Comparing Nutrient Removal from Membrane Filtered and Unfiltered Domestic Wastewater Using Chlorella vulgaris
Abstract
:1. Introduction
- (1)
- Evaluate the application of microalgae to current wastewater treatment with a focus on improving the quality of final effluent.
- (2)
- Assess the use of membrane technology to be included as a part of the wastewater treatment process.
2. Materials and Methods
2.1. Wastewater Collection
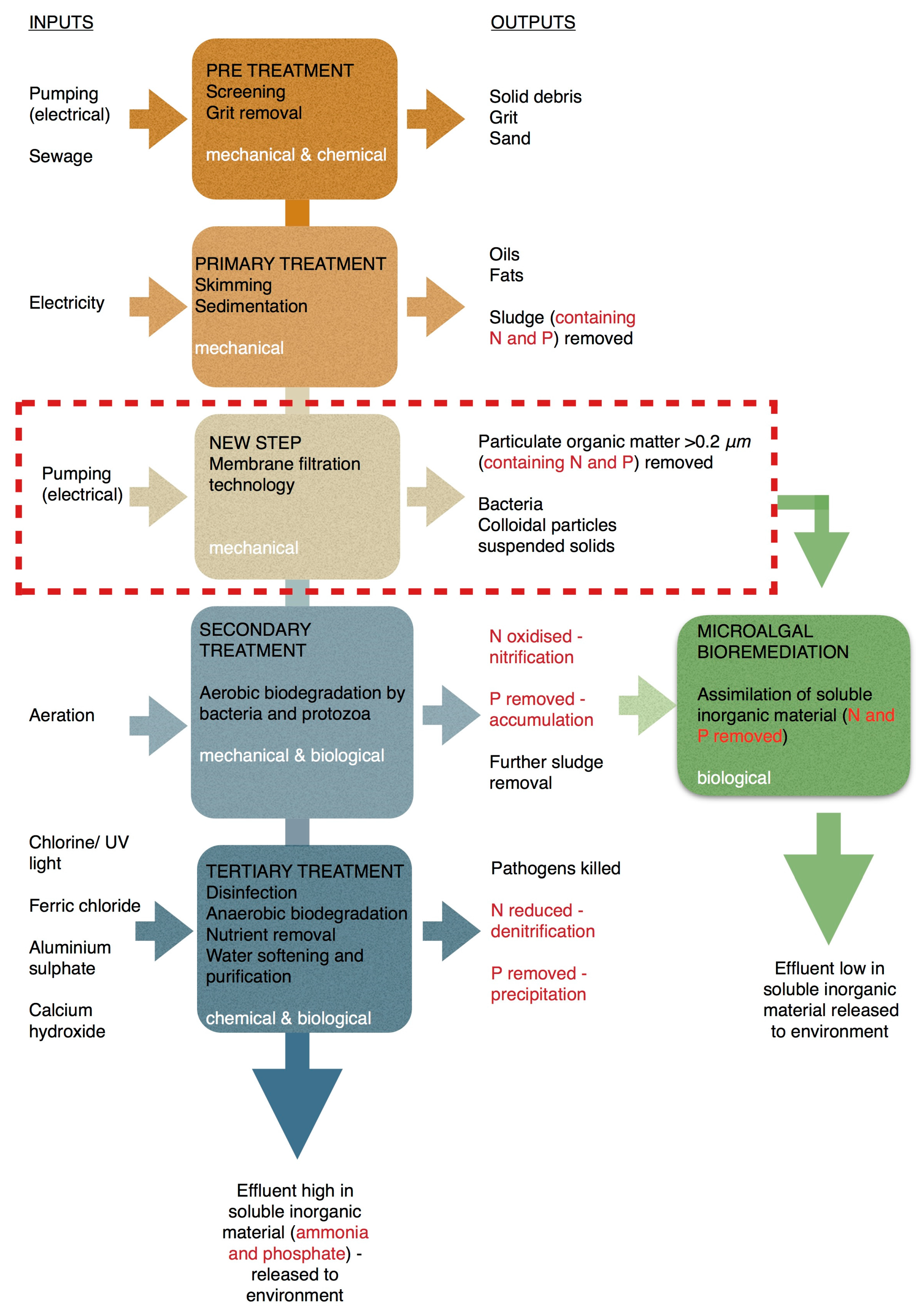
2.2. Pre-Treatments and Characteristics of Wastewater
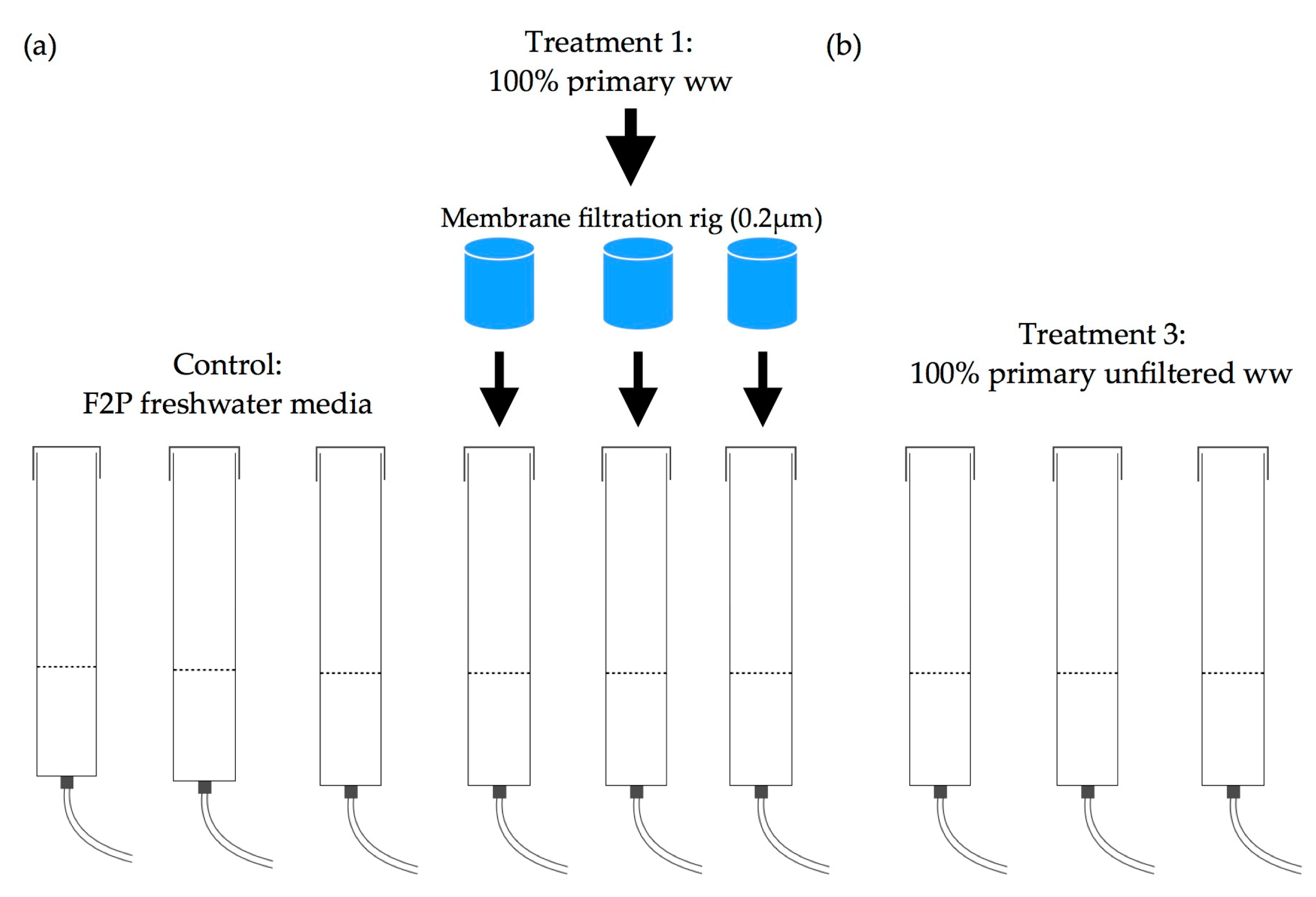
