Media Screening for Obtaining Haematococcus pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Culture and Purification Procedure
2.2. Investigating the Influence of Medium Formulation on Cell Density in a Two Stage Process
2.3. Growth Measurement (Cell Number)
2.4. Dry Weight (DW) Measurement
2.5. Sample Preparation for Carotenoid, Astaxanthin and Fatty Acid Analysis
2.6. Total Carotenoid Analysis
2.7. Astaxanthin Analysis by Liquid Chromatography-Mass Spectrometry (LC-MS)
2.8. Fatty Acid Analysis
2.8.1. Direct-Derivatisation
2.8.2. Gas Chromatography-Flame Ionisation Detection (GC-FID)
2.9. Data Analysis
3. Results and Discussion
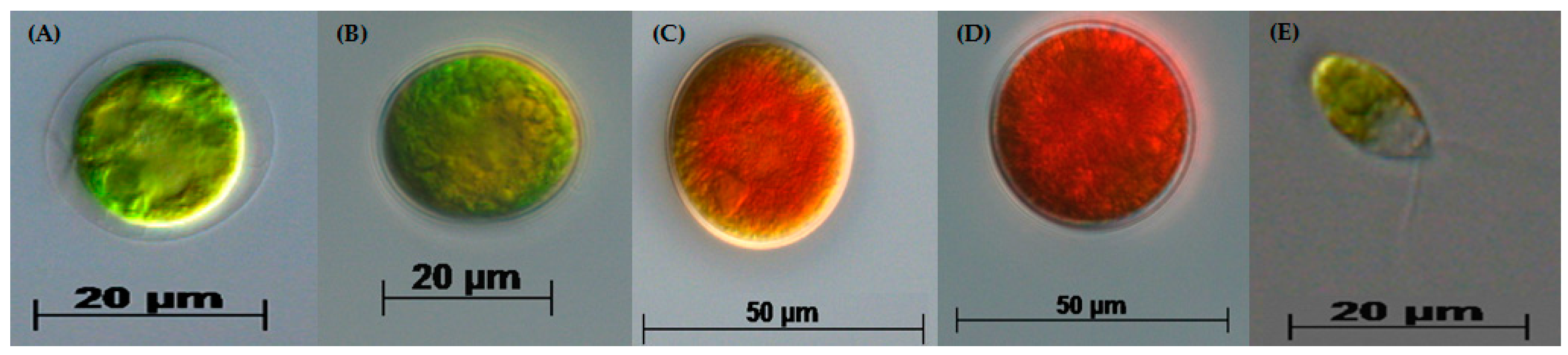
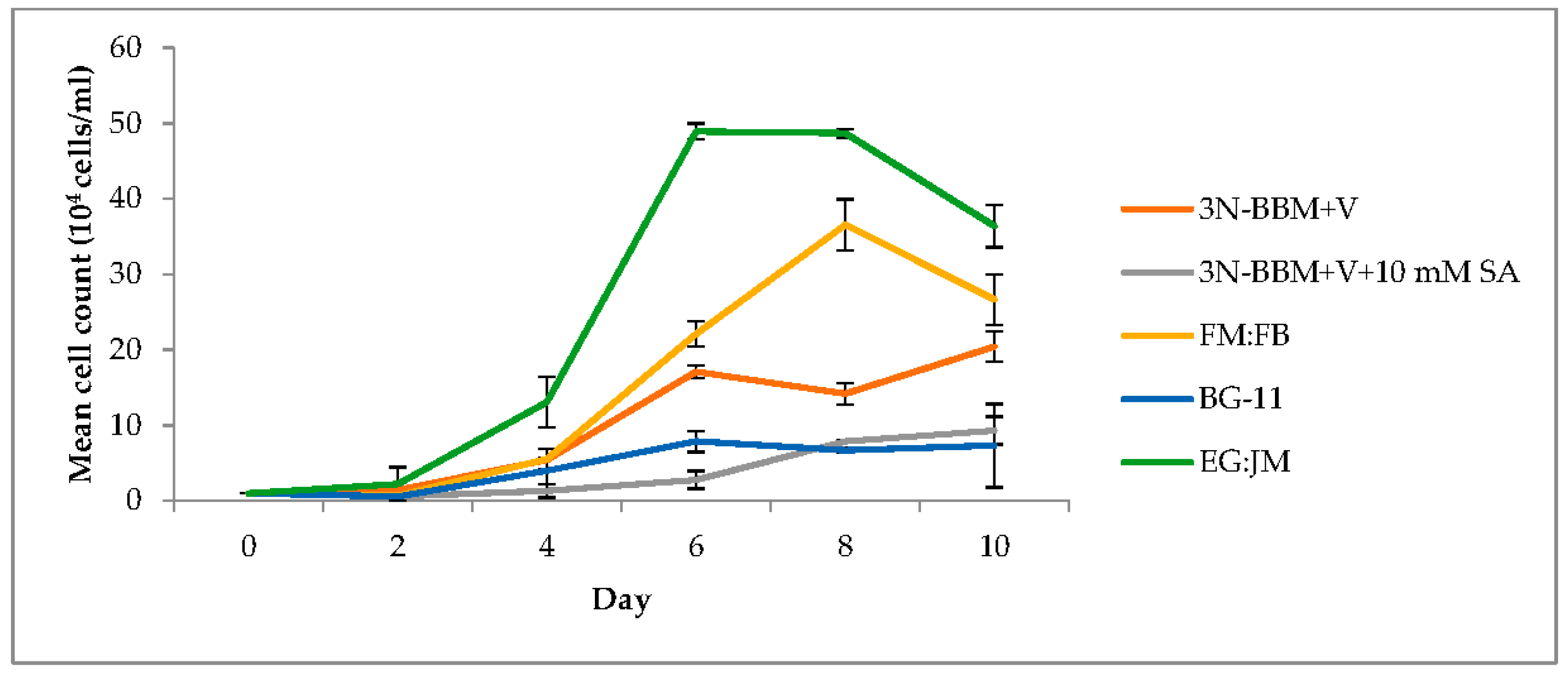
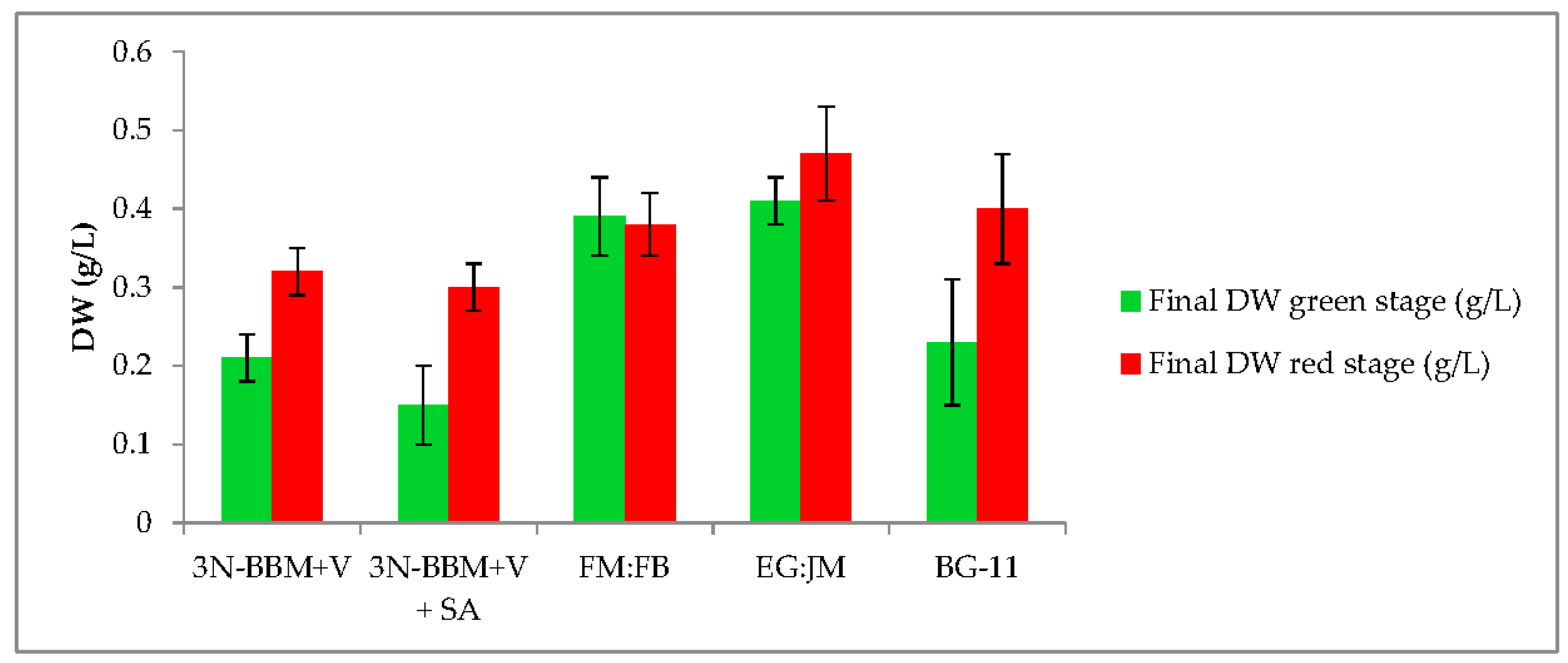
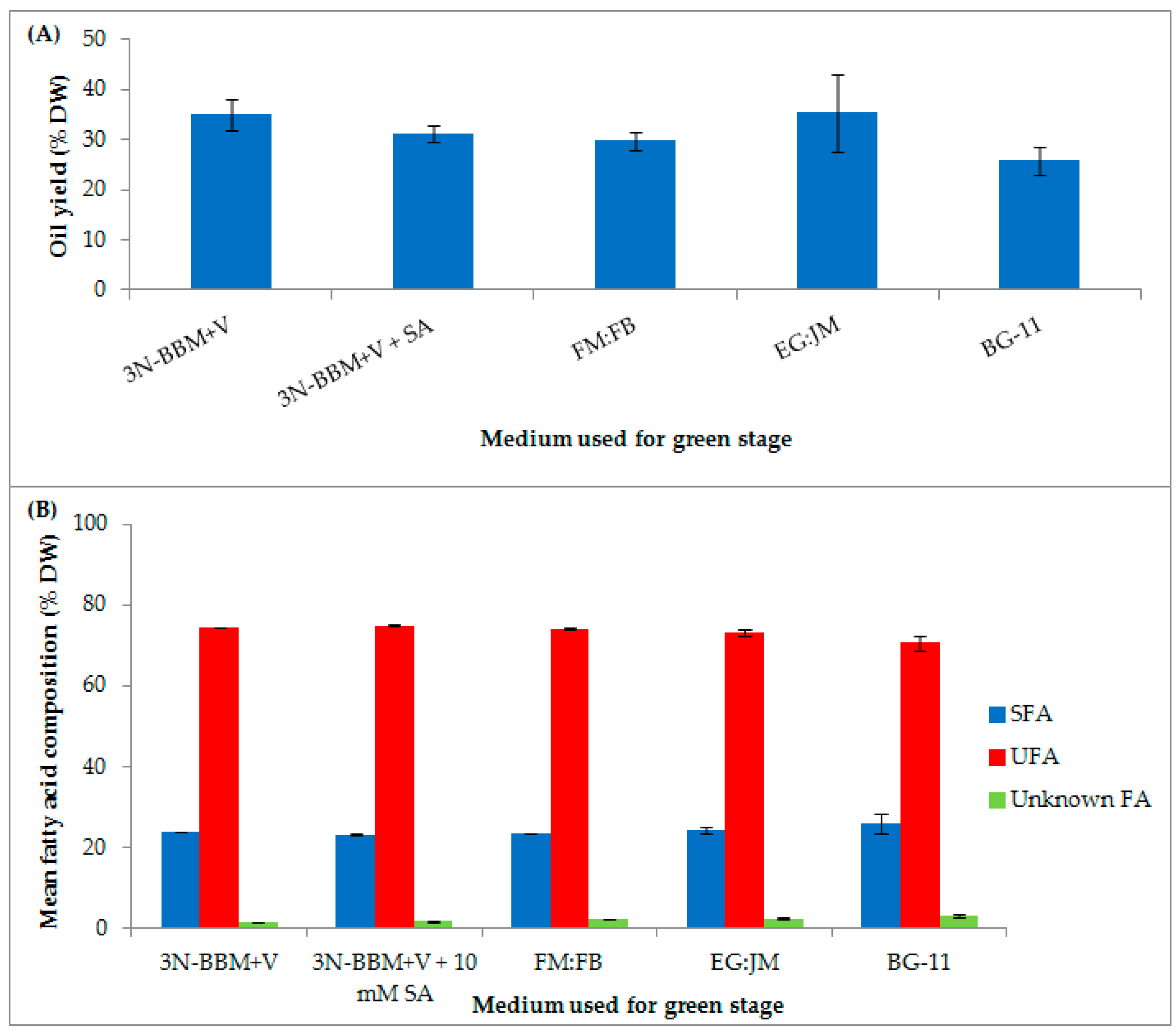
3.1. Two-Stage Process—Green Stage
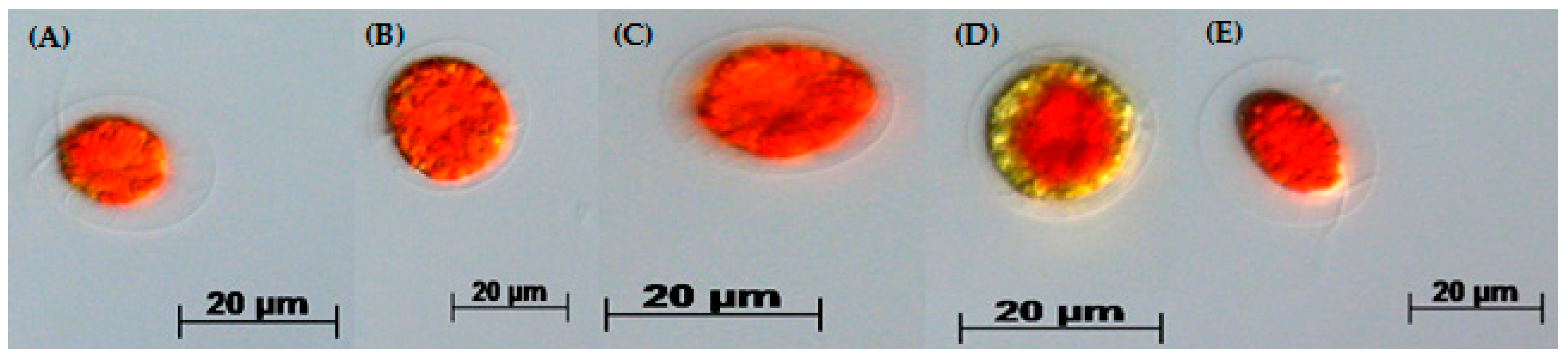
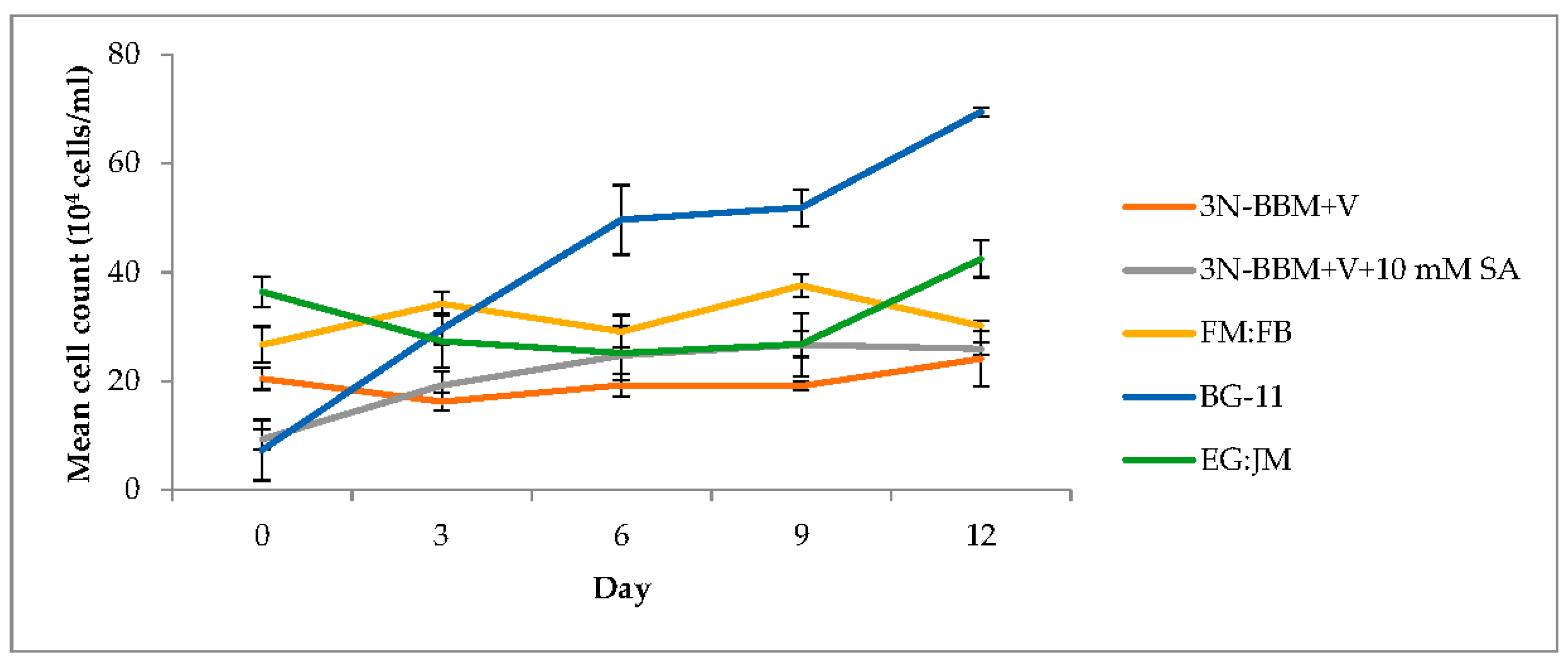
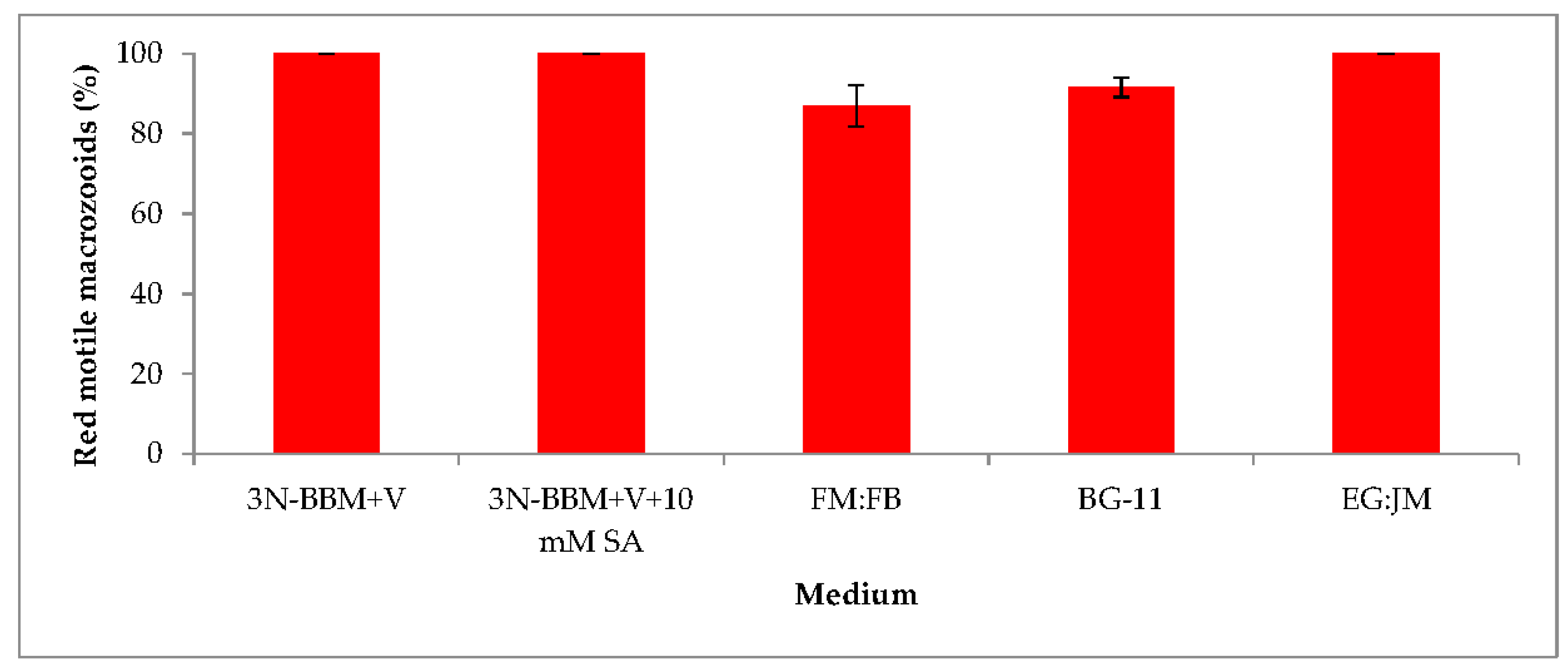
3.2. Two-Stage Process—Red Stage
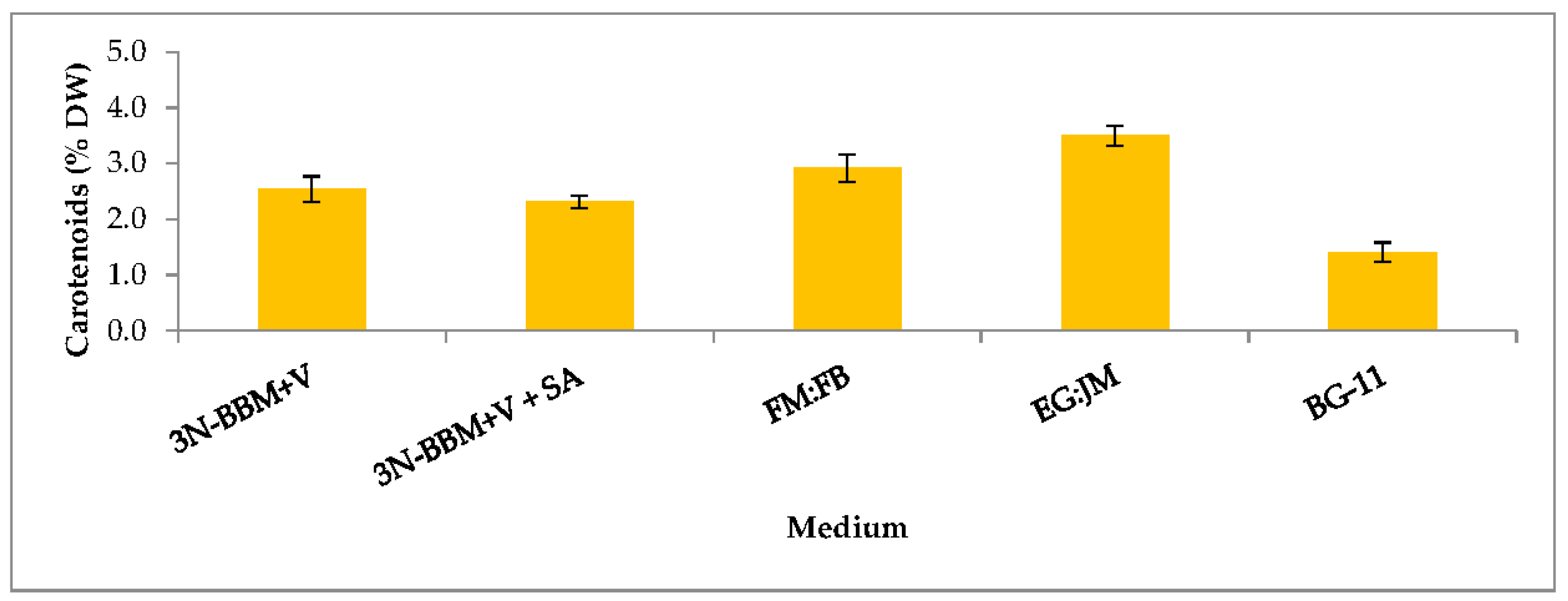
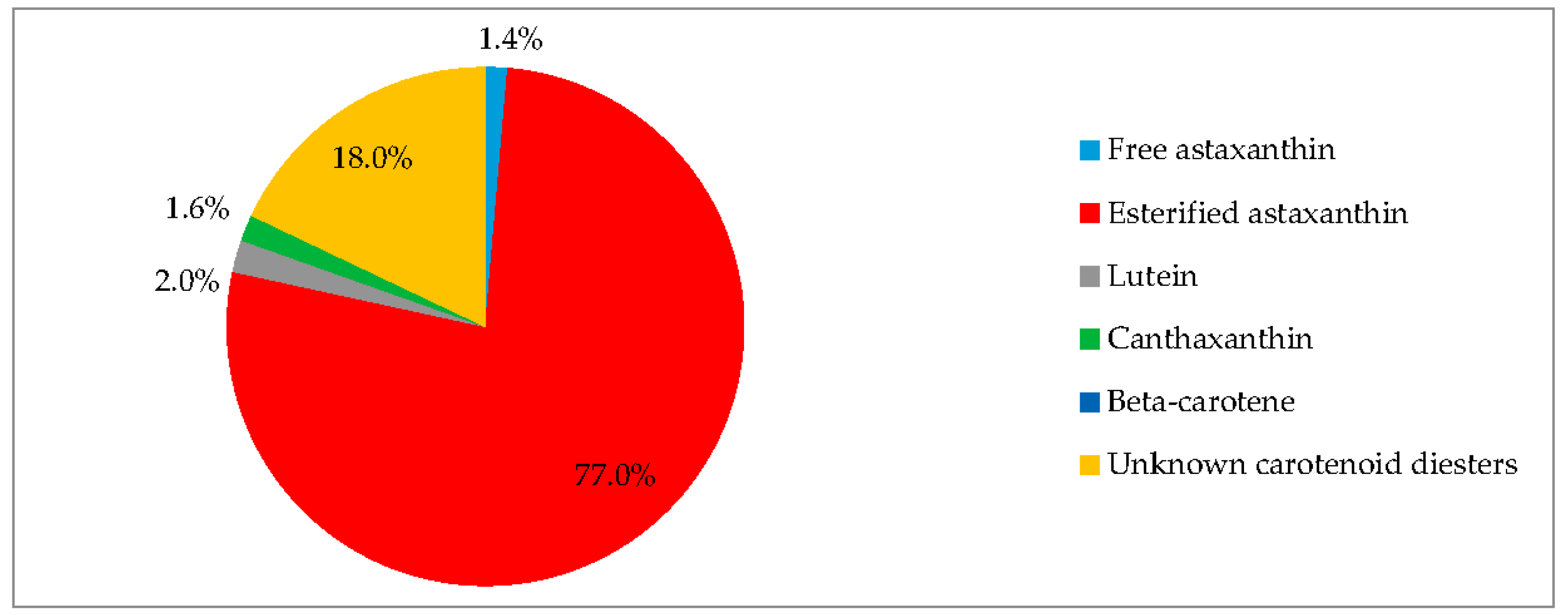
3.3. Biochemical Composition in the Red Stage
3.4. Red Motile Macrozooids—Rich in PUFAs and Astaxanthin
4. Conclusions and Future Research Direction
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haematococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- Dore, J.E.; Cysewski, G.R. Haematococcus Algae Meal as a Source of Natural Astaxanthin for Aquaculture Feeds. Cyanotech Corporation: Kailua, HI, USA, 2003. Available online: http://www.ruscom.com/cyan/web02/pdfs/naturose/nrtl09.pdf (accessed on 10 January 2015).
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Droop, M.R. Haematococcus pluvialis and its allies. I. The Sphaerellaceae. Rev. Algologique 1956, 2, 53–71. [Google Scholar]
