Human Intrinsic Factor Expression for Bioavailable Vitamin B12 Enrichment in Microalgae
Abstract
:1. Introduction
2. Materials and Methods
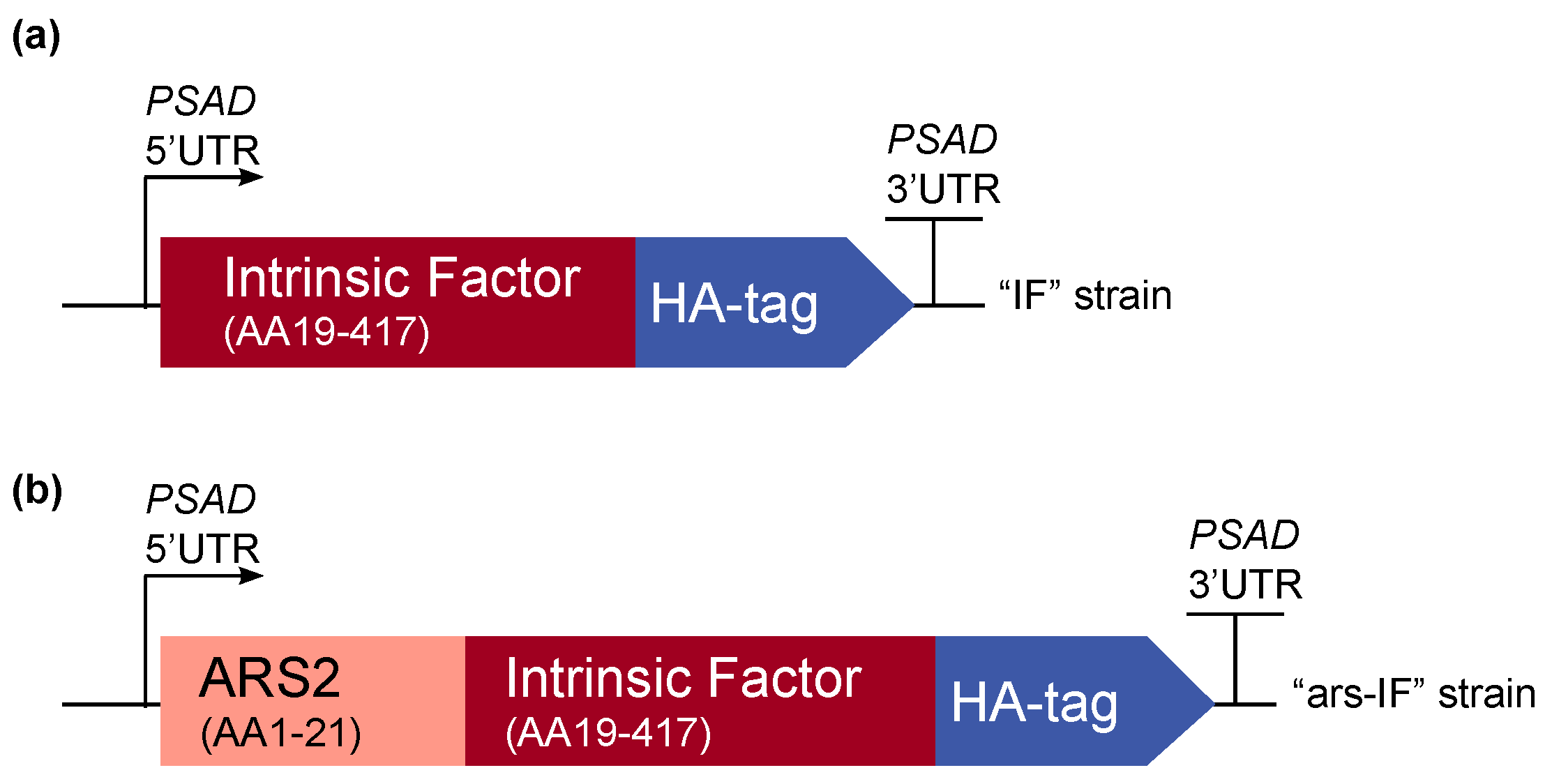
2.1. Construct Design and Plasmid Construction
2.2. Strains and Cultivation Conditions
2.3. Nuclear Transformation
2.4. PCR Analysis
2.5. Preparation of Cellular Lysates and Medium Samples for Protein Expression Analysis
2.6. Protein Concentration and Ion Exchange Chromatography
2.7. Vitamin B12 Assay
2.8. Reducing SDS-PAGE, Native PAGE and Western Blotting
2.9. Densitometric Analysis
3. Results
3.1. Generation of C. reinhardtii Nuclear Transformants “IF” and “ars-IF”
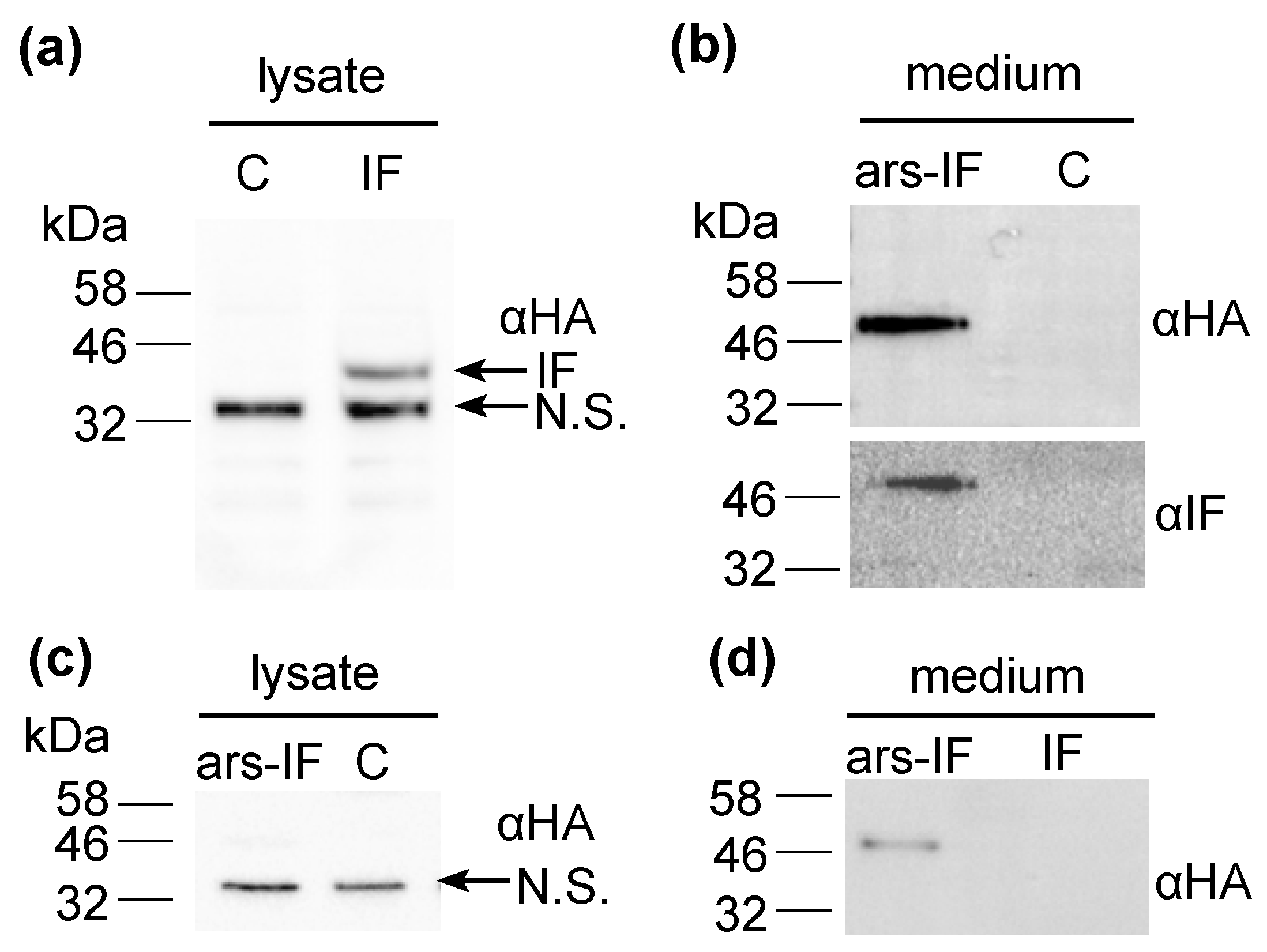
3.2. Expression of Human Intrinsic Factor in C. reinhardtii
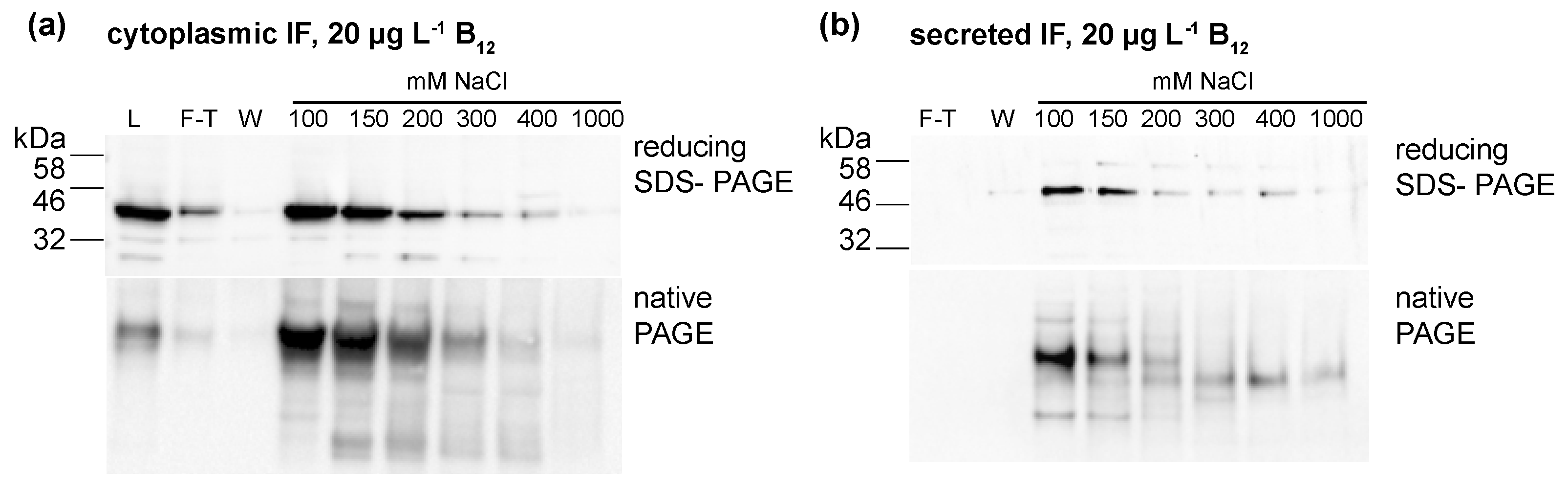
3.3. C. reinhardtii-Produced IF Stability Increases with External Vitamin B12 Supplementation
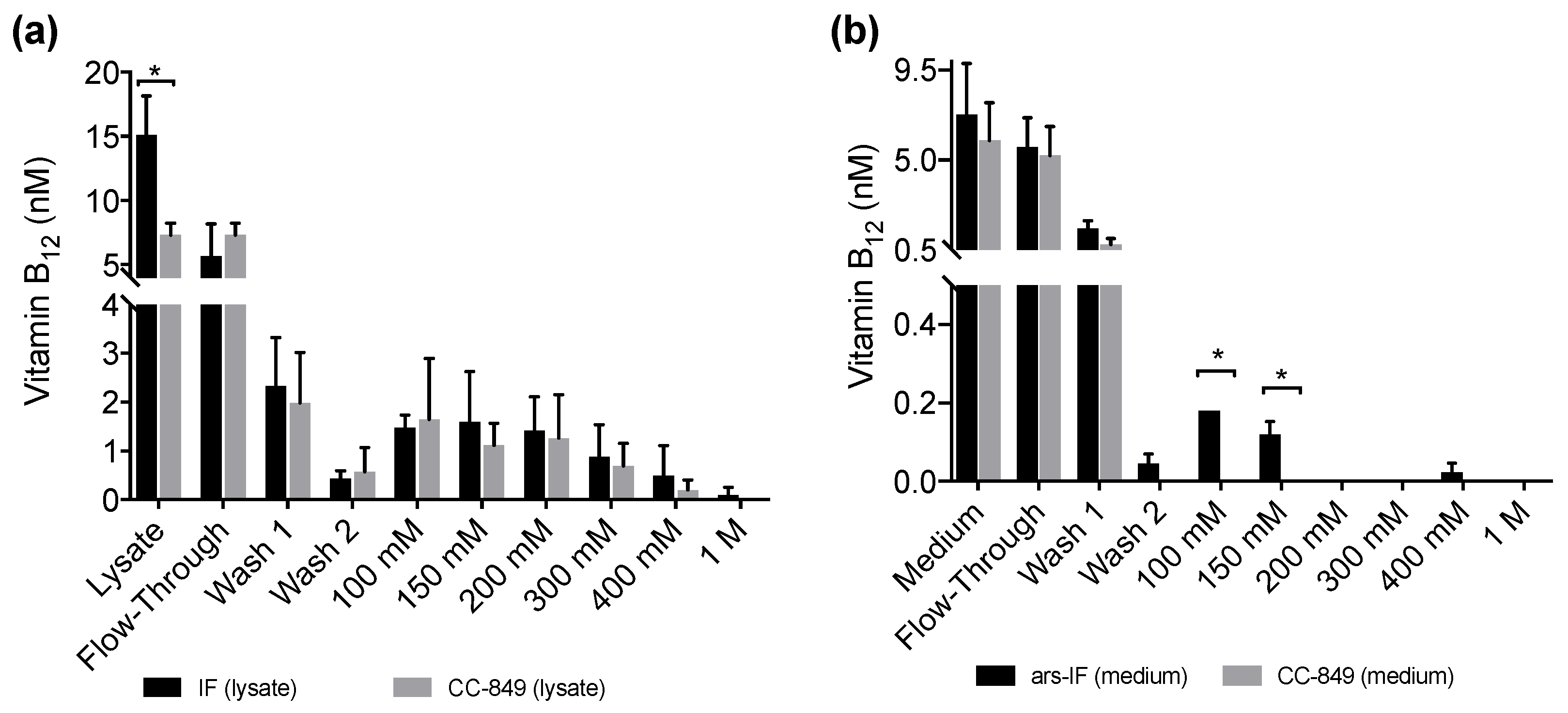
3.4. Correlation of Vitamin B12 and IF Protein Levels
4. Discussion
4.1. Expression and Efficient Secretion of a Human Glycoprotein Intrinsic Factor in Microalgae
4.2. Posttranslational-Modification of the Secreted IF Protein
4.3. Vitamin B12 Enrichment in IF Expressing C. reinhardtii Strains
4.4. Intrinsic Factor for Developing B12-Enriched Microalgae
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Warren, M.J.; Raux, E.; Schubert, H.L.; Escalante-Semerena, J.C. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 2002, 19, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.F.; Nexø, E.; Moestrup, S.K. Vitamin B12 transport from food to the body’s cells—A sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Alpers, D.H.; Russell-Jones, G. Gastric intrinsic factor: The gastric and small intestinal stages of cobalamin absorption. A personal journey. Biochimie 