2.3. Experimental Layout
2.3.1. Algal Species and Culture Conditions
- a
- (Control) F2P media in freshwater
- b
- (T1) 100% primary filtered ww
- c
- (T2) 100% primary unfiltered ww
2.3.2. Bubble Column System
2.3.3. Aeration
2.3.4. Daily Illumination
2.3.5. Experimental Design
2.4. Sampling
2.5. Growth Determination
2.6. Nutrient Analysis and pH Monitoring
2.7. Statistical Analysis
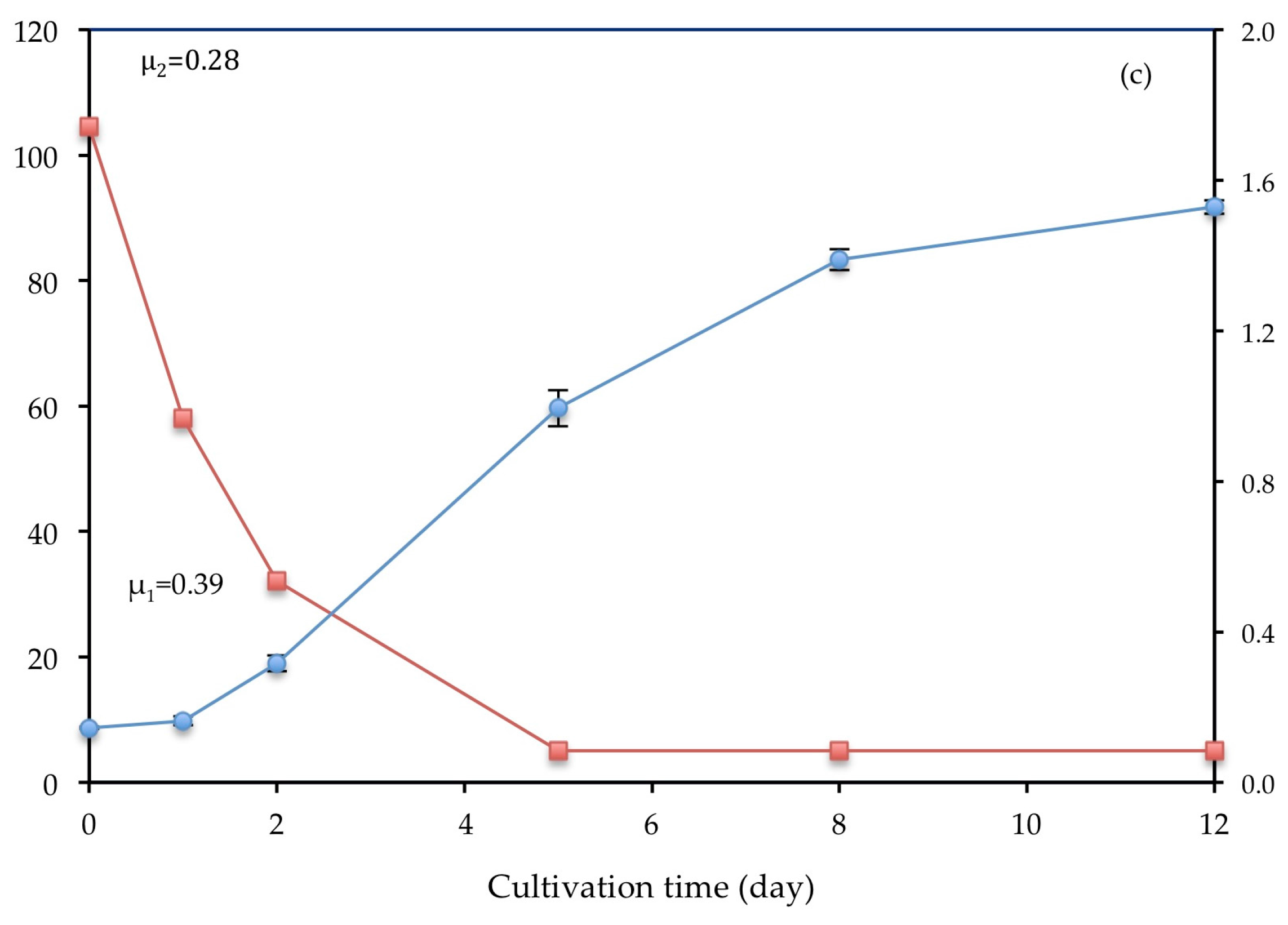
3. Results
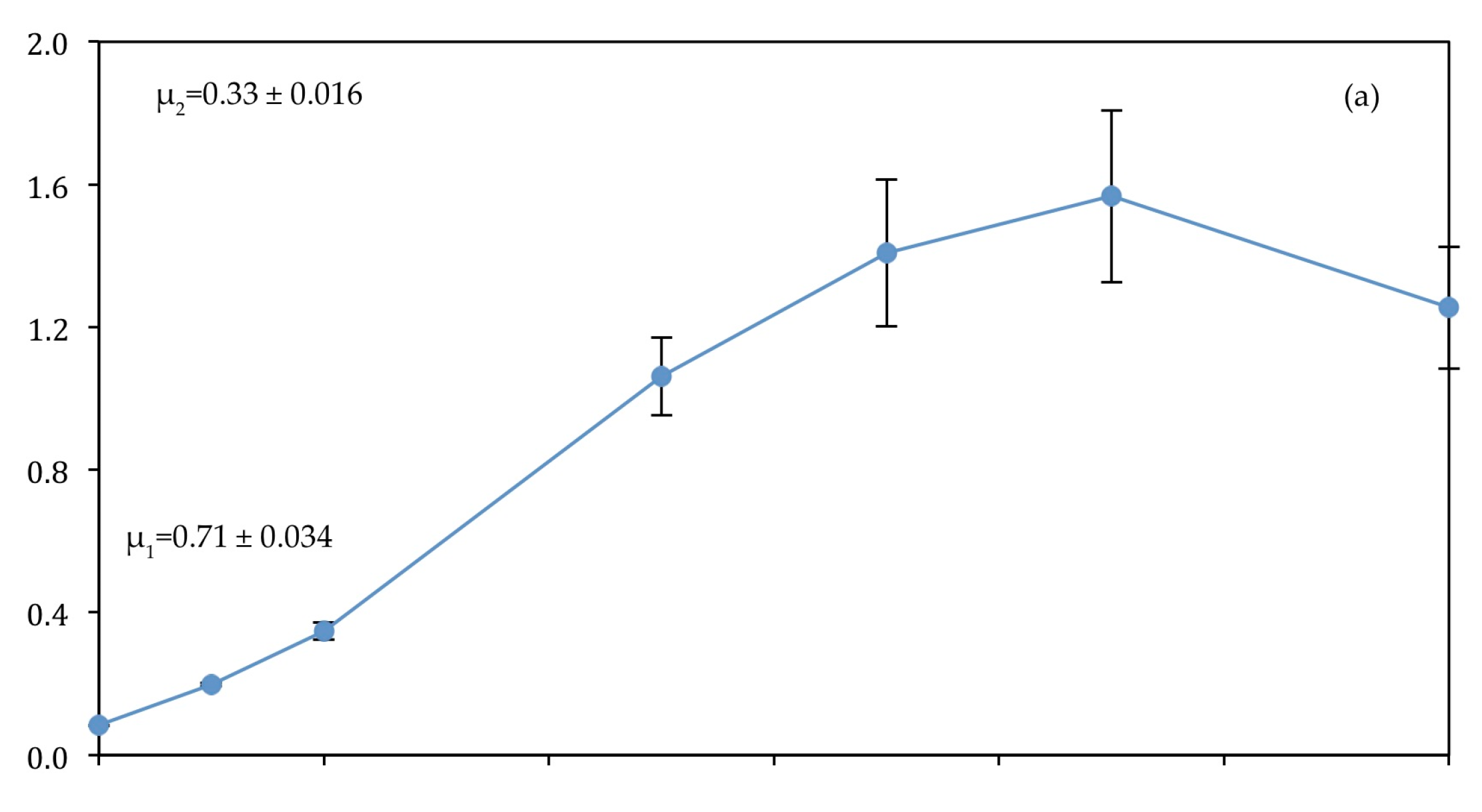
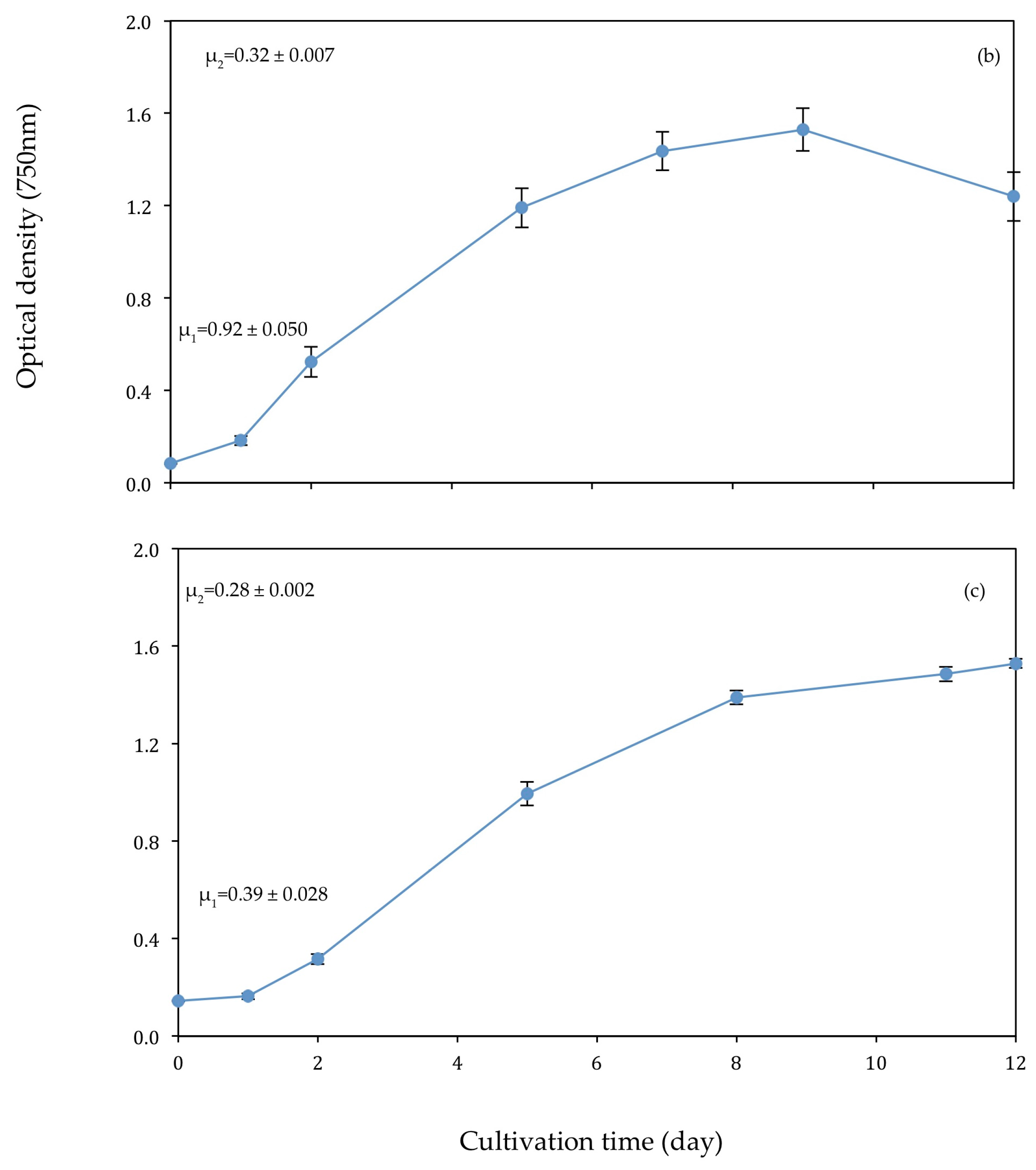
3.1. Algal Growth
