Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Determination of Grain Initial Moisture Content
2.3. Estimating Grain Moisture Content over Time
- Individual coated grain (coat share >75%) − average uncoated grain = weight of coating
- Weight of imbibed grain − weight imbibed seed within = weight of imbibed coating
- Weight of imbibed coating − weight of coating = amount of water
- Moisture content of coating = (amount of water/Imbibed coating) × 100
2.4. Estimating Grain Imbibition Rate and Capacity
2.5. Measurement of Oxygen Concentrations in the Coated and Uncoated Seeds
2.6. Extraction and Quantification of Sugars
2.7. Calculation of the Amount of Sugars
2.8. Quantification of Enzymatic Activity
2.9. Statistical Analysis
3. Results
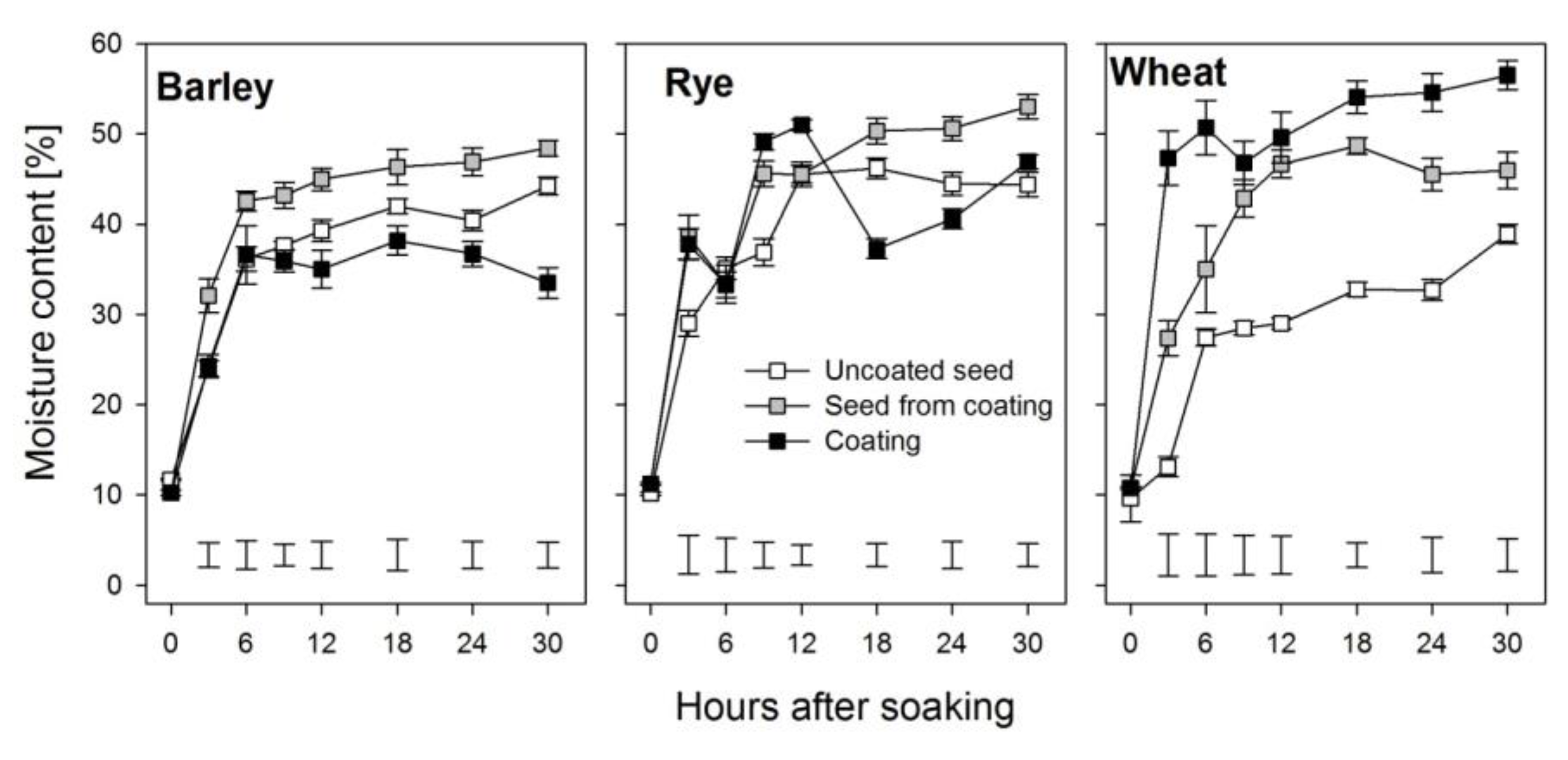
3.1. Effects of Coating on Imbibition
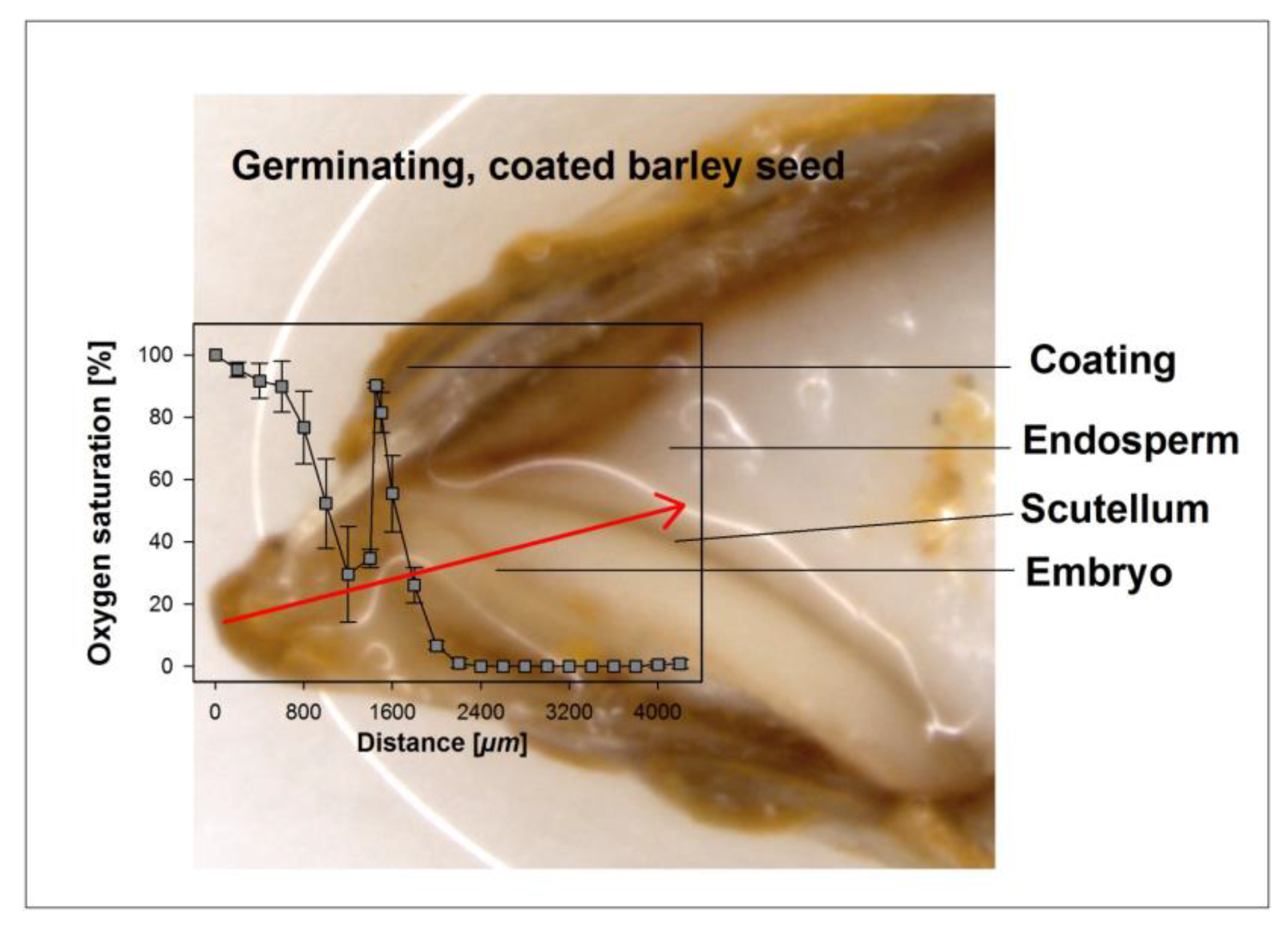
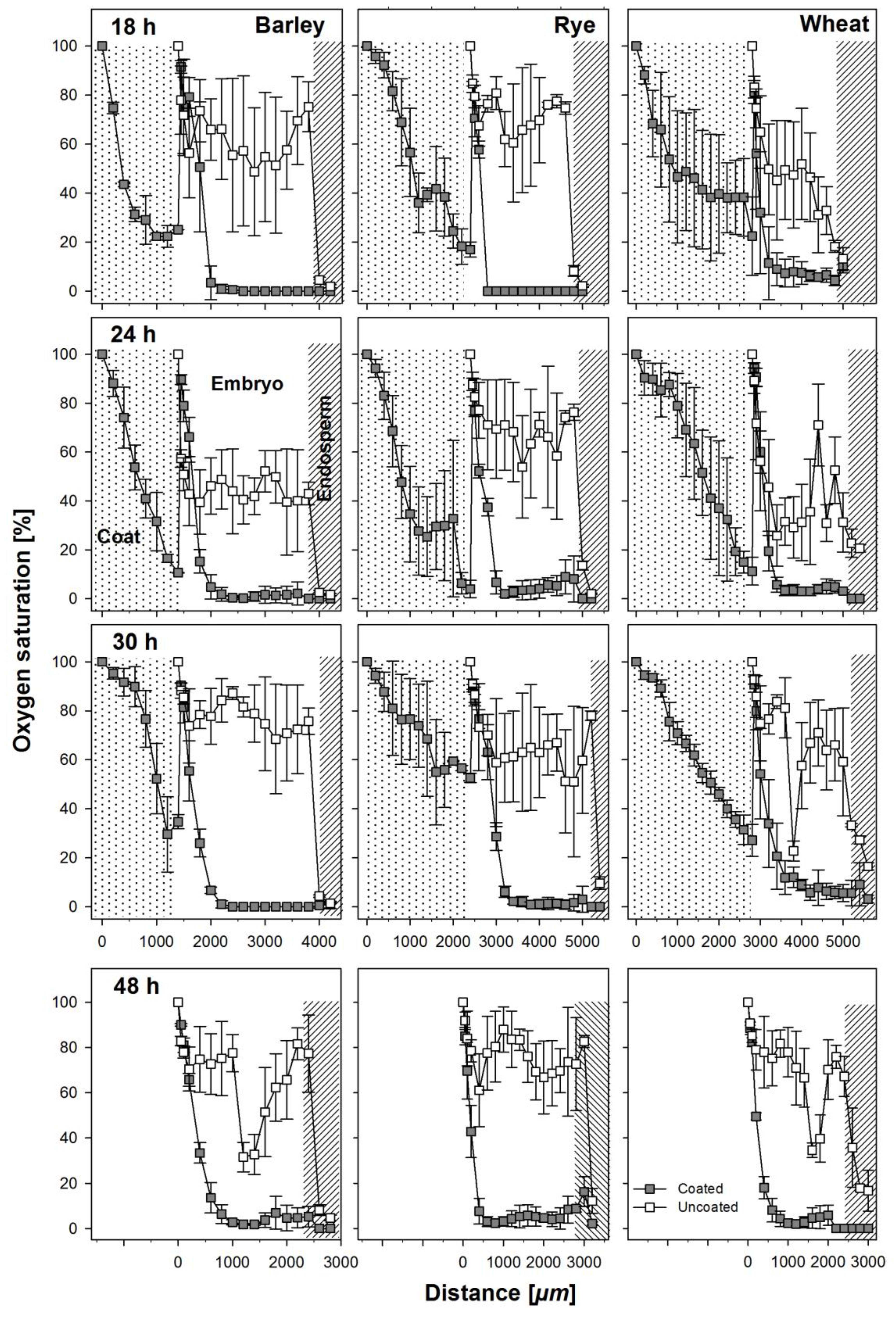
3.2. Oxygen Profiles in Coated and Uncoated Seeds
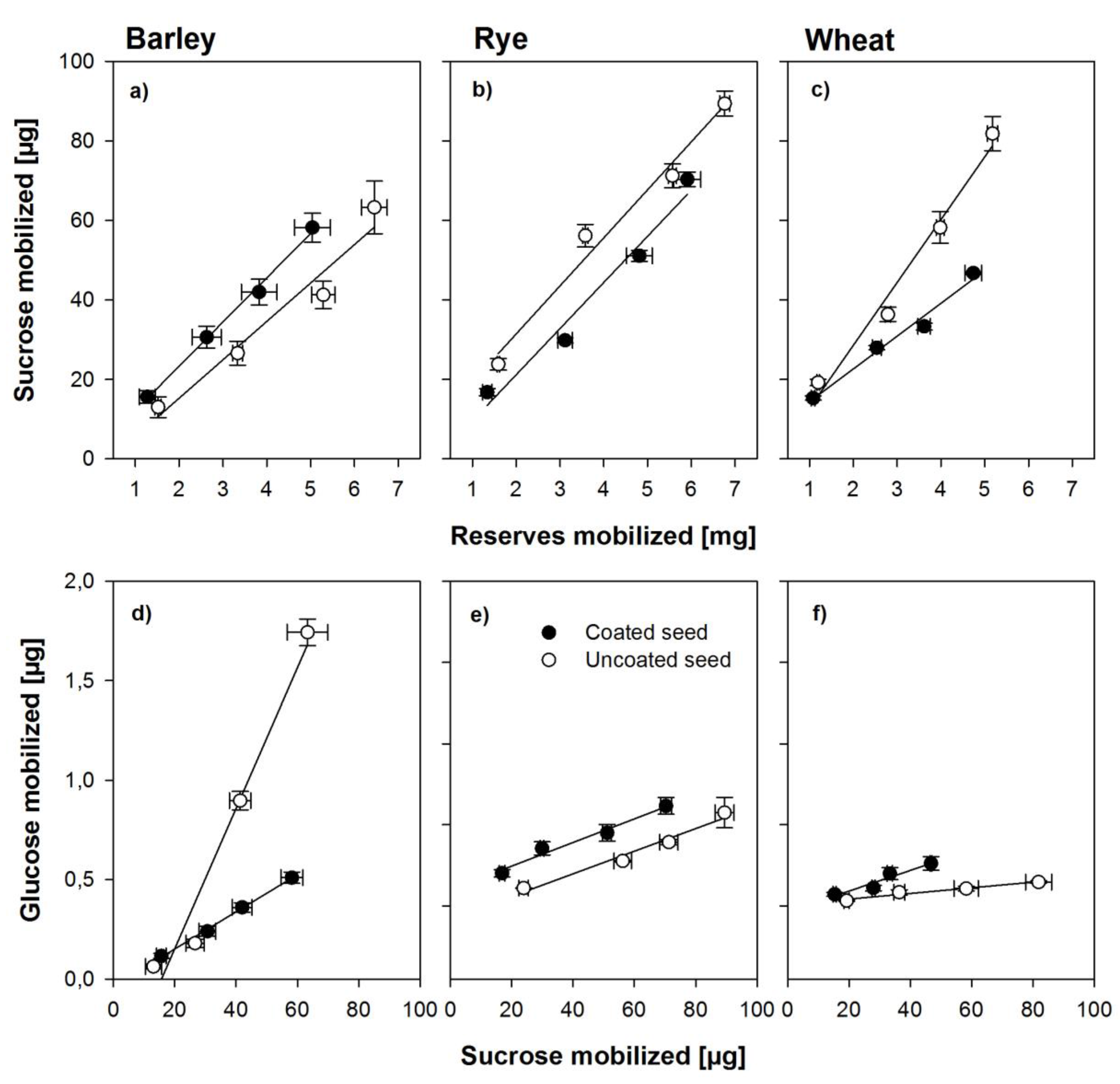
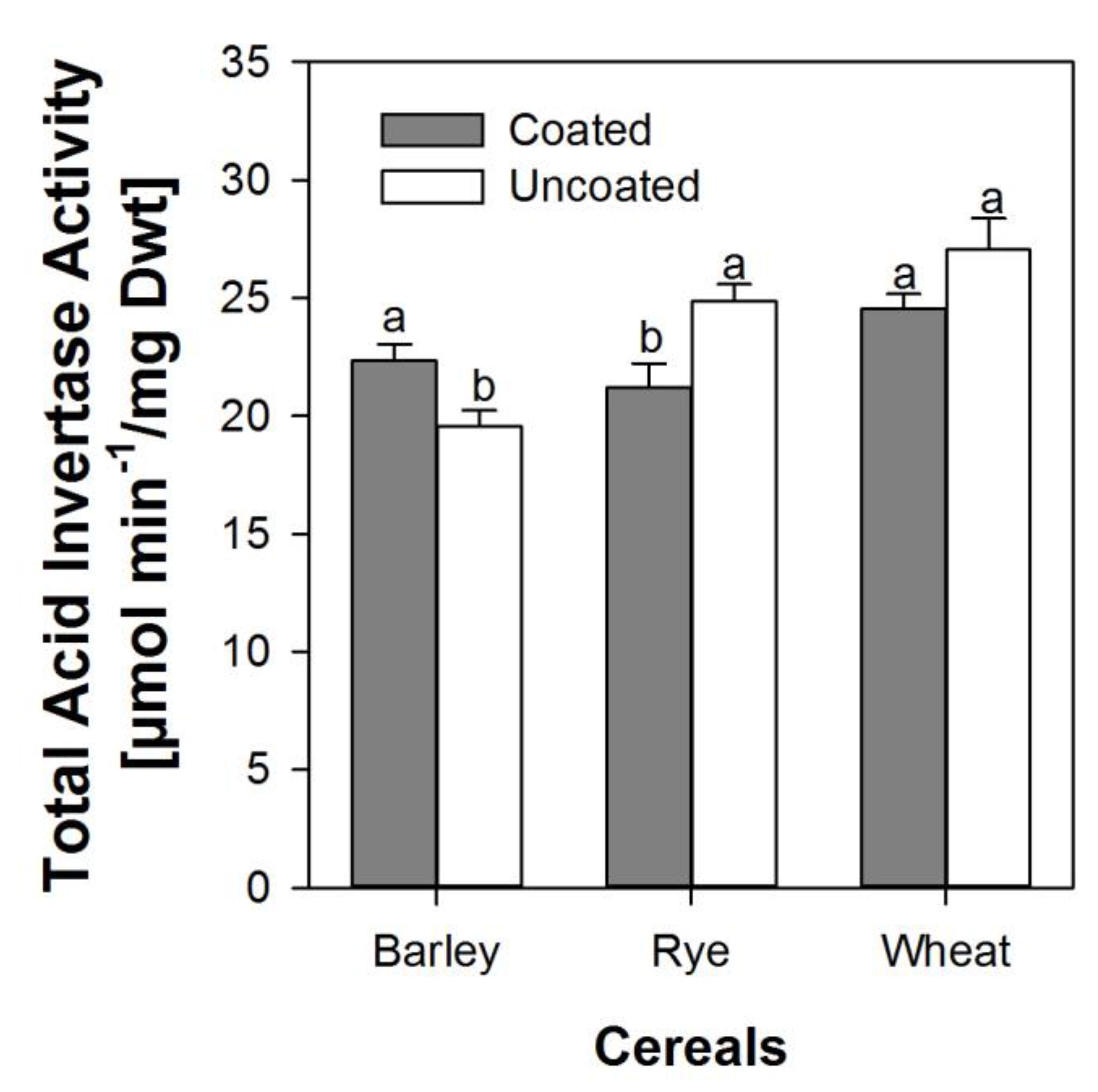
3.3. Effects of Coating on Soluble and Insoluble Invertase Activity