- Tjahjono, A.E.; Hayama, Y.; Kakizono, T.; Terada, Y.; Nishio, N.; Nagai, S. Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol. Lett. 1994, 16, 133–138. [Google Scholar] [CrossRef]
- Hagen, C.; Grünewald, K.; Xyländer, M.; Rothe, E. Effect of cultivation parameters on growth and pigment biosynthesis in flagellated cells of Haematococcus pluvialis. J. Appl. Phycol. 2001, 13, 79–87. [Google Scholar] [CrossRef]
- García-Malea, M.C.; Acién, F.G.; Del Río, E.; Fernández, J.M.; Cerón, M.C.; Guerrero, M.G.; Molina-Grima, E. Production of astaxanthin by Haematococcus pluvialis: Taking the one-step system outdoors. Biotechnol. Bioeng. 2009, 102, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, M.A.; Sutherland, D.M.; Buchheim, J.A.; Wolf, M. The blood alga: Phylogeny of Haematococcus (Chlorophyceae) inferred from ribosomal RNA gene sequence data. Eur. J. Phycol. 2013, 48, 318–329. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic vs. Natural Production. Available online: https://trace.tennessee.edu/cgi/viewcontent.cgi?article=1094&context=utk_chembiopubs (accessed on 12 May 2016).
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Chen, F. Identification of astaxanthin isomers in Haematococcus lacustris by HPLC-photodiode array detection. Biotechnol. Tech. 1997, 11, 455–459. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Grewe, C.B.; Griehl, C. The carotenoid astaxanthin from Haematococcus pluvialis. In Microalgal Biotechnology: Integration and Economy; Posten, C., Walter, C., Eds.; Walter de Gruyter: Berlin, Germany, 2012; pp. 129–144. [Google Scholar]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Olaizola, M.; Huntley, M.E. Recent advances in commercial production of Astaxanthin from microalgae. In Recent Advances in Marine Biotechnology; Fingerman, M., Nagabhushanam, R., Eds.; Science Publishers: Hauppauge, NY, USA, 2003; Volume 9, pp. 143–164. [Google Scholar]