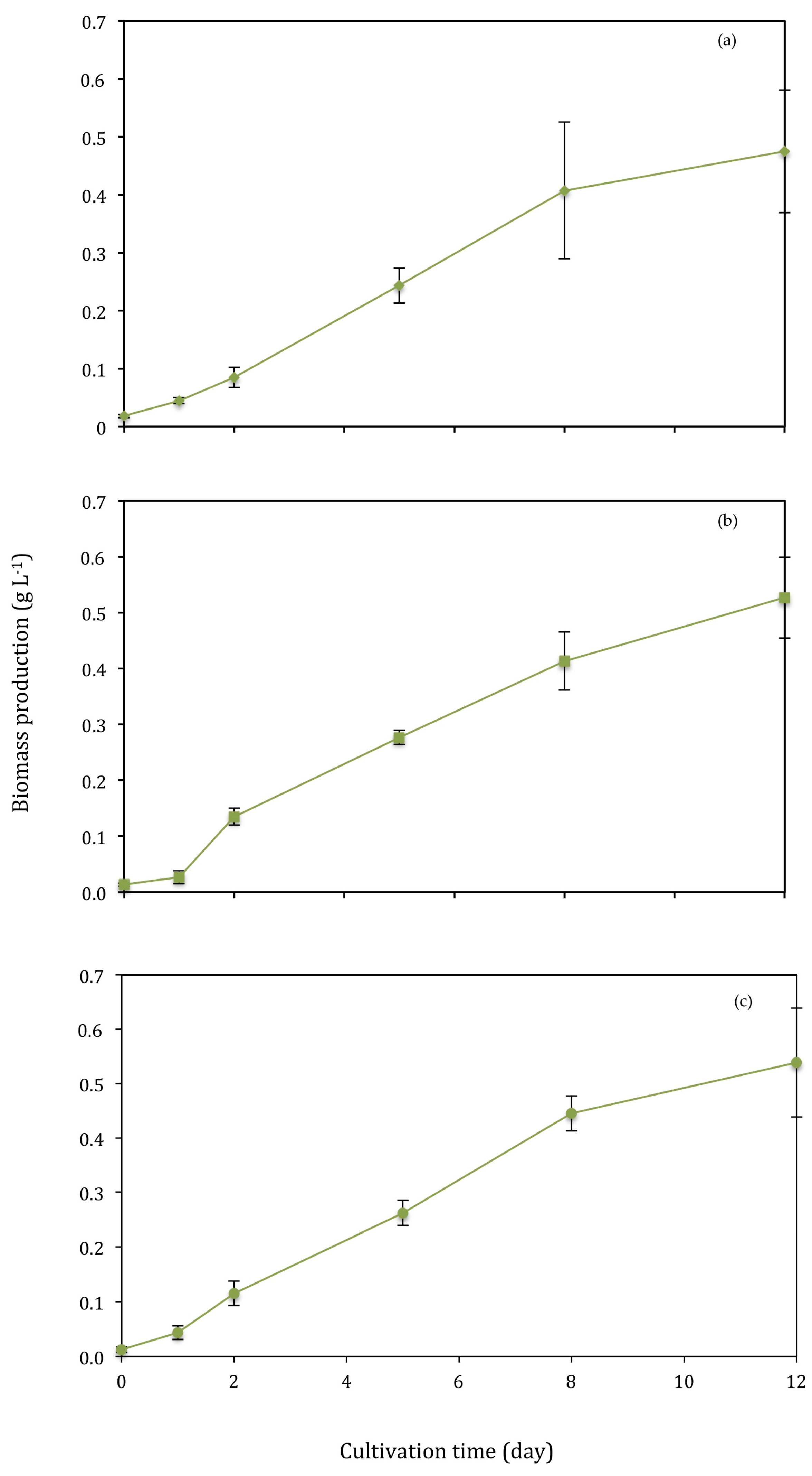
3.2. Biomass Production
3.3. Bioremediation of Nutrients from Wastewater
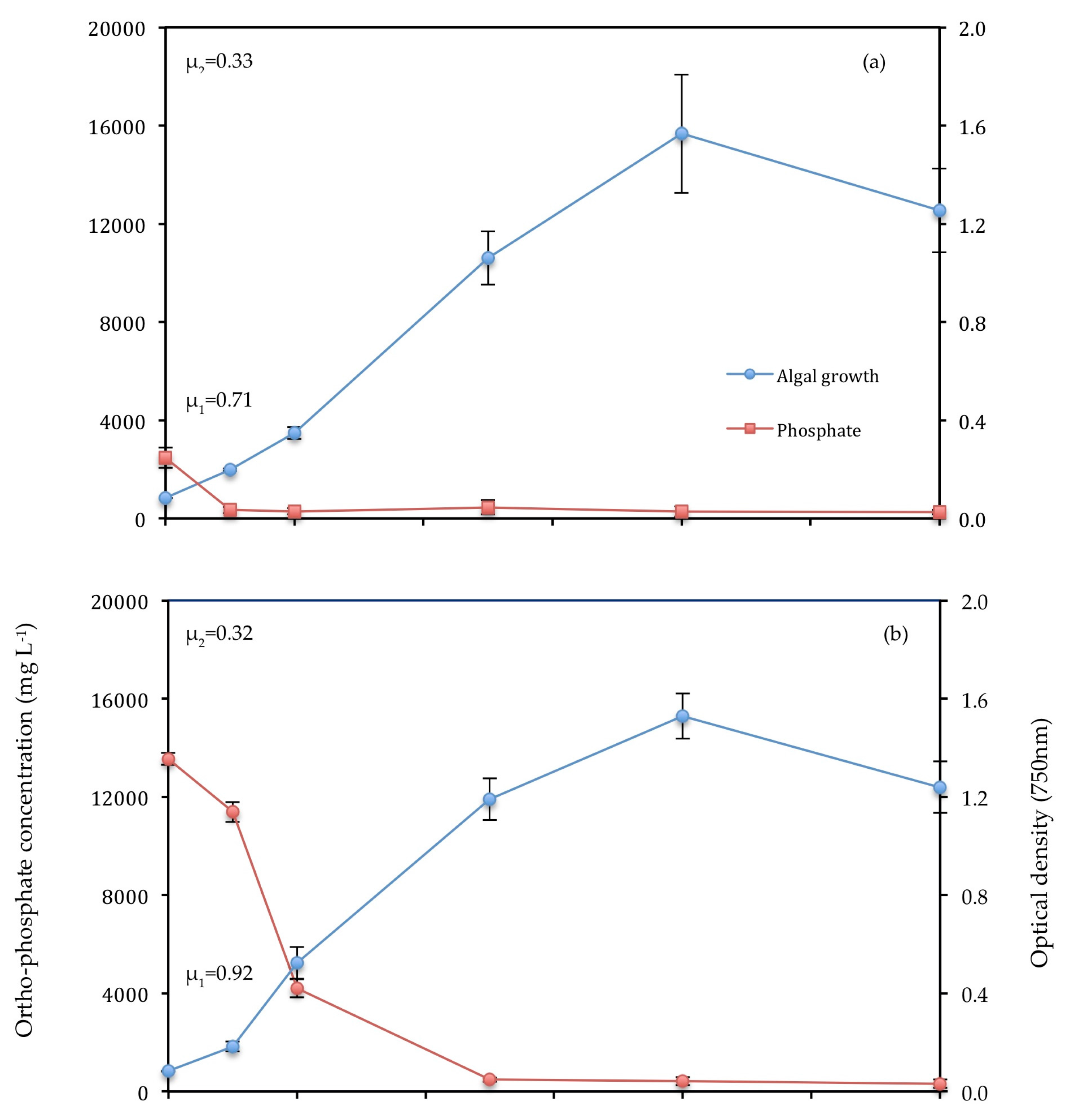
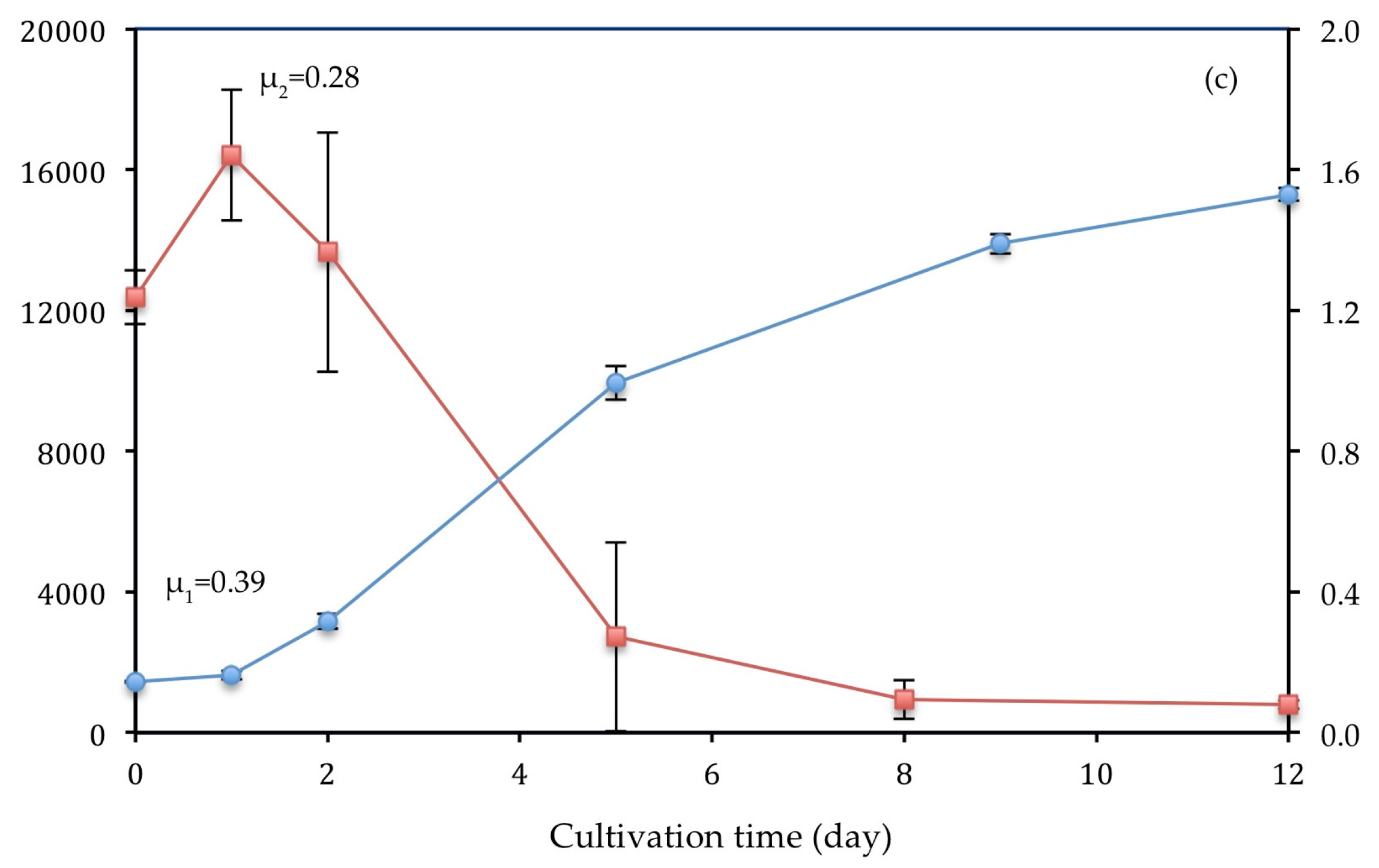
3.3.1. Ortho-Phosphate
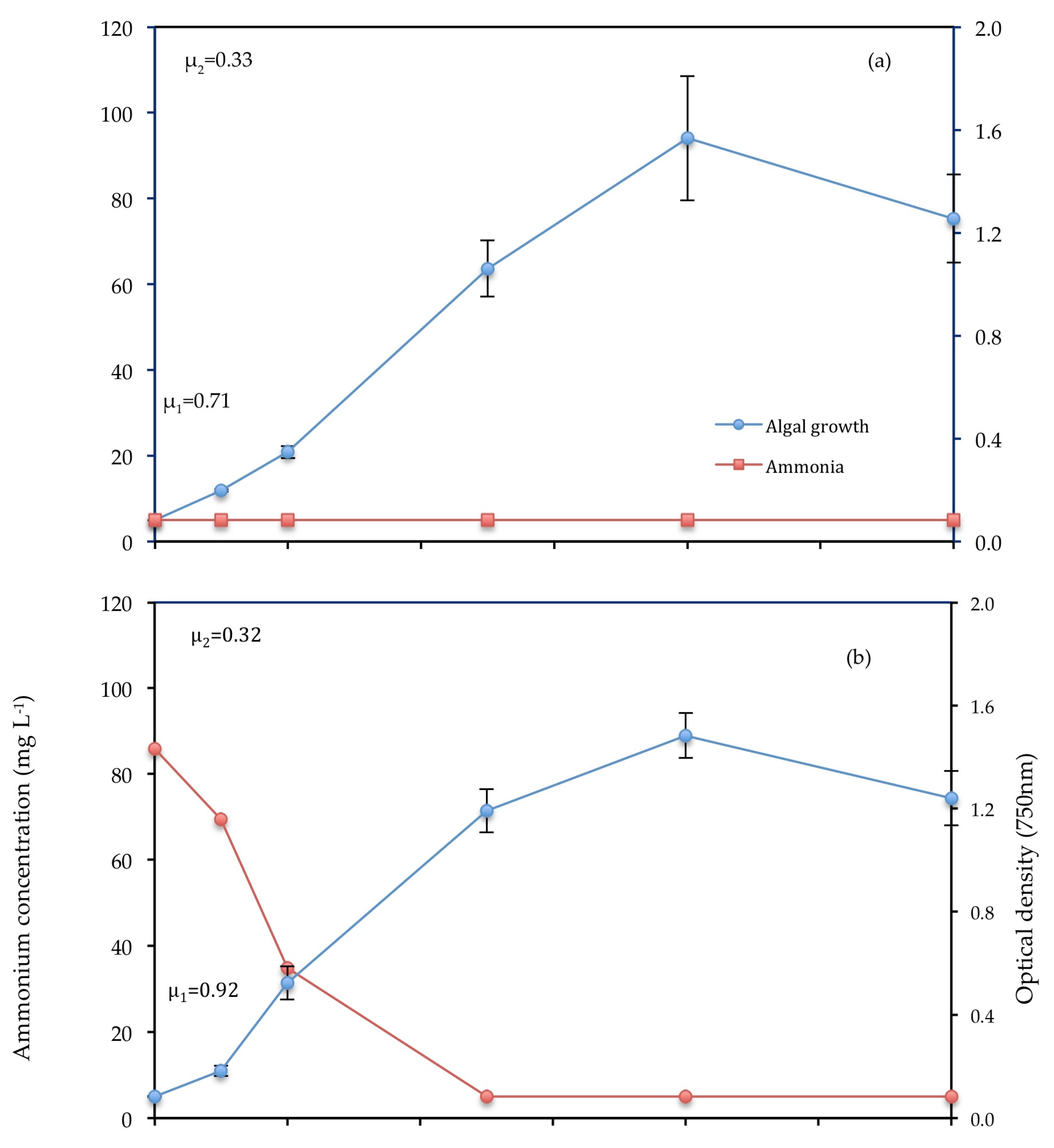
3.3.2. Ammonium
3.4. Comparison of the Results of Bioremediation to Current Wastewater Treatment
4. Discussion
4.1. Algal Growth
4.2. Effect of Wastewater Composition on Nutrient Removal
4.3. Mechanisms of Nutrient Removal
4.3.1. Nitrogen
4.3.2. Ammonium
4.3.3. Phosphorous
4.3.4. Ortho-phosphate