4. Discussion
4.1. Effects of Coating on Water Imbibition
4.2. Effect of Coating on Oxygen Availability
4.3. Effects of Coating on Sugar Metabolism and Enzyme Activity
4.4. Effect of Hydro-Absorber Coating on Enzyme Activity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berdahl, J.D.; Barker, R.E. Germination and emergence of Russian wildrye seeds coated with hydrophilic materials. Agron. J. 1980, 72, 1006–1008. [Google Scholar] [CrossRef]
- Schneider, A.; Renault, P. Effects of coating on seed imbibitions: I. Model estimates of water transport coefficient. Crop Sci. 1997, 37, 1841–1849. [Google Scholar] [CrossRef]
- Willenborg, C.J.; Gulden, R.H.; Johnson, E.N.; Shirtliffe, S.J. Germination characteristics of polymer-coated canola (Brassica napus L.) seeds subjected to moisture stress at different temperatures. Agron. J. 2004, 96, 786–791. [Google Scholar] [CrossRef]
- Gorim, L.; Asch, F. Effects of composition and share of seed coatings on the mobilization efficiency of cereal seeds during germination. J. Agron. Crop Sci. 2012, 198, 81–91. [Google Scholar] [CrossRef]
- Baxter, L.; Waters, L. Effect of a hydrophilic polymer seed coating on the imbibitions, respiration and germination of sweet corn at four matrix potentials. J. Am. Soc. Hortic. Sci. 1986, 111, 517–520. [Google Scholar]
- Klein, J.D.; Sachs, M. Measurement of water uptake and volatile production by coated wheat seeds and subsequent seedling growth. Seed Sci. Technol. 1992, 20, 299–305. [Google Scholar]
- Gorim., L.; Asch, F. Seed coating reduces respiration losses and affects sugar metabolism during germination in cereals. Funct. Plant Biol. 2015, 42, 209–218. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Y.; Avigne, T.W.; Koch, E.K. Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Phys. 1998, 116, 1573–1583. [Google Scholar] [CrossRef]
- Howell, K.A.; Narsai, R.; Carroll, A.; Ivanova, A.; Lohse, M.; Usadel, B.; Millar, A.H.; Whelan, J. Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Phys. 2009, 149, 961–980. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination-still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Usadel, B.; Winter, A.; Radchuk, V.; Scholz, U.; Stein, N.; Weschke, W.; Strickert, M.; Close, T.J.; Stitt, M.; et al. Barley grain maturation and germination: Metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Phys. 2008, 146, 1738–1758. [Google Scholar] [CrossRef] [PubMed]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Guglielminetti, L.; Alpi, A. Mobilization of endosperm reserves in cereal seeds under anoxia. Ann. Bot. 1997, 79, 49–56. [Google Scholar] [CrossRef]
- Rolletschek, H.; Koch, K.; Wobus, U.; Borisjuk, L. Positional cue for the starch/lipid balance in maize kernels and resource partitioning to the embryo. Plant J. 2005, 42, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Rolletschek, H.; Stangelmayer, A.; Borisjuk, L. Methodology and significance of micro-sensor-based oxygen mapping in plant seeds—An overview. Sensors 2009, 9, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, V.; Sankat, C.K. Rehydration characteristics and quality of dehydrated dasheen leaves. Can. Agric. Eng. 2000, 42, 81–85. [Google Scholar]
- Turhan, M.; Sayar, S.; Gunasekaran, S. Application of Peleg model to study water absorption in chickpea during soaking. J. Food Eng. 2002, 53, 153–159. [Google Scholar] [CrossRef]
- Peleg, M. An empirical model for the description of moisture sorption curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2010. [Google Scholar]
- Rolletschek, H.; Borisjuk, L.; Koschorreck, M.; Wobus, U.; Weber, H. Legume embryo develop in a hypoxic environment. J. Exp. Bot. 2002, 53, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.M.; Asch, F.; Wu, Y.; Jensen, R.C.; Naested, H.; Mogensen, O.V.; Koch, E.K. Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Phys. 2002, 130, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Salamini, F.; Nelson, O.E. Enzymes in carbohydrate metabolism in the developing endosperm of maize. Plant Phys. 1970, 46, 299–306. [Google Scholar] [CrossRef]
- Leopold, A.C. Volumetric components of seed imbibition. Plant Phys. 1983, 73, 677–680. [Google Scholar] [CrossRef]