2013, 95, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Bito, M.; Asai, Y.; Takenaka, S.; Yabuta, Y.; Tago, K.; Ohnishi, M.; Mizoguchi, T.; Watanabe, F. Characterization and Quantitation of Vitamin B12 Compounds in Various Chlorella Supplements. J. Agric. Food. Chem. 2016, 64, 8516–8524. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae–their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J.Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Gangl, D.; Zedler, J.A.Z.; Rajakumar, P.D.; Ramos Martinez, E.M.; Riseley, A.; Włodarczyk, A.; Purton, S.; Sakuragi, Y.; Howe, C.J.; Jensen, P.E.; et al. Biotechnological exploitation of microalgae. J. Exp. Bot. 2015, 66, 6975–6990. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.Z.; Mellor, S.B.; Vavitsas, K.; Wlodarczyk, A.J.; Gnanasekaran, T.; Perestrello Ramos H de Jesus, M.; King, B.C.; Bakowski, K.; Jensen, P.E. Extending the biosynthetic repertoires of cyanobacteria and chloroplasts. Plant J. 2016, 87, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Scaife, M.A.; Nguyen, G.T.D.T.; Rico, J.; Lambert, D.; Helliwell, K.E.; Smith, A.G. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015, 82, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Mayfield, S.P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth. Res. 2015, 123, 227–239. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.C.; Kelly, S. Engineering biosynthesis of high-value compounds in photosynthetic organisms. Crit. Rev. Biotechnol. 2017, 37, 779–802. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.; Ness, J.E.; Gustafsson, C.; Minshull, J.; Govindarajan, S. Gene Designer: A synthetic biology tool for constructing artificial DNA segments. BMC Bioinform. 2006, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Eichler-Stahlberg, A.; Weisheit, W.; Ruecker, O.; Heitzer, M. Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta 2009, 229, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Rochaix, J.-D.; Purton, S. The bacterial phleomycin resistance geneble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet. 1996, 251, 23–30. [Google Scholar] [PubMed]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Kropat, J.; Hong-Hermesdorf, A.; Casero, D.; Ent, P.; Castruita, M.; Pellegrini, M.; Merchant, S.S.; Malasarn, D. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011, 66, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1990, 87, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras, V.; Stevens, D.R.; Purton, S. Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J. 1998, 14, 441–447. [Google Scholar] [CrossRef]
- Raux, E.; Lanois, A.; Levillayer, F.; Warren, M.J.; Brody, E.; Rambach, A.; Thermes, C. Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: Functional studies in S. typhimurium and Escherichia coli. J. Bacteriol. 1996, 178, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.; Wannathong, T.; Szaub, J.B.; Purton, S. A Simple, Low-Cost Method for Chloroplast Transformation of the Green Alga Chlamydomonas reinhardtii. Methods Mol. Biol. 2014, 1132, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zedler, J.A.Z.; Gangl, D.; Hamberger, B.; Purton, S.; Robinson, C. Stable expression of a bifunctional diterpene synthase in the chloroplast of Chlamydomonas reinhardtii. J. Appl. Phycol. 2015, 27, 2271–2277. [Google Scholar] [CrossRef]