4.4. Microalgal Uses and Current Technologies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- UKTI. Water and Treated Water. UK Trade and Investment. Available online: https://www.gov.uk/government/publications/water-and-treated-water/water-and-treated-water (accesed on 20 December 2016).
- De la Noue, J.; de Pauw, N. The potential of microalgal biotechnology: A review of production and uses of microalgae. Biotechnol. Adv. 1988, 6, 725–770. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. Res. Int. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- DEFRA. Wastewater Treatment in the United Kingdom; DEFRA: London, UK, 2012; p. 47.
- Mainston, C.P.; Parr, W. Phosphorus in rivers—Ecology and management. Sci. Total Environ. 2002, 282, 25–47. [Google Scholar] [CrossRef]
- UNESCO. Water for a Sustainable World; UNESCO: Paris, France, 2015; p. 126. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Gray, N. Biology of Wastewater Treatment, 2nd ed.; Imperial College Press: London, UK, 2004; pp. 26–29. [Google Scholar]
- Carey, R.O.; Migliaccio, K.W. Contribution of wastewater treatment plant effluents to nutrient dynamics in aquatic systems: A review. Environ. Manag. 2009, 44, 205–217. [Google Scholar] [CrossRef] [PubMed]
- EEA. Eutrophication in Europe’s Coastal Waters; European Environment Agency: Copenhagen, Denmark, 2001; p. 86. [Google Scholar]
- Khan, F.A.; Ansari, A.A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Carlisle, S. Productive Filtration: Living System Infrastructure in Calcutta. Available online: http://scenariojournal.com/article/productive-filtration-living-system-infrastructure-in-calcutta/ (accessed on 20 December 2016).