- Meyer, C.; Steudle, E.; Peterson, C.A. Patterns and kinetics of water uptake by soybean seeds. J. Exp. Bot. 2007, 58, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Phys. Plant Mol. Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Aoki, N.; Scofield, G.N.; Wang, X.-D.; Offler, C.E.; Patrick, J.W.; Furbank, R.T. Pathway of sugar transport in germinating wheat seeds. Plant Phys. 2006, 141, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Rathjen, J.R.; Strounina, E.V.; Mares, D.J. Water movements into dormant and non dormant wheat (Triticum aestivum L.) grains. J. Exp. Bot. 2009, 60, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Chrispeels, M.J.; Tenner, A.J.; Johnson, K.D. Synthesis and release of sucrose by the aleurone layer of barley: Regulation by gibberelic acid. Planta 1973, 113, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, Y; Avigne, W.T.; Koch, K.E. Rapid repression of maize invertases by low oxygen. Invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Phys. 1999, 121, 599–608. [Google Scholar] [CrossRef]
- Guglielminetti, L.; Perata, P.; Alpi, A. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Phys. 1995, 108, 735–741. [Google Scholar] [CrossRef]
| Cereals | Treatments | K1 × 10−2 (h %−1) | K2 × 10−2 (%−1) | IC (%) | Me (%) |
