Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production
Abstract
:1. Introduction
2. Materials and Methods
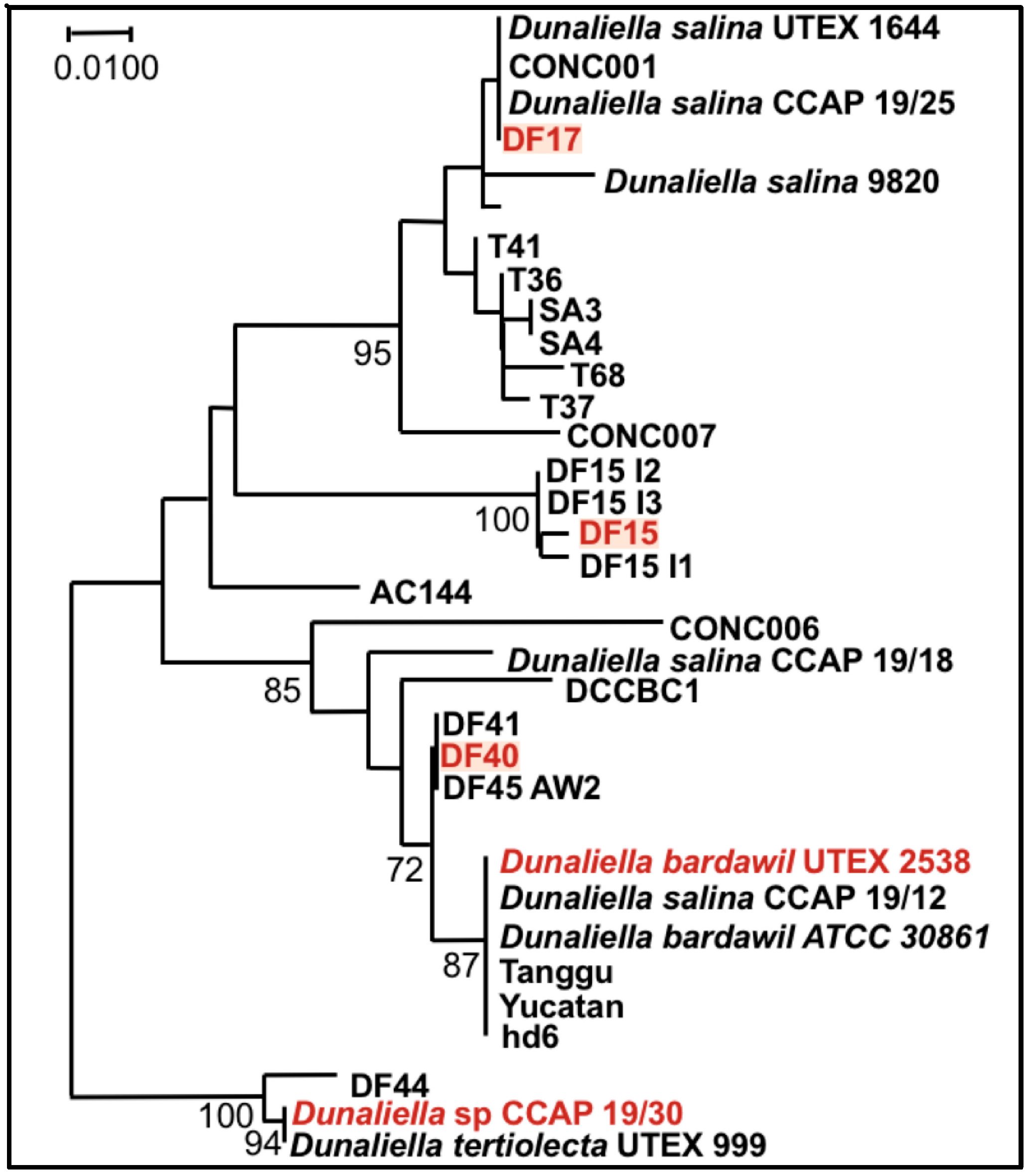
2.1. Algal Strains and Cultivation
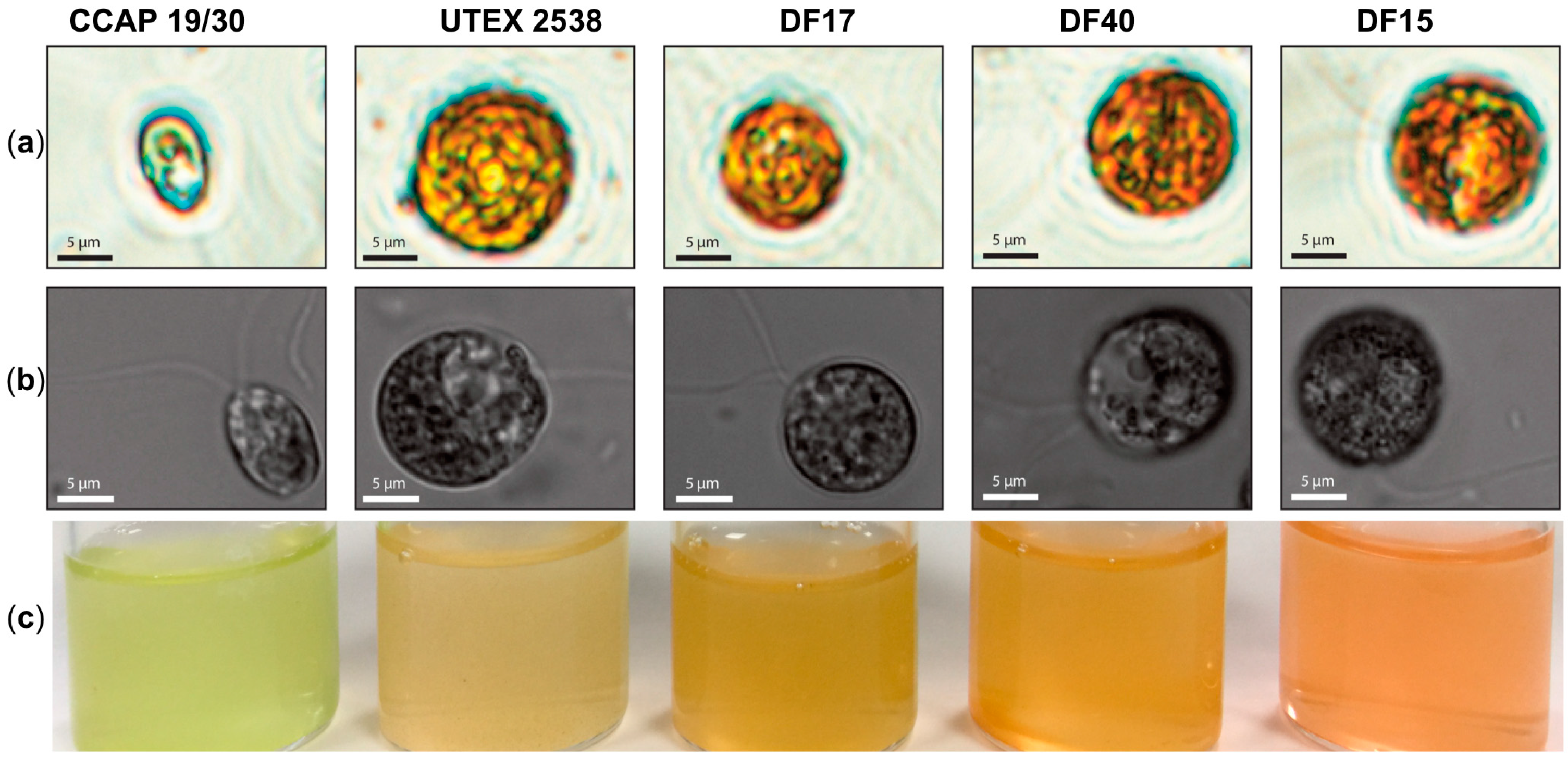
2.2. Microscopy Observations
2.3. Algal Biomass Analysis
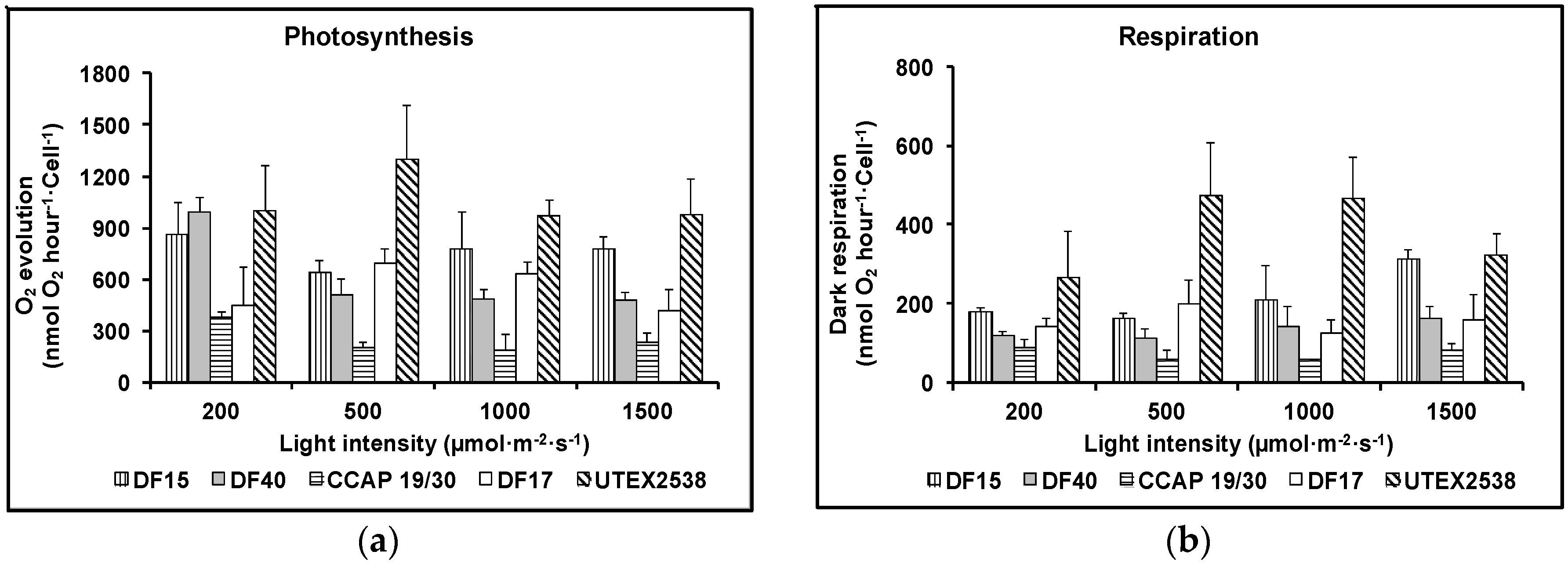
2.4. Oxygen Evolution and Dark Respiration
2.5. Statistical Analysis
3. Results
3.1. Cell Growth
3.2. Photosynthesis and Respiration
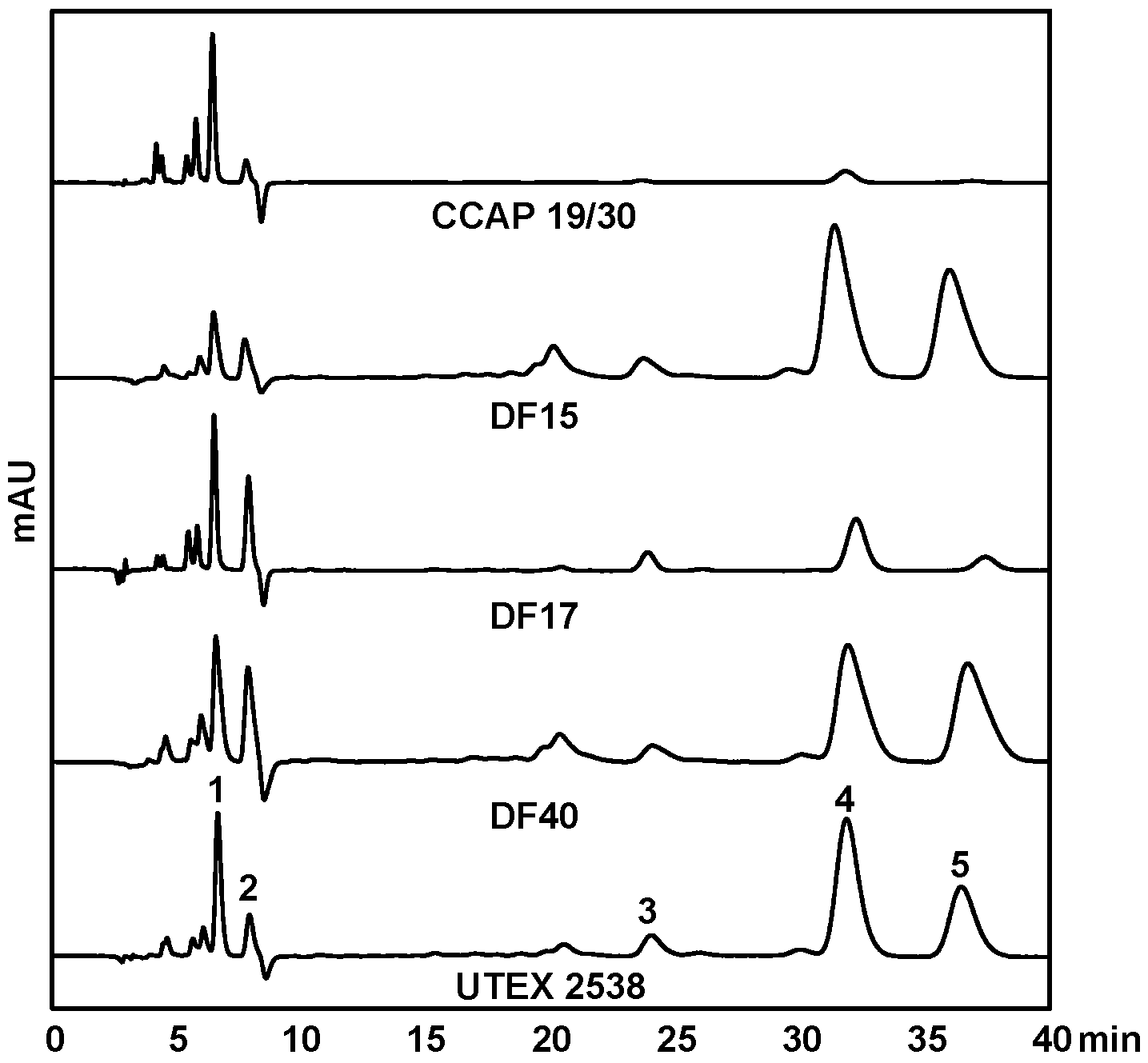
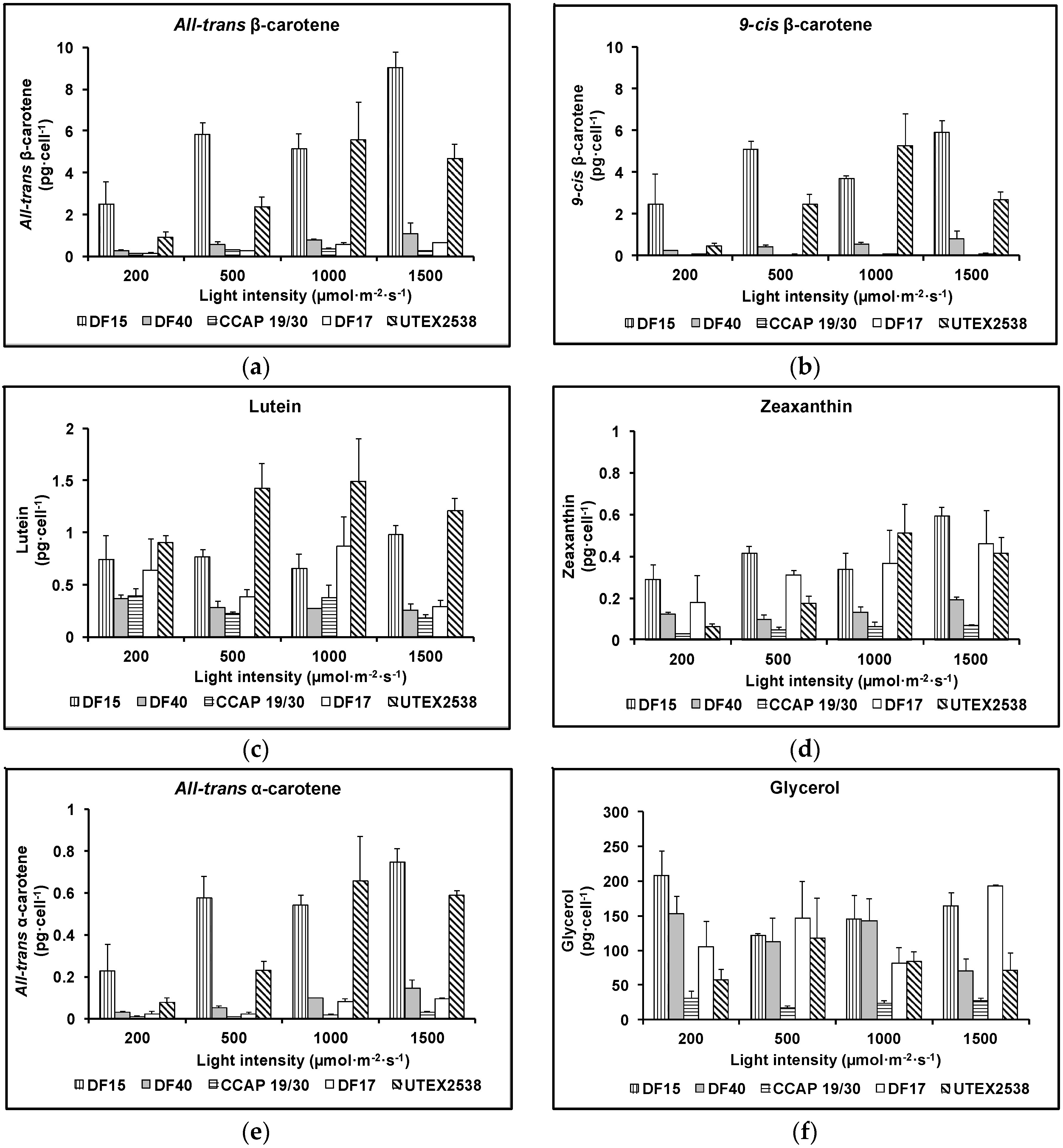
3.3. Pigment Composition
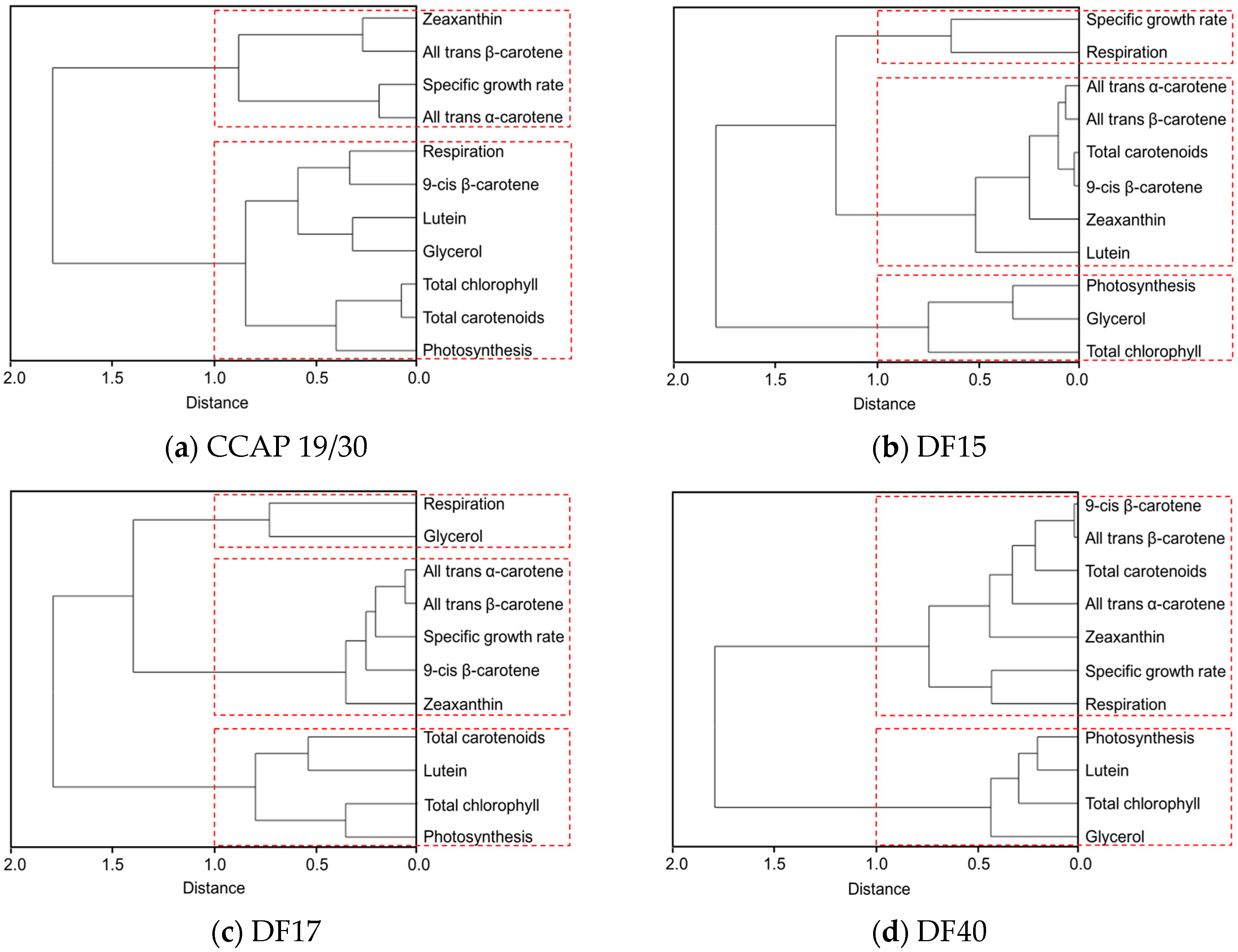
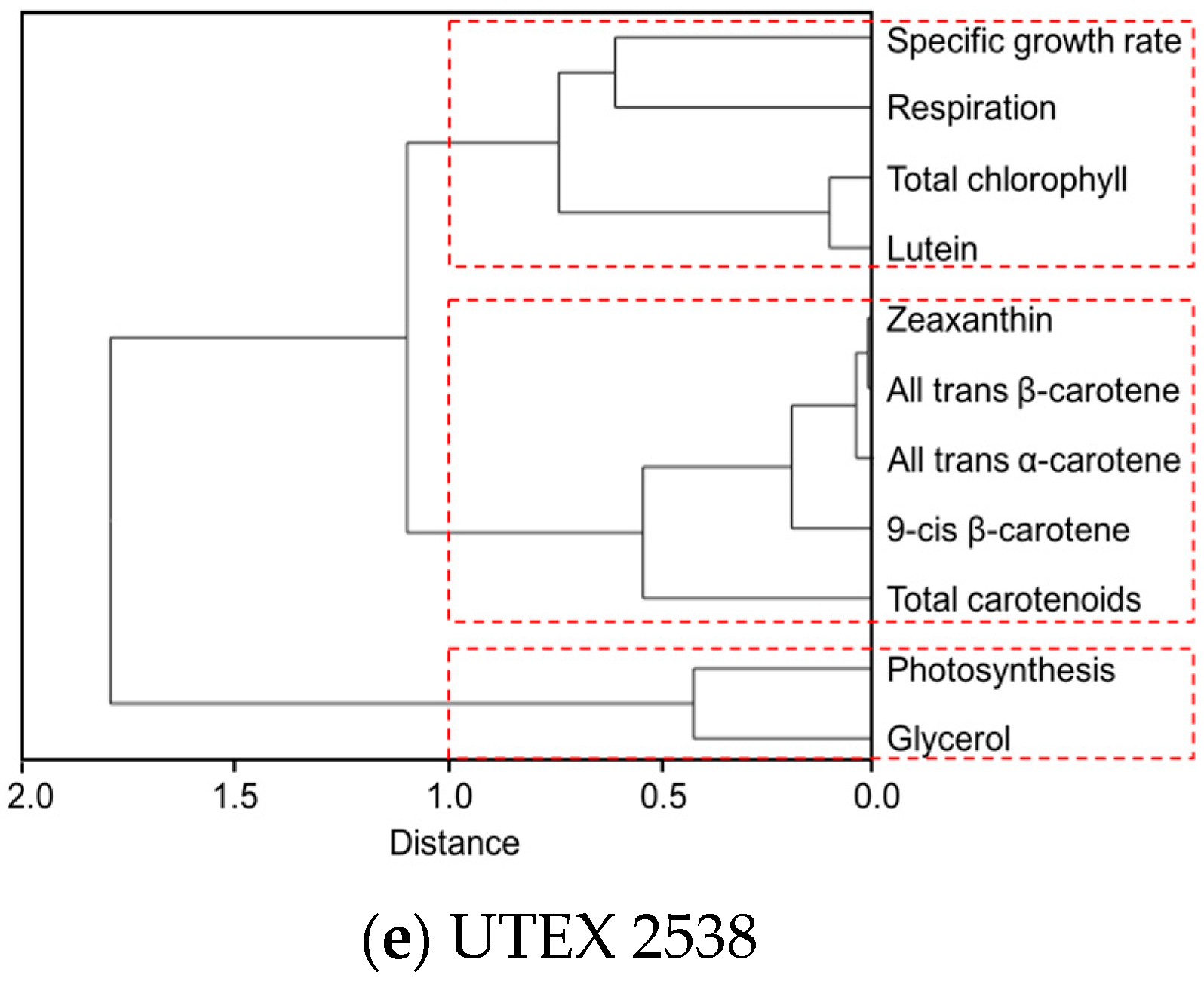
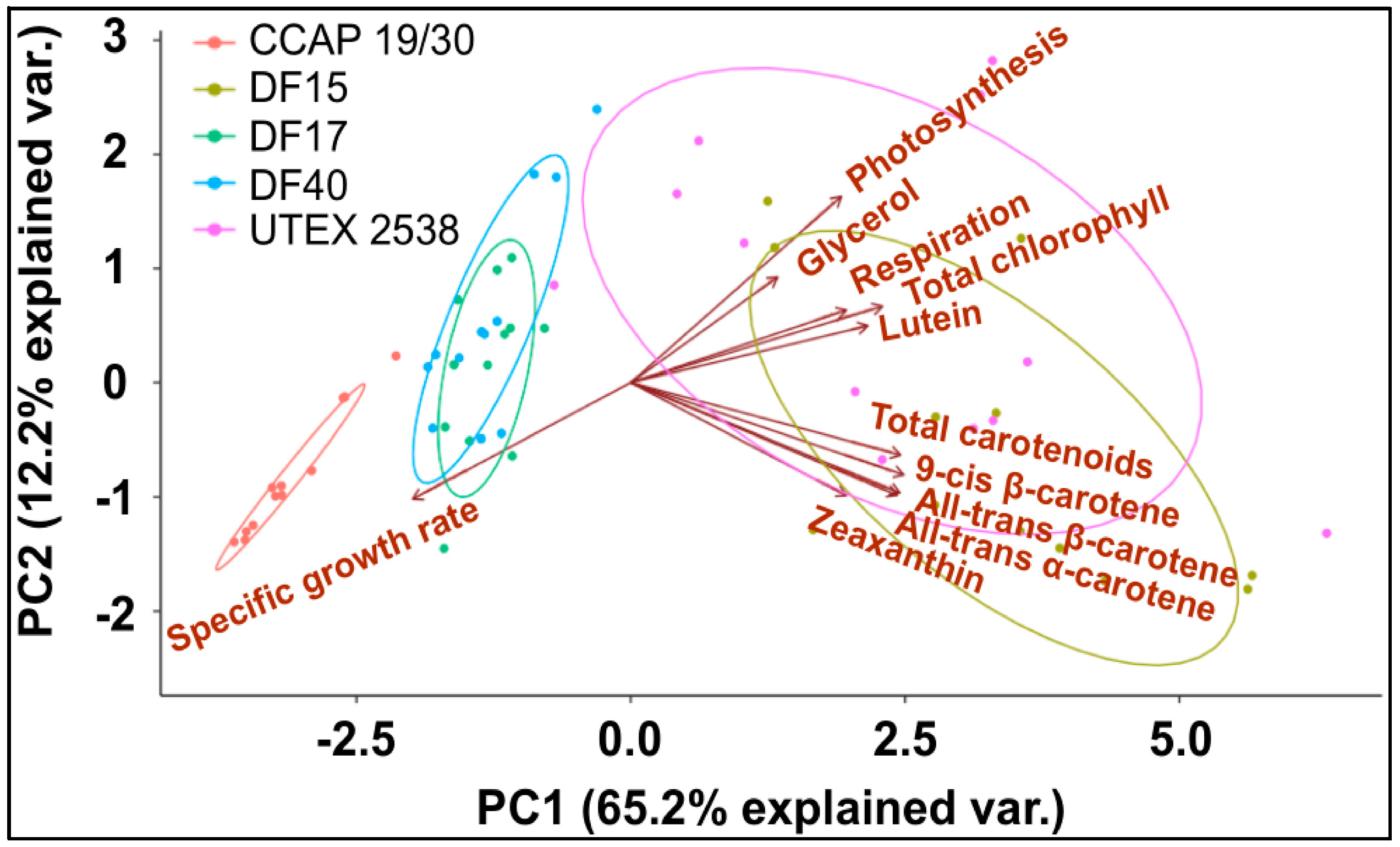
3.4. Statistical Analysis
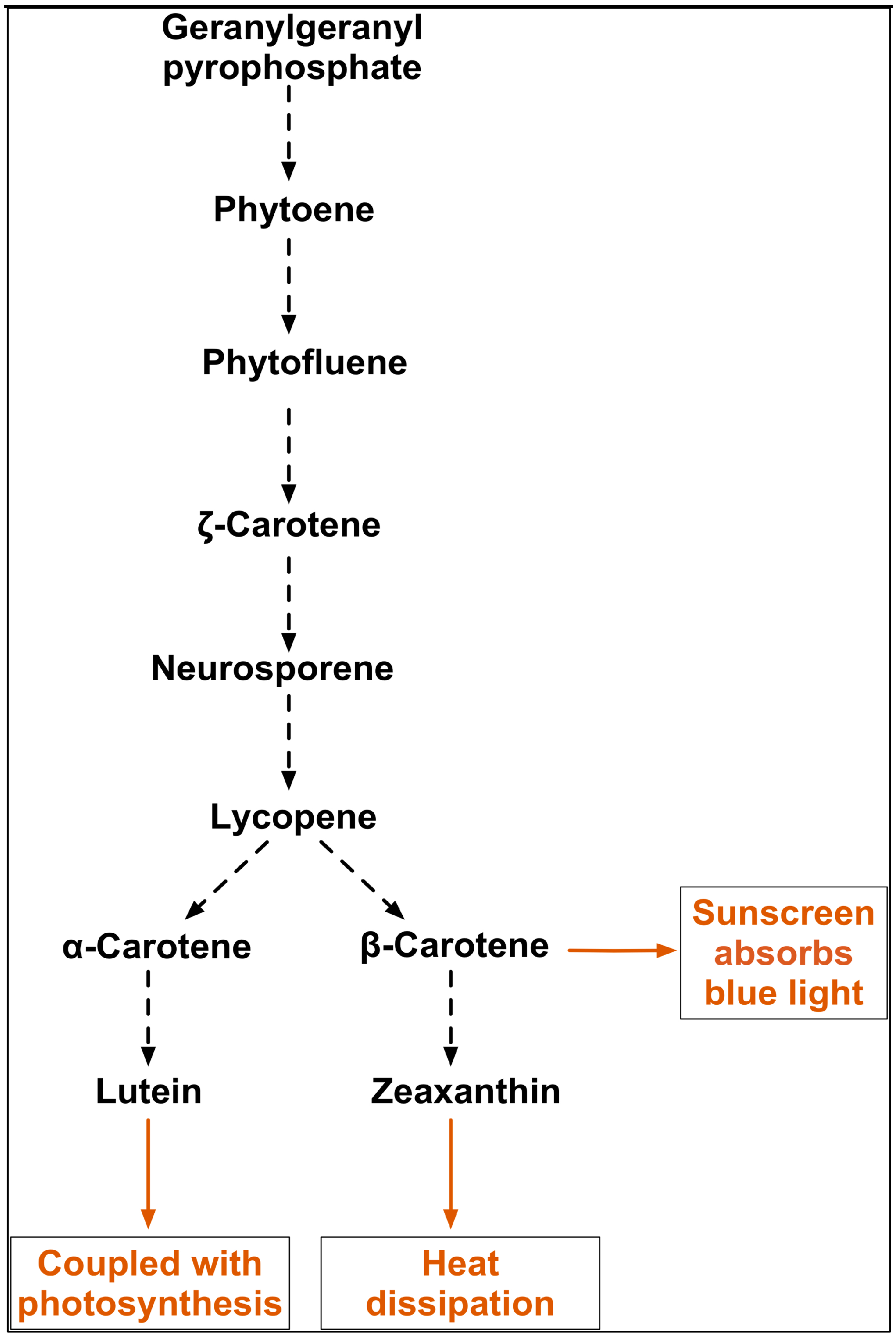
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated β-Carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Carle, R. Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci. Technol. 2005, 16, 416–422. [Google Scholar] [CrossRef]