- UKTAG. Environmental Standards And Conditions (Phase 2); UK Technical Advisory Group on the Water Framework Directive: London, UK, 2008; p. 84. [Google Scholar]
- De la Noüe, J.; Laliberté, G.; Proulx, D. Algae and waste water. J. Appl. Phycol. 1992, 4, 247–254. [Google Scholar] [CrossRef]
- Palmer, C. Algae in american sewage stabilization ponds. Rev. Microbiol. (S-Paulo) 1974, 75–80. [Google Scholar]
- Przytocka-Jusiak, M.; Duszota, M.; Matusiak, K.; Mycielski, R. Intensive culture of chlorella vulgaris/aa as the second stage of biological purification of nitrogen industry wastewaters. Water Res. 1984, 18, 1–7. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Sahu, D.; Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Technol. 2011, 3, 299–3004. [Google Scholar]
- Comber, S.D.W.; Gardner, M.J.; Churchley, J. Aluminium speciation: Implications of wastewater effluent dosing on river water quality. Chem. Speciat. Bioavailab. 2005, 17, 117–128. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Lovitt, R.W. Adding value to wastewater by resource recovery and reformulation as growth media: Current prospects and potential. J. Water Reuse Desalination 2015, 5, 473–479. [Google Scholar] [CrossRef]
- Silkina, A.; Zacharof, M.-P.; Hery, G.; Nouvel, T.; Lovitt, R.W. Formulation and utilisation of spent anaerobic digestate fluids for the growth and product formation of single cell algal cultures in heterotrophic and autotrophic conditions. Bioresour. Technol. 2017, 244, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chase, H.A. Applications of membrane techniques for purification of natural products. Biotechnol. Lett. 2010, 32, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Luong, T.T.; Lee, D.; Oh, Y.K.; Lee, T. Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour. Technol. 2011, 102, 8639–8645. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P.; Lovitt, R.W. The filtration characteristics of anaerobic digester effluents employing cross flow ceramic membrane microfiltration for nutrient recovery. Desalination 2014, 341, 27–37. [Google Scholar] [CrossRef]
- Henze, M.C.Y. Wastewater characterization. In Biological Wastewater Treatment: Principles, Modelling and Design; Henze, M.v.L., van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 33–52. [Google Scholar]
- Li, Y.; Chen, Y.F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. Well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.S.; Singh, G.P.; Sharma, V.K. Effects of culture conditions on growth and biochemical profile of Chlorella vulgaris. J. Plant Pathol. Microb. 2012, 3, doi.org/10.4172/2157–747110001. [Google Scholar] [CrossRef]
- Dickinson, K.E.; Whitney, C.G.; McGinn, P.J. Nutrient remediation rates in municipal wastewater and their effect on biochemical composition of the microalga Scenedesmus sp. AMDD. Algal Res. 2013, 2, 127–134. [Google Scholar] [CrossRef]
- Ji, M.-K.; Abou-Shanab, R.A.I.; Kim, S.-H.; Salama, E.-S.; Lee, S.-H.; Kabra, A.N.; Lee, Y.-S.; Hong, S.; Jeon, B.-H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Stein, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press Archive: Cambridge, UK, 1973; Volume 1, p. 265. [Google Scholar]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Whitton, R.; Le Mével, A.; Pidou, M.; Ometto, F.; Villa, R.; Jefferson, B. Influence of microalgal n and p composition on wastewater nutrient remediation. Water Res. 2016, 91, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Bashan, L.E.; Moreno, M.; Hernandez, J.-P.; Bashan, Y. Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium azospirillum brasilense. Water Res. 2002, 36, 2941–2948. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lananan, F.; Jusoh, A.; Ali, N.a.; Lam, S.S.; Endut, A. Effect of conway medium and f/2 medium on the growth of six genera of south china sea marine microalgae. Bioresour. Technol. 2013, 141, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, L.T.; Del Campo, C.M.; Rodriguez, C.M.; de- Bashan, L.E.; Bashan, Y. Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyte Lemna minuscula. Water Res. 2002, 36, 4185–4192. [Google Scholar] [CrossRef]
- Lim, S.-L.; Chu, W.-L.; Phang, S.-M. Use of chlorella vulgaris for bioremediation of textile wastewater. Bioresour. Technol. 2010, 101, 7314–7322. [Google Scholar] [CrossRef] [PubMed]
- Riaño, B.; Molinuevo, B.; García-González, M.C. Treatment of fish processing wastewater with microalgae-containing microbiota. Bioresour. Technol. 2011, 102, 10829–10833. [Google Scholar] [CrossRef] [PubMed]
- Pipes, W.O.; Gotaas, H.B. Utilization of organic matter by chlorella grown in sewage. Appl. Microbiol. 1960, 8, 163–169. [Google Scholar] [PubMed]
- Bouterfas, R.B.M.; Dauta, A. The effects of irradiance and photoperiod on the growth rate of three freshwater green algae isolated from a eutrophic lake. Limnetica 2006, 25, 647–656. [Google Scholar]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. In different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Effect of turbidity on algal growth; Illinois State Water Survey: Champaign, IL, USA, 1974. [Google Scholar]
- Myers, J. Physiology of the algae. Ann. Rev. Microb. 1951, 5, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Iasimone, F.; De Felice, V.; Panico, A.; Pirozzi, F. Experimental study for the reduction of CO2 emissions in wastewater treatment plant using microalgal cultivation. J. CO2 Utili. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Croft, M.T.; Warren, M.J.; Smith, A.G. Algae need their vitamins. Eukaryot. Cell 2006, 5, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- CCAP. F/2 Medium. Available online: www.Ccap.Ac.Uk/media/documents/f2 (accesed on 6 February 2017).
- Hammouda, O.; Gaber, A.; Abdel-Raouf, N. Microalgae and wastewater treatment. Ecotoxicol. Environ. Saf. 1995, 31, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Darley, W. Algal Biology: A Physiological Approach; Basic Microbiology: Oxford, UK, 1982. [Google Scholar]
- Reynolds, C. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Martin, C.; De la Noüe, J.; Picard, G. Intensive cultivation of freshwater microalgae on aerated pig manure. Biomass 1985, 7, 245–259. [Google Scholar] [CrossRef]
- Hillebrand, H.; Sommer, U. The nutrient stoichiometry of benthic microalgal growth: Redfield proportions are optimal. Limnol. Oceanogr. 1999, 44, 440–446. [Google Scholar] [CrossRef]