- Ramos-Martinez, E.M.; Fimognari, L.; Sakuragi, Y. High-yield secretion of recombinant proteins from the microalga Chlamydomonas reinhardtii. Plant Biotechnol. J. 2017, 15, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J.; Berger, H.; Mussgnug, J.H.; Kruse, O. Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii. J. Biotechnol. 2013, 167, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J.; Vanderveer, T.L.; Berger, H.; Kaluza, I.; Mussgnug, J.H.; Walker, V.K.; Kruse, O. Ice recrystallization inhibition mediated by a nuclear-expressed and -secreted recombinant ice-binding protein in the microalga Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2013, 97, 9763–9772. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Lee, P.A.; Shen, Z.X.; Briggs, S.P.; Mendez, M.; Mayfield, S.P. Robust Expression and Secretion of Xylanase1 in Chlamydomonas reinhardtii by Fusion to a Selcetion Gene and Processing with the FMDV 2A Peptide. PLoS ONE 2012, 7, e43349. [Google Scholar] [CrossRef] [PubMed]
- Hempel, F.; Maier, U.G. An engineered diatom acting like a plasma cell secreting human IgG antibodies with high efficiency. Microb. Cell. Fact. 2012, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Vanier, G.; Hempel, F.; Chan, P.; Rodamer, M.; Vaudry, D.; Maier, U.G.; Lerouge, P.; Bardor, M. Biochemical Characterization of Human Anti-Hepatitis B Monoclonal Antibody Produced in the Microalgae Phaeodactylum tricornutum. PLoS ONE 2015, 10, e0139282. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.S.; Gordon, M.M.; Chen, Z.; Rajashankar, K.R.; Ealick, S.E.; Alpers, D.H.; Sukumar, N. Crystal structure of human intrinsic factor: Cobalamin complex at 2.6-Å resolution. Proc. Natl. Acad. Sci. USA 2007, 104, 17311–17316. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Laursen, N.B.; Nexø, E.; Moestrup, S.K.; Petersen, T.E.; Jensen, E.Ø.; Berglund, L. Human intrinsic factor expressed in the plant Arabidopsis thaliana. Eur. J. Biochem. 