack, B. Activation of long-term memory by alpha oscillations in a working-memory task? Behav. Brain Sci. 2003. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G. Induced oscillations in the alpha band: Functional meaning. Epilepsia 2003, 44, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, I.C.; Rushworth, M.F.; Nobre, A.C. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J. Neurophysiol. 2011, 105, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Haegens, S.; Handel, B.F.; Jensen, O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J. Neurosci. 2011, 31, 5197–5204. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Doppelmayr, M.; Pecherstorfer, T.; Freunberger, R.; Hanslmayr, S. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum. Brain Mapp. 2005, 26, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Gelfand, J.; Kounios, J.; Lisman, J.E. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb. Cortex 2002, 12, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Staudigl, T.; Fellner, M.-C. Oscillatory power decreases and long-term memory: The information via desynchronization hypothesis. Front. Hum. Neurosci. 2012, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Spitzer, B.; Bäuml, K.-H. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb. Cortex 2009, 19, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Smith, M.E.; McAvoy, L.; Yu, D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Gruber, W.; Doppelmayr, M.; Stadler, W.; Schabus, M. The interplay between theta and alpha oscillations in the electroencephalogram reflects the transfer of information between memory systems. Neurosci. Lett. 2002, 324, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Kizilirmak, J.M.; Rösler, F.; Khader, P.H. Control processes during selective long-term memory retrieval. NeuroImage 2012, 59, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R.; Shinosaki, K.; Ukai, S.; Inouye, T.; Ishihara, T.; Yoshimine, T.; Hirabuki, N.; Asada, H.; Kihara, T.; Robinson, S.E.; Takeda, M. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 1999, 10, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Khader, P.H.; Rösler, F. EEG power changes reflect distinct mechanisms during long-term memory retrieval. Psychophysiology 2011, 48, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Griesmayr, B.; Gruber, W.; Klimesch, W.; Sauseng, P. Human frontal midline theta and its synchronization to gamma during a verbal delayed match to sample task. Neurobiol. Learn. Mem. 2010, 93, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Seijnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Pernier, J.; Bertrand, O.; Echallier, J.F. Sperical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Jokisch, D.; Jensen, O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal and ventral stream. J. Neurosci. 2007, 27, 3244–3251. [Google Scholar] [CrossRef]
- Palva, J.M.; Palva, S.; Kaila, K. Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 2005, 25, 3962–3972. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Gruber, W.; Birbaumer, N. Cross-frequency phase synchronization: A brain mechanism of memory matching and attention. NeuroImage 2008, 40, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Heise, K.F.; Gruber, W.; Holz, E.; Karim, A.A.; Glennon, M.; Gerloff, C.; Birbaumer, N.; Hummel, F.C. Brain oscillatory substrates of visual short-term memory capacity. Curr. Biol. 2009, 15, 1846–1852. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D. Standardized low resolution electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar] [PubMed]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Rihs, T.A.; Michel, C.M.; Thut, G. A bias for posterior α-band power suppression versus enhancement during shifting versus maintenance of spatial attention. NeuroImage 2009, 44, 190–199. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, B.; Omer, S.; Minarik, T.; Sterr, A.; Sauseng, P. Interacting Memory Systems—Does EEG Alpha Activity Respond to Semantic Long-Term Memory Access in a Working Memory Task? Biology 2015, 4, 1-16. https://doi.org/10.3390/biology4010001
Berger B, Omer S, Minarik T, Sterr A, Sauseng P. Interacting Memory Systems—Does EEG Alpha Activity Respond to Semantic Long-Term Memory Access in a Working Memory Task? Biology. 2015; 4(1):1-16. https://doi.org/10.3390/biology4010001
Chicago/Turabian StyleBerger, Barbara, Serif Omer, Tamas Minarik, Annette Sterr, and Paul Sauseng. 2015. "Interacting Memory Systems—Does EEG Alpha Activity Respond to Semantic Long-Term Memory Access in a Working Memory Task?" Biology 4, no. 1: 1-16. https://doi.org/10.3390/biology4010001