- Levy, Y.; Zaltsberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. Dietary supplementation of a natural isomer mixture of beta-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann. Nutr. Metab. 2000, 44, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bechor, S.; Zolberg Relevy, N.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-cis β-Carotene increased cholesterol efflux to HDL in macrophages. Nutrients 2016, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, S.; Harats, D.; Salameh, F.; Lubish, T.; Harari, A.; Trau, H.; Shaish, A. 9-cis-rich β-carotene powder of the alga Dunaliella reduces the severity of chronic plaque psoriasis: A randomized, double-blind, placebo-controlled clinical trial. J. Am. Coll. Nutr. 2012, 31, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Rotenstreich, Y.; Belkin, M.; Sadetzki, S.; Chetrit, A.; Ferman-Attar, G.; Sher, I.; Harari, A.; Shaish, A.; Harats, D. Treatment with 9-cis β-carotene-rich powder in patients with retinitis pigmentosa: A randomized crossover trial. JAMA Ophthalmol. 2013, 131, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Krinsky, N.I.; Benotti, P.N.; Russell, R.M. Biosynthesis of 9-cis-retinoic acid from 9-cis-beta-carotene in human intestinal mucosa in vitro. Arch. Biochem. Biophys. 1994, 313, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Melis, A. Microalgal biotechnology: Carotenoid production by the green algae Dunaliella salina. Biotechnol. Bioprocess Eng. 2003, 8, 331–337. [Google Scholar] [CrossRef]
- Tafreshi, A.H.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009, 107, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Guðmundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, Ó.S.; Palsson, B.Ø.; Brynjólfsson, S. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Siva, C.J. The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J. Appl. Phycol. 2007, 19, 567–590. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Harvey, P.J. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (Chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Algal growth media and sources of cultures. In Microalgal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 456–465. [Google Scholar]