2003, 270, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.M.; Hu, C.; Chokshi, H.; Hewitt, J.E.; Alpers, D.H. Glycosylation is not required for ligand or receptor binding by expressed rat intrinsic factor. Am. J. Physiol. 1991, 260, G736–G742. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Kiefer-Meyer, M.-C.; Vanier, G.; Ovide, C.; Burel, C.; Lerouge, P.; Bardor, M. Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals. Front. Plant Sci. 2014, 5, 359. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Scholz, M.; Arias, C.; Dardelle, F.; Schulze, S.; Le Mauff, F.; Teo, G.; Hochmal, A.K.; Blanco-Rivero, A.; Loutelier-Bourhis, C.; et al. Exploring the N-glycosylation Pathway in Chlamydomonas reinhardtii Unravels Novel Complex Structures. Mol. Cell. Proteom. 2013, 12, 3160–3183. [Google Scholar] [CrossRef] [PubMed]
- Barahimipour, R.; Neupert, J.; Bock, R. Efficient expression of nuclear transgenes in the green alga Chlamydomonas: Synthesis of an HIV antigen and development of a new selectable marker. Plant Mol. Biol. 2016, 90, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, H.; Johnson, A.M.; Gillham, N.W.; Boynton, J.E. Epigenetic Silencing of a Foreign Gene in Nuclear Transformants of Chlamydomonas. Plant Cell 1997, 9, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Jinkerson, R.E.; Jonikas, M.C. Molecular techniques to interrogate and edit the Chlamydomonas nuclear genome. Plant J. 2015, 82, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Z.; Brueggeman, A.J.; Horken, K.M.; Plucinak, T.M.; Weeks, D.P. Successful Transient Expression of Cas9 and Single Guide RNA Genes in Chlamydomonas reinhardtii. Eukaryot. Cell 2014, 13, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-E.; Lim, J.-M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.-S.; Lee, B.; Hwangbo, K.; et al. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 27810. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Kelterborn, S.; Evers, H.; Kreimer, G.; Sizova, I.; Hegemann, P. Targeting of Photoreceptor Genes in Chlamydomonas reinhardtii via Zinc-finger Nucleases and CRISPR/Cas9. Plant Cell 2017, 29, 2498–2518. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Wheeler, G.L.; Leptos, K.C.; Goldstein, R.E.; Smith, A.G. Insights into the Evolution of Vitamin B12 Auxotrophy from Sequenced Algal Genomes. Mol. Biol. Evol. 2011, 28, 2921–2933. [Google Scholar] [CrossRef] [PubMed]
- Fumio, W.; Yoshihisa, N.; Yoshiyuki, T.; Hiroyuki, Y. Vitamin B12 metabolism in a photosynthesizing green alga, Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1991, 1075, 36–41. [Google Scholar] [CrossRef]
- Bertrand, E.M.; Allen, A.E.; Dupont, C.L.; Norden-Krichmar, T.M.; Bai, J.; Valas, R.E.; Saito, M.A. Influence of cobalamin scarcity on diatom molecular physiology and identification of a cobalamin acquisition protein. Proc. Natl. Acad. Sci. USA 2012, 109, E1762–E1771. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Lawrence, A.D.; Holzer, A.; Kudahl, U.J.; Sasso, S.; Kräutler, B.; Scanlan, D.J.; Warren, M.J.; Smith, A.G. Cyanobacteria and Eukaryotic Algae Use Different Chemical Variants of Vitamin B12. Curr. Biol. 2016, 26, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Stupperich, E.; Nexø, E. Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin and haptocorrin. Eur. J. Biochem. 1991, 199, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, J.A.; Hyun, J.S.; Schoepp, N.G.; Mayfield, S.P. Production of Recombinant Proteins in Microalgae at Pilot Greenhouse Scale. Biotechnol. Bioeng. 2015, 112, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Zedler, J.A.Z.; Gangl, D.; Guerra, T.; Santos, E.; Verdelho Vieira, V.; Robinson, C. Pilot-scale cultivation of wall-deficient transgenic Chlamydomonas reinhardtii strains expressing recombinant proteins in the chloroplast. Appl. Microbiol. Biotechnol. 2016, 100, 7061–7070. [Google Scholar] [CrossRef] [PubMed]
