Treatment of Real-World HCV Genotype 2-Infected Japanese Patients with Sofosbuvir plus Ribavirin
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Clinical and Laboratory Assessments
2.3. Statistical Analysis
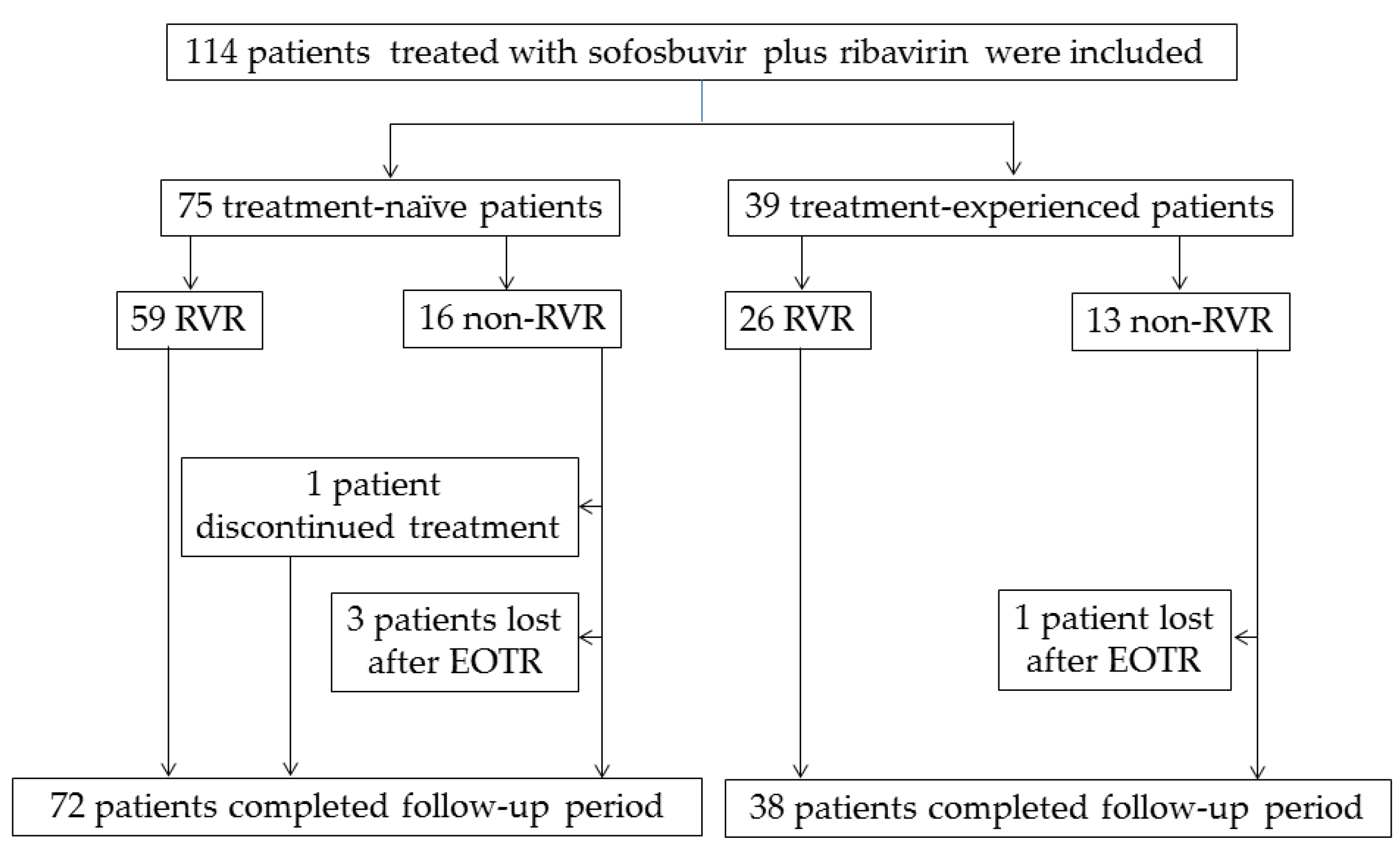
3. Results
3.1. Characteristics of the Patients in the Present Study
3.2. Virologic Response
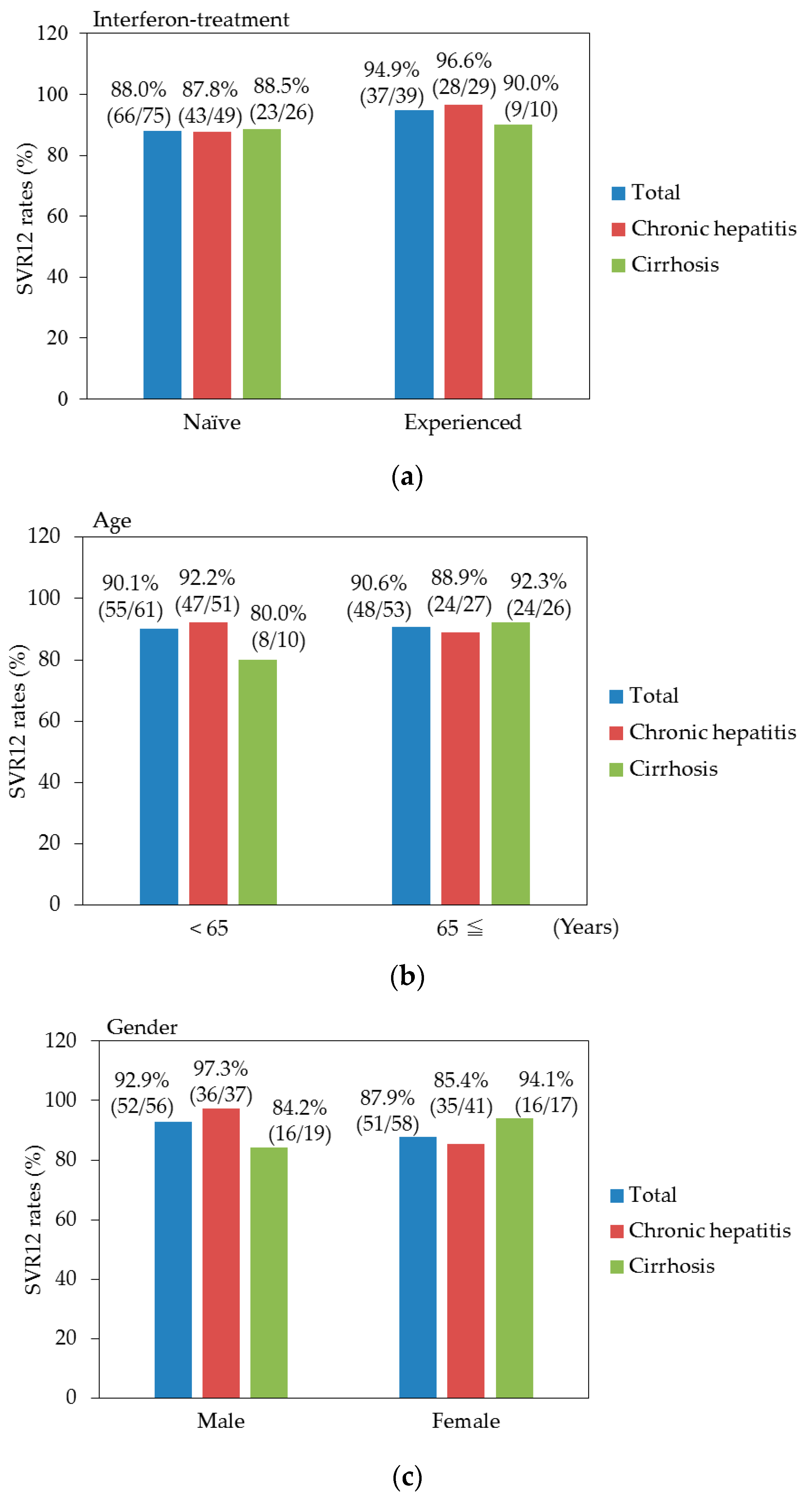
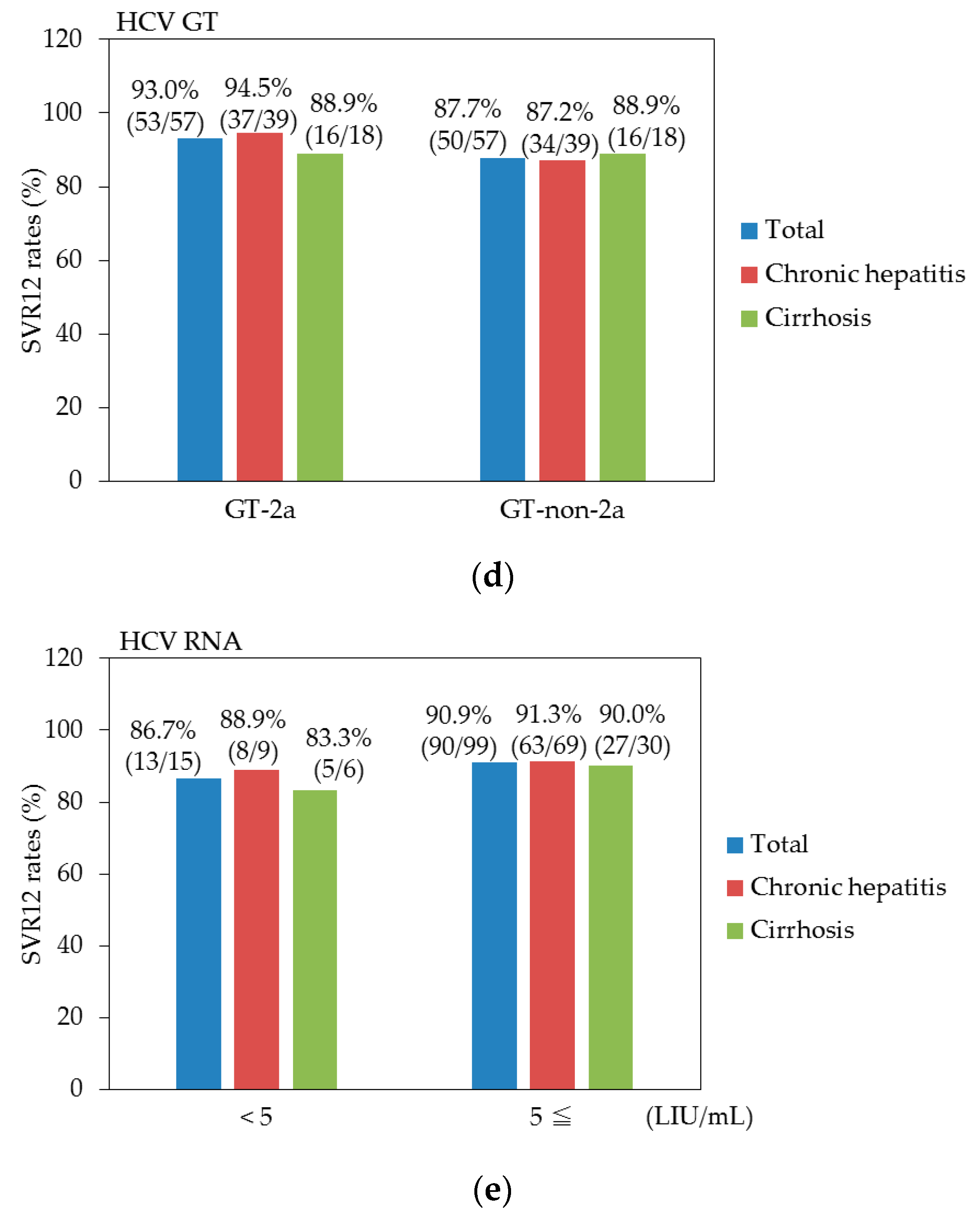
3.3. Efficacy for Patients with or without Cirrhosis
3.4. Factors at Baseline Associated with SVR
3.5. Adherence and Safety
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Bisceglie, A.M. Hepatitis C and hepatocellular carcinoma. Hepatology 1997, 26, 34S–38S. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Miyamura, T.; Ohbayashi, A.; Harada, H.; Katayama, T.; Kikuchi, S.; Watanabe, Y.; Koi, S.; Onji, M.; Ohta, Y.; et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1990, 87, 6547–6549. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.R.; Ghany, M.G.; Kim, H.Y.; Snow, K.K.; Shiffman, M.L.; De Santo, J.L.; Lee, W.M.; Di Bisceglie, A.M.; Bonkovsky, H.L.; Dienstag, J.L.; et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010, 52, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Imazeki, F.; Yokosuka, O. New antiviral therapies for chronic hepatitis C. Hepatol. Int. 2010, 4, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T. Interferon-free treatment for HCV-infected patients with decompensated cirrhosis. Hepatol. Int. 2017, 11, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Kanda, T.; Yokosuka, O.; Crawford, D.; Al-Mahtab, M.; Wei, L.; Ibrahim, A.; Lau, G.K.; Sharma, B.C.; Hamid, S.S.; et al. Features of hepatitis C virus infection, current therapies and ongoing clinical trials in ten Asian Pacific countries. Hepatol. Int. 2015, 9, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Imazeki, F.; Azemoto, R.; Yonemitsu, Y.; Mikami, S.; Kita, K.; Takashi, M.; Sunaga, M.; Wu, S.; Nakamoto, S.; et al. Response to peginterferon-alfa 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 2. Dig. Dis. Sci. 2011, 56, 3335–3342. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Nakamoto, S.; Nishino, T.; Takada, N.; Tsubota, A.; Kato, K.; Miyamura, T.; Maruoka, D.; Wu, S.; Tanaka, T.; et al. Peginterferon Alfa-2a plus ribavirin in Japanese patients infected with hepatitis C virus genotype 2 who failed previous interferon therapy. Int. J. Med. Sci. 2013, 10, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.M.; Gordon, S.C.; Kowdley, K.V.; Yoshida, E.M.; Rodriguez-Torres, M.; Sulkowski, M.S.; Shiffman, M.L.; Lawitz, E.; Everson, G.; Bennett, M.; et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 2013, 368, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Tanaka, M.; Shino, Y.; Shimada, H.; Tomonaga, T.; Nomura, F.; Nagao, K.; Ochiai, T.; et al. Inhibition of subgenomic hepatitis C virus RNA in Huh-7 cells: Ribavirin induces mutagenesis in HCV RNA. J. Viral Hepat. 2004, 11, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Nishiguchi, S.; Ueno, Y.; Mochizuki, H.; Izumi, N.; Ikeda, F.; Toyoda, H.; Yokosuka, O.; Nirei, K.; Genda, T.; et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: An open-label, phase 3 trial. J. Viral Hepat. 2014, 21, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Etoh, R.; Imazeki, F.; Kurihara, T.; Fukai, K.; Fujiwara, K.; Arai, M.; Kanda, T.; Mikata, R.; Yonemitsu, Y.; Yokosuka, O. Pegylated interferon-alfa-2a monotherapy in patients infected with HCV genotype 2 and importance of rapid virological response. BMC Res. Notes 2011, 4, 316. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Imazeki, F.; Wu, S.; Nakamoto, S.; Yokosuka, O. The assessment of serum hepatitis C virus RNA 12 weeks after the end of treatment using TaqMan polymerase chain reaction is less relevant than after 24 weeks for predicting sustained virological response. Hepatology 2011, 54, 1482. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Florian, J.; Carter, W.; Fleischer, R.D.; Hammerstrom, T.S.; Jadhav, P.R.; Zeng, W.; Murray, J.; Birnkrant, D.D. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology 2013, 144, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Nakamoto, S.; Sasaki, R.; Nakamura, M.; Yasui, S.; Haga, Y.; Ogasawara, S.; Tawada, A.; Arai, M.; Mikami, S.; et al. Sustained Virologic Response at 24 Weeks after the End of Treatment Is a Better Predictor for Treatment Outcome in Real-World HCV-Infected Patients Treated by HCV NS3/4A Protease Inhibitors with Peginterferon plus Ribavirin. Int. J. Med. Sci. 2016, 13, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kanda, T.; Katsuno, T.; Yasui, S.; Haga, Y.; Sasaki, R.; Nakamura, M.; Wu, S.; Nakamoto, S.; Arai, M.; et al. Successful sofosbuvir treatment with ribavirin dose reduction for chronic hepatitis C virus genotype 2 infection in a patient with ulcerative colitis: A case report. BMC Gastroenterol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Kanda, T.; Yasui, S.; Haga, Y.; Nakamura, M.; Yamato, M.; Wu, S.; Nakamoto, S.; Arai, M.; Mikami, S.; et al. Successful Eradication of Hepatitis C Virus by Interferon-Free Regimens in Two Patients with Advanced Liver Fibrosis following Kidney Transplantation. Case Rep. Gastroenterol. 2016, 10, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Kanda, T.; Ohtsuka, M.; Yasui, S.; Haga, Y.; Nakamura, M.; Yokoyama, M.; Wu, S.; Nakamoto, S.; Arai, M.; et al. Successful Management of Graft Reinfection of HCV Genotype 2 in Living Donor Liver Transplantation from a Hepatitis B Core Antibody-Positive Donor with Sofosbuvir and Ribavirin. Case Rep. Gastroenterol. 2016, 10, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Win, N.N.; Nakamoto, S.; Kanda, T.; Takahashi, H.; Takahashi-Nakaguchi, A.; Yasui, S.; Nakamura, M.; Wu, S.; Imazeki, F.; Mikami, S.; et al. Discrepancy between Hepatitis C Virus Genotypes and NS4-Based Serotypes: Association with Their Subgenomic Sequences. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- McHutchison, J.G.; Manns, M.; Patel, K.; Poynard, T.; Lindsay, K.L.; Trepo, C.; Dienstag, J.; Lee, W.M.; Mak, C.; Garaud, J.J.; et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002, 123, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Aloia, A.L.; Locarnini, S.; Beard, M.R. Antiviral resistance and direct-acting antiviral agents for HCV. Antivir. Ther. 2012, 17, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kozak, R.A.; Biondi, M.J.; Pilon, R.; Vallee, D.; Liang, B.B.; La, D.; Kim, J.; Van Domselaar, G.; Leonard, L.; et al. Next generation sequencing of the hepatitis C virus NS5B gene reveals potential novel S282 drug resistance mutations. Virology 2015, 477, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Bacon, B.R.; Curry, M.P.; Dieterich, D.T.; Flamm, S.L.; Guest, L.E.; Kowdley, K.V.; Lee, Y.; Tsai, N.C.; Younossi, Z.M.; et al. Evaluation of proton pump inhibitor use on treatment outcomes with ledipasvir and sofosbuvir in a real-world cohort study. Hepatology 2016, 64, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Furusyo, N.; Nomura, H.; Takahashi, K.; Higashi, N.; Kawano, A.; Dohmen, K.; Satoh, T.; Azuma, K.; Nakamuta, M.; et al. Effectiveness and safety of sofosbuvir plus ribavirin for HCV genotype 2 patients 65 and over with or without cirrhosis. Antivir. Res. 2016, 136, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Morio, K.; Imamura, M.; Kawakami, Y.; Nakahara, T.; Nagaoki, Y.; Kawaoka, T.; Tsuge, M.; Hiramatsu, A.; Aikata, H.; Hayes, C.N.; et al. ITPA polymorphism effects on decrease of hemoglobin during sofosbuvir and ribavirin combination treatment for chronic hepatitis C. J. Gastroenterol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Nguyen, P.; Le, A.; Zhao, C.; Ahmed, A.; Daugherty, T.; Garcia, G.; Lutchman, G.; Kumari, R.; Nguyen, M.H. Real-world experience with interferon-free, direct acting antiviral therapies in Asian Americans with chronic hepatitis C and advanced liver disease. Medicine (Baltimore) 2017, 96, e6128. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, T.; Kanda, T.; Nakamoto, S.; Wu, S.; Jiang, X.; Arai, M.; Fujiwara, K.; Imazeki, F.; Yokosuka, O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses 2012, 4, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Kim, S.K.; Kim, S.R.; Kobayashi, M.; Kato, A.; Morimoto, E.; Imoto, S.; Kim, C.W.; Tanaka, Y.; Kudo, M.; et al. Efficacy and Safety of Sofosbuvir Plus Ribavirin Treatment for Patients with Chronic Hepatitis C Genotype 2. Dig. Dis. 2016, 34, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, R.; Hai, H.; Teranishi, Y.; Motoyama, H.; Kawamura, E.; Hagihara, A.; Uchida-Kobayashi, S.; Morikawa, H.; Enomoto, M.; Murakami, Y.; et al. ITPA polymorphism correlates with the reductions in hemoglobin concentration and ribavirin dose during sofosbuvir and ribavirin therapy. J. Gastroenterol. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A.; Elshemey, W.M.; Gawad, W.A.; Desoky, O.S. Molecular modeling comparison of the performance of NS5b polymerase inhibitor (PSI-7977) on prevalent HCV genotypes. Protein J. 2013, 32, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.J.; Hyland, R.H.; Yang, Y.; Svarovskaia, E.; Stamm, L.M.; Brainard, D.M.; McHutchison, J.G.; Stedman, C.A. Efficacy of Ledipasvir Plus Sofosbuvir for 8 or 12 Weeks in Patients With Hepatitis C Virus Genotype 2 Infection. Gastroenterology 2017. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y.; Badshah, M.B. New hepatitis C virus therapies: Drug classes and metabolism, drug interactions relevant in the transplant settings, drug options in decompensated cirrhosis, and drug options in end-stage renal disease. Curr. Opin. Organ Transplant. 2015, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; Janssen, H.L.A.; Boonstra, A. Hepatitis C treatment and liver cancer recurrence: Cause for concern? Lancet Gastroenterol. Hepatol. 2017, 2, 78–80. [Google Scholar] [CrossRef]
- Collins, J.M.; Raphael, K.L.; Terry, C.; Cartwright, E.J.; Pillai, A.; Anania, F.A.; Farley, M.M. Hepatitis B Virus Reactivation During Successful Treatment of Hepatitis C Virus With Sofosbuvir and Simeprevir. Clin. Infect. Dis. 2015, 61, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Sato, T.; Ikeda, F.; Fujiki, S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol. Res. 2016, 46, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Orkin, C.; Iser, D.M.; Zamora, F.X.; Nelson, M.; Stephan, C.; Massetto, B.; Gaggar, A.; Ni, L.; Svarovskaia, E.; et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): A multicentre, open-label, non-randomised, phase 3 study. Lancet 2015, 385, 1098–1106. [Google Scholar] [CrossRef]
| Characteristics | All (n = 114) | Treatment-Naïve (n = 75) | Treatment-Experienced (n = 39) | p-Values 1 |