- Strickland, J.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, Canada, 1972; pp. 185–190. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Brindley, C.; Acién, F.G.; Fernández Sevilla, J.M. The oxygen evolution methodology affects photosynthetic rate measurements of microalgae in well-defined light regimes. Biotechnol. Bioeng. 2010, 106, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Delieu, T.J.; Walker, D.A. Simultaneous measurement of oxygen evolution and chlorophyll fluorescence from leaf. Plant Physiol. 1983, 73, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A. Effect of low temperature on the stereoisomer composition of β-carotene in the halotolerant alga Dunaliella Bardawil (Chlorophyta). J. Phycol. 1996, 32, 272–275. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Shaish, A.; Avron, M.; Pick, U.; Ben-Amotz, A. Are active oxygen species involved in induction of β-carotene in Dunaliella bardawil? Planta 1993, 190, 363–368. [Google Scholar] [CrossRef]
- Levin, G.; Mokady, S. Antioxidant activity of 9-cis compared to all-trans β-carotene in vitro. Free Radic. Biol. Med. 1994, 17, 77–82. [Google Scholar] [CrossRef]
- Orset, S.C.; Young, A.J. Exposure to low irradiances favors the synthesis of 9-cis beta, beta-carotene in Dunaliella salina (Teod.). Plant Physiol. 2000, 122, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Davidi, L.; Levin, Y.; Ben-Dor, S.; Pick, U. Proteome analysis of cytoplasmatic and plastidic β-carotene lipid droplets in Dunaliella bardawil. Plant Physiol. 2015, 167, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.W.; Smyth, R.D. Light Antennas in phototactic algae. Microbiol. Rev. 1980, 44, 572–630. [Google Scholar] [PubMed]
- Eitzinger, N.; Wagner, V.; Weisheit, W.; Geimer, S.; Boness, D.; Kreimer, G.; Mittag, M. Proteomic analysis of a fraction with intact eyespots of Chlamydomonas reinhardtii and assignment of protein methylation. Front. Plant Sci. 2015, 6, 3389. [Google