- Solovchenko, A.; Aflalo, C.; Lukyanov, A.; Boussiba, S. Nondestructive monitoring of carotenogenesis in Haematococcus pluvialis via whole-cell optical density spectra. Appl. Microbiol. Biotechnol. 2013, 97, 4533–4541. [Google Scholar] [CrossRef] [PubMed]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. 1. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996, 55, 207–214. [Google Scholar] [CrossRef]
- Burczyk, J. Cell wall carotenoids in green algae which form sporopollenins. Phytochemistry 1987, 26, 121–128. [Google Scholar] [CrossRef]
- Sommer, T.R.; Potts, W.T.; Morrissy, N.M. Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 94, 79–88. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of Different Cell Disruption Processes on Encysted Cells of Haematococcus pluvialis: Effects on Astaxanthin Recovery and Implications for Bio-Availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. Food Chem. 2006, 54, 7585–7588. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 1–7. [Google Scholar]
- Pan, J.L.; Wang, H.M.; Chen, C.Y.; Chang, J.S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Bubrick, P. Production of astaxanthin from Haematococcus. Bioresour. Technol. 1991, 38, 237–239. [Google Scholar] [CrossRef]
- Margalith, P.Z. Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 1999, 51, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Gutman, J.; Zarka, A.; Boussiba, S. The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur. J. Phycol. 2009, 44, 509–514. [Google Scholar] [CrossRef]