|---|---|---|---|---|---|
| Barley | SFC | 5.0 (±0.8)aB | 2.5 (±0.1)aB | 40.4 (±0.8)aB | 51.1 (±0.8)aB |
| Uncoated | 5.9 (±1.2)aB | 2.8 (±0.1)aB | 35.8 (±1.7)aA | 47.5 (±1.7)aA | |
| Rye | SFC | 8.9 (±0.9)aA | 2.1 (±0.1)bC | 47.1 (±2.1)aA | 58.3 (±2.1)aA |
| Uncoated | 5.4 (±0.3)bB | 2.6 (±0.2)aB | 37.9 (±0.3)bA | 48.1 (±0.3)bA | |
| Wheat | SFC | 2.5 (±0.6)bB | 2.8 (±0.1)bA | 36.2 (±1.0)aB | 47.0 (±1.0)aB |
| Uncoated | 11.8 (±2.1)aA | 4.1 (±0.2)aA | 24.3 (±0.9)bB | 33.9 (±0.9)bB |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorim, L.; Asch, F. Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds. Biology 2017, 6, 31. https://doi.org/10.3390/biology6020031
Gorim L, Asch F. Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds. Biology. 2017; 6(2):31. https://doi.org/10.3390/biology6020031
Chicago/Turabian StyleGorim, Linda, and Folkard Asch. 2017. "Seed Coating Increases Seed Moisture Uptake and Restricts Embryonic Oxygen Availability in Germinating Cereal Seeds" Biology 6, no. 2: 31. https://doi.org/10.3390/biology6020031