|---|---|---|---|---|
| Age (years) | 62.0 ± 12.5 | 60.2 ± 13.3 | 65.6 ± 10.0 | 0.028 |
| Gender (male/female) | 56/58 | 38/37 | 18/21 | 0.795 |
| Interferon (naive/experienced) | 75/39 | 75/0 | 0/39 | |
| HCV GT (2a/2b/unknown) | 57/46/11 | 39/31/5 | 18/15/6 | 0.920 |
| HCV RNA (L/H) | 15/99 | 11/64 | 4/35 | 0.712 |
| Body weight (kg) | 61.9 ± 12.1 | 61.8 ± 12.7 | 62.2 ± 11.2 | 0.869 |
| Body length (cm) | 162.3 ± 8.8 | 163.2 ± 8.8 | 160.4 ± 8.7 | 0.108 |
| Histories of HCC +/− | 8/106 | 5/70 | 3/36 | 0.855 |
| Chronic hepatitis/cirrhosis | 78/36 | 49/26 | 29/10 | 0.441 |
| Liver stiffness (kPa) | 10.2 ± 8.3 | 10.5 ± 8.7 | 9.6 ± 7.5 | 0.584 |
| AST (IU/L) | 50.6 ± 38.7 | 56.1 ± 43.8 | 38.8 ± 20.7 | 0.022 |
| ALT (IU/L) | 54.5 ± 51.6 | 62.8 ± 58.4 | 36.9 ± 25.7 | 0.010 |
| Hemoglobin (g/dL) | 13.5 ± 1.4 | 13.6 ± 1.5 | 13.3 ± 1.3 | 0.291 |
| Platelets (×104/μL) | 16.5 ± 6.1 | 16.8 ± 6.7 | 15.8 ± 4.7 | 0.407 |
| eGFR (mL/min/1.73 m2) | 78.0 ± 20.2 | 80.7 ± 20.5 | 72.2 ± 18.6 | 0.032 |
| Characteristics | All (n = 114) | Treatment-Naïve (n = 75) | Treatment-Experienced (n = 39) | p-Values 1 |
|---|---|---|---|---|
| HCV undetectable no. (%) | ||||
| During treatment | ||||
| At 4 w | 85 (74.6) | 59 (78.7) | 26 (66.7) | 0.242 |
| At 8 w | 111 (97.4) | 72 (96.0) | 39 (100) | 0.516 |
| At 12 w | 113 (99.1) | 74 (98.7) | 39 (100) | 0.738 |
| After treatment | ||||
| Post 4 w | 108 (94.7) | 72 (96.0) | 37 (94.9) | 0.839 |
| Post 8 w | 104 (91.2) | 67 (89.3) | 37 (94.9) | 0.520 |
| Post 12 w | 103 (90.4) | 66 (88.0) | 37 (94.9) | 0.398 |
| Virologic failure | ||||
| Discontinuation | 1 | 1/75 (1.3) | 0/39 (0) | 0.738 |
| Relapse | 6 | 5/72 (6.9) | 1/38 (2.6) | 0.613 |
| Characteristics | SVR (n = 103) | Non-SVR (n = 7) | Univariate | Multivariate |
|---|---|---|---|---|
| p-values 1 | p-values 1 | |||
| Age (years) | 62.4 ± 12.4 | 61.6 ± 13.7 | 0.870 | |
| Gender (male/female) | 52/51 | 2/5 | 0.464 | |
| Interferon (naive/experienced) | 66/37 | 6/1 | 0.451 | |
| HCV GT (2a/others) | 53/50 | 1/6 | 0.131 | 0.094 |
| HCV RNA (L/H) | 13/90 | 1/6 | 0.647 | |