- Gutman, J.; Zarka, A.; Boussiba, S. Evidence for the involvement of surface carbohydrates in the recognition of Haematococcus pluvialis by the parasitic blastoclad Paraphysoderma sedebokerensis. Fungal Biol. 2011, 115, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, Y.; Aflalo, C.; Zarka, A.; Gutman, J.; James, T.Y.; Boussiba, S. Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota), parasitic on the green alga Haematococcus. Mycol. Res. 2008, 112, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Li, H.; Zhao, P.; Yu, R.; Pan, G.; Gao, S.; Xie, X.; Huang, A.; He, L.; Wang, G. Quantitative proteomic analysis of thylakoid from two microalgae (Haematococcus pluvialis and Dunaliella salina) reveals two different high light-responsive strategies. Sci. Rep. 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hill, R.T.; Zheng, T.; Hu, X.; Wang, B. Effects of bacterial communities on biofuel-producing microalgae: Stimulation, inhibition and harvesting. Crit. Rev. Biotechnol. 2016, 36, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Del Río, E.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol. Bioeng. 2008, 100, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Del Río, E.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol. Bioeng. 2005, 91, 808–815. [Google Scholar] [PubMed]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojidek, J. Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar] [CrossRef]
- Kan, Y.; Pan, J. A one-shot solution to bacterial and fungal contamination in the green alga Chlamydomonas reinhardtii culture by using an antibiotic cocktail. J. Phycol. 2010, 46, 1356–1358. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella Nana Hustedt and Detonula Confervacea (CLEVE) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Lorenz, R.T. HPLC and spectrophotometric analysis of carotenoids from Haematococcus algae oleoresin. BioAstin/NaturoseTM Tech. Bull. 2001, 20, 1–10. Available online: http://www.ruscom.com/cyan/web02 /pdfs/bioastin/axbul20.pdf (accessed on 22 November 2016).
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids handbook. J. Appl. Cosmetol. 2004, 22, 118–120. [Google Scholar]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI) MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, S.P.; Zhang, Q.Y.; Black, K.D.; Day, J.G.; Stanley, M.S. Comparison of screening methods for high-throughput determination of oil yields in micro-algal biofuel strains. J. Appl. Phycol. 2013, 25, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Tocquin, P.; Fratamico, A.; Franck, F. Screening for a low-cost Haematococcus pluvialis medium reveals an unexpected impact of a low N/P ratio on vegetative growth. J. Appl. Phycol. 2012, 24, 365–373. [Google Scholar] [CrossRef]
- Kakizono, T.; Kobayashi, M.; Nagai, S. Effect of carbon/nitrogen ratio on encystment accompanied with astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992, 74, 403–405. [Google Scholar] [CrossRef]
- Harker, M.; Young, A.J. Inhibition of astaxanthin synthesis in the green alga, Haematococcus pluvialis. Eur. J. Phycol. 1995, 30, 179–187. [Google Scholar] [CrossRef]
- Gong, X.; Chen, F. Influence of medium components on astaxanthin content and production of Haematococcus pluvialis. Process Biochem. 1998, 33, 385–391. [Google Scholar] [CrossRef]