Scholar] [CrossRef] [PubMed]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Yokthongwattana, K.; Polle, J.E.W.; Melis, A. Role of the reversible xanthophyll cycle in the photosystem II damage and repair cycle in Dunaliella salina. Plant Physiol. 2003, 132, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and brain function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; Lin, D. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, M.H.N.; Atkin, O.K.; Wiskich, J.T. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 235–255. [Google Scholar] [CrossRef]
- Hansberg, W.; Aguirre, J. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J. Theor. Biol. 1990, 142, 201–221. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Paglia, G.; Magnúsdóttir, M.; Steinarsdóttir, E.A.; Gudmundsson, S.; Palsson, B.Ø.; Andrésson, Ó.S.; Brynjólfsson, S. Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microb. Cell Fact. 2014, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schroeder, D.; Harvey, P. Dunaliella sp. from hypersaline environments. Presented at the Algae Biorefineries for Europe, Brussels, Belgium, 17–18 October 2017; Algae Biorefineries for Europe Web site. Available online: https://algaebiorefineryconference.eu/wp-content/uploads/2017/11/S16.pdf (accessed on 19 October 2017).


| Response | Light Intensity | Strain | Light Intensity*Strain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | F Values | P Values | Significance Level | Df | F Values | P Values | Significance Level | Df | F Values | P Values | Significance Level | |
| Photosynthesis | 3 | 8.1825 | 0.0002 | *** | 4 | 71.2528 | <2.2e−16 | *** | 12 | 2.7966 | 0.0073 | ** |
| Respiration | 3 | 1.7925 | 0.1641 | 4 | 52.7992 | 1.96e−15 | *** | 12 | 2.4328 | 0.0176 | * | |
| Total carotenoids | 3 | 2.9403 | 0.0446 | * | 4 | 693.560 | <2.2e−16 | *** | 12 | 7.9749 | 2.52e−07 | *** |
| Total chlorophyll | 3 | 36.529 | 1.55e−11 | *** | 4 | 161.782 | <2.2e−16 | *** | 12 | 10.285 | 8.41e−09 | *** |
| All-trans β-carotene | 3 | 88.922 | <2.2e−16 | *** | 4 | 474.255 | <2.2e−16 | *** | 12 | 3.6878 | 0.0009 | *** |