| Body weight (kg) | 61.7 ± 12.2 | 63.4 ± 17.2 | 0.729 | |
| Body length (cm) | 162.7 ± 8.8 | 159.5 ± 9.9 | 0.357 | |
| Histories of HCC +/− | 7/96 | 1/7 | 0.913 | |
| Chronic hepatitis/cirrhosis | 71/32 | 5/2 | 0.776 | |
| Liver stiffness (kPa) | 10.2 ± 8.3 | 8.4 ± 3.1 | 0.571 | |
| AST (IU/L) | 50.6 ± 38.7 | 48.0 ± 29.2 | 0.862 | |
| ALT (IU/L) | 54.5 ± 51.6 | 48.3 ± 29.9 | 0.754 | |
| Hemoglobin (g/dL) | 13.5 ± 1.4 | 13.3 ± 1.3 | 0.714 | |
| Platelets (×104/μL) | 16.5 ± 6.1 | 17.0 ± 9.8 | 0.841 | |
| eGFR (mL/min/1.73 m2) | 70.5 ± 17.5 | 88.4 ± 26.7 | 0.0129 | 0.116 |
| No. | Age/Gender | Treatment | GT | Cirrhosis | Efficacies | Adherence >80% | NS5B-S282 | Others |
|---|---|---|---|---|---|---|---|---|
| 1 | 62/Male | Naive | 2b | Yes | Discontinued at 2 w | No | Wild | |
| 2 | 43/Female | Naive | 2b | No | Relapse (post 4 w) | Yes | Wild | |
| 3 | 63/Female | Naive | 2a | No | Relapse (post 4 w) | Yes | Wild | |
| 4 | 74/Female | Naive | 2 | No | Relapse (post 8 w) | No 1 | Wild | |
| 5 | 79/Male | Naive | 2b | Yes 1 | Relapse (post 8 w) | Yes | Wild | SR-BII 2 |
| 6 | 44/Female | Naive | 2b | No | Relapse (post 12 w) | Yes | Wild | |
| 7 | 66/Female | Experienced | 2b | No | Relapse (post 4 w) | Yes | Wild |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Nakamura, M.; Yasui, S.; Haga, Y.; Tawada, A.; Suzuki, E.; Ooka, Y.; Takahashi, K.; Sasaki, R.; Wu, S.; et al. Treatment of Real-World HCV Genotype 2-Infected Japanese Patients with Sofosbuvir plus Ribavirin. Biology 2017, 6, 30. https://doi.org/10.3390/biology6020030
Kanda T, Nakamura M, Yasui S, Haga Y, Tawada A, Suzuki E, Ooka Y, Takahashi K, Sasaki R, Wu S, et al. Treatment of Real-World HCV Genotype 2-Infected Japanese Patients with Sofosbuvir plus Ribavirin. Biology. 2017; 6(2):30. https://doi.org/10.3390/biology6020030
Chicago/Turabian StyleKanda, Tatsuo, Masato Nakamura, Shin Yasui, Yuki Haga, Akinobu Tawada, Eiichiro Suzuki, Yoshihiko Ooka, Koji Takahashi, Reina Sasaki, Shuang Wu, and et al. 2017. "Treatment of Real-World HCV Genotype 2-Infected Japanese Patients with Sofosbuvir plus Ribavirin" Biology 6, no. 2: 30. https://doi.org/10.3390/biology6020030