- Fábregas, J.; Domínguez, A.; Regueiro, M.; Maseda, A.; Otero, A. Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 53, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Sarada, R.; Bhattacharya, S.; Ravishankar, G. Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J. Microbiol. Biotechnol. 2002, 18, 517–521. [Google Scholar] [CrossRef]
- García-Malea, M.; Brindley, C.; Del Río, E.; Acíen, F.G.; Fernández, J.M.; Molina, E. Modelling of growth and accumulation of carotenoids in Haematococcus pluvialis as a function of irradiance and nutrients supply. Biochem. Eng. J. 2005, 26, 107–114. [Google Scholar] [CrossRef]
- Dalay, M.C.; Imamoglu, E.; Demirel, Z. Agricultural fertilizers as economical alternative for cultivation of Haematococcus pluvialis. J. Microbiol. Biotechnol. 2007, 17, 393–397. [Google Scholar] [PubMed]
- Suh, I.S.; Joo, H.N.; Lee, C.G. A novel double-layered photobioreactor for simultaneous Haematococcus pluvialis cell growth and astaxanthin accumulation. J. Biotechnol. 2006, 125, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhao, X.; Xing, X.; Hu, J.; Gong, J. NH Secretion during astaxanthin synthesis in Haematococcus pluvialis under high irradiance and nitrogen deficient conditions. Chin. J. Chem. Eng. 2007, 15, 162–166. [Google Scholar] [CrossRef]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Oncel, S.S.; Imamoglu, E.; Gunerken, E.; Sukan, F.V. Comparison of different cultivation modes and light intensities using mono-cultures and co-cultures of Haematococcus pluvialis and Chlorella zofingiensis. J. Chem. Technol. Biotechnol. 2011, 86, 414–420. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Geng, Y.H.; Li, Z.K.; Hu, H.J.; Li, Y.G. Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture 2009, 295, 275–281. [Google Scholar] [CrossRef]