- Redfield, A.C. On the Proportions of Organic Derivatives in Sea Water and Their Relation to the Composition of Plankton; University Press of Liverpool: Liverpool, UK, 1934. [Google Scholar]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioproc. Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.F.Y.; Wong, Y.S. Effect of ammonia concentrations on growth of Chlorella vulgaris and nitrogen removal from media. Bioresour. Technol. 1996, 57, 45–50. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Van Den Hende, S.; Vervaeren, H.; Coward, T.; Skill, S.C. Harvesting of microalgae within a biorefinery approach: A review of the developments and case studies from pilot-plants. Algal Res. 2015, 11, 248–262. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y.B. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Becker, E. Microalgae: Biotechnology and Microbiolog; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.; Chien, C.C.; Ju, Y.H. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Espen, G.; Stãle, K.; Sverre, M.M. Cellular and extracellular production of carbohydrates and amino acids by the marine diatom skeletonema costatum: Diel variations and effects of N depletion. Marine Ecol. Prog. Ser. 2002, 242, 83–94. [Google Scholar]
- Wang, Y.; Guo, W.; Yen, H.-W.; Ho, S.-H.; Lo, Y.-C.; Cheng, C.-L.; Ren, N.; Chang, J.-S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Pick, U.; Rental, M.; Chitlaru, E.; Weiss, M. Polyphosphate-hydrolysis—A protective mechanism against alkaline stress? Febs. Lett. 1990, 274, 15–18. [Google Scholar] [PubMed]
- Maestrini, S.Y.; Robert, J.M.; Leftley, J.W.; Collos, Y. Ammonium thresholds for simultaneous uptake of ammonium and nitrate by oyster-pond algae. J. Exp. Mar. Biol. Ecol. 1986, 102, 75–98. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science Ltd.: Oxford, UK, 2004. [Google Scholar]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.L.; Stauffer, J.F.; Umbreit, W.W. Relationships between phosphorylation and photosynthesis in chlorella. Am. J. Bot. 1944, 31, 107–120. [Google Scholar] [CrossRef]
- Yang, C.; Hua, Q.; Shimizu, K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 2000, 6, 87–102. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Shu, Q.; Takala, J.; Hiltunen, E.; Feng, P.; Yuan, Z. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Riesing, T. Cultivating algae for liquid fuel production. Permac. Activist 2006, 48, 59. [Google Scholar]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cabanelas, I.T.D.; Ruiz, J.; Arbib, Z.; Chinalia, F.A.; Garrido-Pérez, C.; Rogalla, F.; Nascimento, I.A.; Perales, J.A. Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour. Technol. 2013, 131, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Bayliss, C.; Zanain, M.; Pooley, C.; Scolamacchia, M.; Silkina, A. Evaluation of batch and semi-continuous culture of porphyridium purpureum in a photobioreactor in high latitudes using fourier transform infrared spectroscopy for monitoring biomass composition and metabolites production. Bioresour. Technol. 2015, 189, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Kuo, C.-M.; Lin, C.-S. Cultivation of microalgal chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.; Daniels, T.C.; Eiler, J.J.; Gunnison, J.B.; Kumler, W.D.; Oneto, J.F.; Strait, L.A.; Spoehr, H.A.; Hardin, G.J.; Milner, H.W.; et al. Chlorellin, an antibacterial substance from chlorella. Science 1944, 99, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Ördög, V.; Stirk, W.A.; Lenobel, R.; Bancířová, M.; Strnad, M.; van Staden, J.; Szigeti, J.; Németh, L. Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J. Appl. Phycol. 2004, 16, 309–314. [Google Scholar] [CrossRef]
- Suarez, E.R.; Kralovec, J.A.; Noseda, M.D.; Ewart, H.S.; Barrow, C.J.; Lumsden, M.D.; Grindley, T.B. Isolation, characterization and structural determination of a unique type of arabinogalactan from an immunostimulatory extract of chlorella pyrenoidosa. Carbohydr. Res. 2005, 340, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Suarez, E.R.; Syvitski, R.; Kralovec, J.A.; Noseda, M.D.; Barrow, C.J.; Ewart, H.S.; Lumsden, M.D.; Grindley, T.B. Immunostimulatory polysaccharides from chlorella pyrenoidosa. A new galactofuranan. Measurement of molecular weight and molecular weight dispersion by dosy NMR. Biomacromolecules 2006, 7, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Muller-Feuga, A. Microalgae for aquaculture. The current global situation and future trends. In Handbook of Microalgal Culture; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 352–364. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nutrients | Concentration (mg L−1) | |
|---|---|---|
| Primary | Secondary (effluent) | |
| NH4-N | 104.51 ± 0.455 | <5 1 |
| PO4-P | 23.65 ± 0.087 | 15.41 ± 0.066 |
| Control | Treatment 1 | Treatment 2 | ||
|---|---|---|---|---|
| Initial | N:P | 2.4:1.0 | 7.5:1.0 | 4.4:1.0 |
| NH4-N (mg L−1) | 5 1 | 85.84 | 104.51 | |
| PO4-P (mg L−1) | 2.47 | 13.54 | 23.65 | |
| Final | NH4-N (mg L−1) | 5 1 | 5 1 | 5 1 |
| PO4-P (mg L−1) | 0.256 | 0.313 | 0.796 | |
| % removed | NH4-N | NA | 94.18% | 95.22% |
| PO4-P | 89.64% | 97.69% | 96.63% |
| Measure of Effluent Quality | Nutrient/Discharge Area | Treatment 1 | Treatment 2 | Current ww Treatment (Table 1) |
|---|---|---|---|---|
| Final level | NH4-N mg L−1 | <5 | <5 | <5 |
| PO4-P mg L−1 | 0.313 | 0.796 | 15.41 | |
| Classification | Rivers | Moderate-Poor | Moderate-Poor | Poor |
| Coastal | Poor | Poor | Poor | |
| Lakes | <Good | <Good | <Good |
| Strain | Culture Medium/Wastewater | Pre-Treatment | Light Intensity (μ mol m−2 s−1) | µ1 (d−1) | % NH4-N | % PO4-P | Biomass Production (mg L−1) | References |
|---|---|---|---|---|---|---|---|---|
| C. vulgaris | Primary (T1) | 0.2 μm filtration | 177 | 0.92 | 94.18 | 97.69 | 0.513 | This work |
| Chlorella sp. | Primary | filtration | 200 | 0.43 | 82.40 | 83.20 | - | Wang et al. [44] |
| Chlorella sp. 227 | Secondary | 0.2 μm filtration | 60 | - | 92.00 | 86.00 | - | Cho et al. [23] |
| Chlorella sp. 10b | Centrate | - | 50 | 0.48 | 93.00 | 79.00 | 1.175 | Li et al. [26] |
| C. vulgaris | Secondary | - | 135 | 0.19 | 60.10 | - | - | Ruiz-Marin et al. [35] |
| C. vulgaris | Primary (T2) | - | 177 | 0.39 | 95.22 | 96.63 | 0.527 | This work |
| C. vulgaris | F2P media | - | 177 | 0.71 | - | 89.64 | 0.457 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayhead, E.; Silkina, A.; Llewellyn, C.A.; Fuentes-Grünewald, C. Comparing Nutrient Removal from Membrane Filtered and Unfiltered Domestic Wastewater Using Chlorella vulgaris. Biology 2018, 7, 12. https://doi.org/10.3390/biology7010012
Mayhead E, Silkina A, Llewellyn CA, Fuentes-Grünewald C. Comparing Nutrient Removal from Membrane Filtered and Unfiltered Domestic Wastewater Using Chlorella vulgaris. Biology. 2018; 7(1):12. https://doi.org/10.3390/biology7010012
Chicago/Turabian StyleMayhead, Elyssia, Alla Silkina, Carole A. Llewellyn, and Claudio Fuentes-Grünewald. 2018. "Comparing Nutrient Removal from Membrane Filtered and Unfiltered Domestic Wastewater Using Chlorella vulgaris" Biology 7, no. 1: 12. https://doi.org/10.3390/biology7010012