- Kazamia, E.; Riseley, A.S.; Howe, C.J.; Smith, A.G. An Engineered Community Approach for Industrial Cultivation of Microalgae. Ind. Biotechnol. 2014, 10, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kazamia, E.; Czesnick, H.; Nguyen, T.T.V.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Pandhal, J.; Cooper, M.B.; Longworth, J.; Kudahl, U.J.; Russo, D.A.; Tomsett, E.V.; Bunbury, F.; Salmon, D.L.; Smirnoff, N.; et al. Quantitative proteomics of a B12-dependent alga grown in coculture with bacteria reveals metabolic tradeoffs required for mutualism. New Phytol. 2017, 217, 599–612. [Google Scholar] [CrossRef] [PubMed]

| Vitamin B12 in Medium (μg·L−1) | ars-IF Strain (Medium) 1 | IF Strain (Cytoplasmic) 2 |
|---|---|---|
| 0 | 1.0 | 1.0 |
| 20 | 1.1 ± 0.01 | 1.4 ± 0.15 |
| 50 | 1.6 ± 0.17 | 2.3 ± 0.17 |
| 100 | 2.1 ± 0.50 | 1.4 ± 0.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, S.; Webb, C.L.; Deery, E.; Robinson, C.; Zedler, J.A.Z. Human Intrinsic Factor Expression for Bioavailable Vitamin B12 Enrichment in Microalgae. Biology 2018, 7, 19. https://doi.org/10.3390/biology7010019
Lima S, Webb CL, Deery E, Robinson C, Zedler JAZ. Human Intrinsic Factor Expression for Bioavailable Vitamin B12 Enrichment in Microalgae. Biology. 2018; 7(1):19. https://doi.org/10.3390/biology7010019
Chicago/Turabian StyleLima, Serena, Conner L. Webb, Evelyne Deery, Colin Robinson, and Julie A. Z. Zedler. 2018. "Human Intrinsic Factor Expression for Bioavailable Vitamin B12 Enrichment in Microalgae" Biology 7, no. 1: 19. https://doi.org/10.3390/biology7010019