- Fábregas, J.; Otero, A.; Maseda, A.; Domínguez, A. Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2001, 89, 65–71. [Google Scholar] [CrossRef]
- Raimbault, P.; Mingazzini, M. Diurnal variations of intracellular nitrate storage by marine diatoms: Effects of nutritional state. J. Exp. Mar. Biol. Ecol. 1987, 112, 217–232. [Google Scholar] [CrossRef]
- Jacobson, L.; Halmann, M. Polyphosphate metabolism in the blue-green alga Microcystis aeru-ginosa. J. Plankton Res. 1982, 4, 481–488. [Google Scholar] [CrossRef]
- Grünewald, K.; Hagen, C.; Braune, W. Secondary carotenoid accumulation in flagellates of the green alga Haematococcus lacustris. Eur. J. Phycol. 1997, 32, 387–392. [Google Scholar] [CrossRef]
- Brinda, B.R.; Sarada, R.; Kamath, B.S.; Ravishankar, G.A. Accumulation of astaxanthin in flagellated cells of Haematococcus pluvialis-cultural and regulatory aspects. Curr. Sci. Bangalore 2004, 87, 1290–1294. [Google Scholar]
- Cerón, M.C.; García-Malea, M.C.; Rivas, J.; Acien, F.G.; Fernandez, J.M.; Del Río, E.; Guerrero, M.G.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Saini, S.; Bhowmick, T.K.; Gayen, K. Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Convers. Manag. 2016, 113, 104–118. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery from biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Razon, L.F.; Tan, R.R. Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl. Energy 2011, 88, 3507–3514. [Google Scholar] [CrossRef]
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Yang, J.; Hu, H.Y.; Yu, Y. Lipid-rich microalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecol. Eng. 2013, 60, 155–159. [Google Scholar] [CrossRef]
| Composition | 3N-BBM+V | 3N-BBM+V + SA | BG-11 | EG:JM | FM:FB |