| 9-cis β-carotene | 3 | 28.119 | 6.02e−10 | *** | 4 | 730.574 | <2.2e−16 | *** | 12 | 6.8407 | 1.67e−06 | *** |
| Lutein | 3 | 7.3679 | 0.0005 | *** | 4 | 118.762 | <2.2e−16 | *** | 12 | 6.4955 | 3.08e−06 | *** |
| Zeaxanthin | 3 | 35.542 | 2.31e−11 | *** | 4 | 83.0526 | <2.2e−16 | *** | 12 | 5.2669 | 3.13e−05 | *** |
| All-trans α-carotene | 3 | 113.39 | <2.2e−16 | *** | 4 | 408.180 | <2.2e−16 | *** | 12 | 5.9987 | 7.64e−06 | *** |
| Glycerol | 3 | 2.1170 | 0.1132 | 4 | 95.5589 | <2.2e−16 | *** | 12 | 5.0858 | 4.50e−05 | *** | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production. Biology 2018, 7, 14. https://doi.org/10.3390/biology7010014
Xu Y, Ibrahim IM, Wosu CI, Ben-Amotz A, Harvey PJ. Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production. Biology. 2018; 7(1):14. https://doi.org/10.3390/biology7010014
Chicago/Turabian StyleXu, Yanan, Iskander M. Ibrahim, Chiziezi I. Wosu, Ami Ben-Amotz, and Patricia J. Harvey. 2018. "Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production" Biology 7, no. 1: 14. https://doi.org/10.3390/biology7010014