|---|---|---|---|---|---|
| NO3 (mM) | 8.82 | 8.82 | 17.65 | 0.47 | 2.70 |
| PO4 (mM) | 1.72 | 1.72 | 0.23 | 0.096 | 4.60 |
| N/P ratio | 5.13 | 5.13 | 76.74 | 4.90 | 0.59:1 |
| Fatty Acid | 3N-BBM+V | 3N-BBM+V + 10 mM SA | FM:FB | EG:JM | BG-11 |
|---|---|---|---|---|---|
| 14:0 | 0.35 ± 0.01 | 0.33 ± 0.00 | 0.36 ± 0.01 | 0.54 ± 0.15 | 0.43 ± 0.04 |
| 16:0 | 22.12 ± 0.08 | 21.35 ± 0.26 | 21.65 ± 0.08 | 22.57 ± 0.62 | 21.98 ± 0.30 |
| 16:3(n-3) | 2.09 ± 0.08 | 2.16 ± 0.12 | 2.14 ± 0.05 | 1.86 ± 0.05 | 2.03 ± 0.08 |
| 16:4(n-3) | 3.53 ± 0.03 | 4.08 ± 0.15 | 4.34 ± 0.41 | 5.37 ± 0.46 | 4.09 ± 0.21 |
| 18:1(n-7) | 3.72 ± 0.03 | 3.76 ± 0.16 | 4.08 ± 0.27 | 4.76 ± 0.45 | 4.17 ± 0.14 |
| 18:1(n-9) | 20.31 ± 0.30 | 18.37 ± 1.09 | 17.30 ± 1.42 | 12.31 ± 0.79 | 19.47 ± 0.99 |
| 18:2(n-6) | 28.49 ± 0.21 | 28.26 ± 1.23 | 27.38 ± 0.66 | 23.63 ± 0.54 | 25.91 ± 0.32 |
| 18:3(n-3) | 10.98 ± 0.05 | 12.35 ± 0.44 | 13.05 ± 0.90 | 15.45 ± 1.51 | 11.96 ± 0.37 |
| 18:4(n-3) | 1.61 ± 0.03 | 1.95 ± 0.08 | 2.14 ± 0.26 | 2.74 ± 0.27 | 1.75 ± 0.06 |
| Other SFA | 1.39 ± 0.03 | 1.41 ± 0.05 | 1.32 ± 0.04 | 2.74 ± 1.57 | 1.77 ± 0.31 |
| Other UFA | 3.53 ± 0.07 | 3.97 ± 0.10 | 3.68 ± 0.34 | 4.55 ± 0.26 | 3.73 ± 0.07 |
| Unknown FA | 1.67 ± 0.04 | 1.84 ± 0.07 | 2.35 ± 0.12 | 3.27 ± 0.54 | 2.45 ± 0.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, T.O.; McDougall, G.J.; Campbell, R.; Stanley, M.S.; Day, J.G. Media Screening for Obtaining Haematococcus pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids. Biology 2018, 7, 2. https://doi.org/10.3390/biology7010002
Butler TO, McDougall GJ, Campbell R, Stanley MS, Day JG. Media Screening for Obtaining Haematococcus pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids. Biology. 2018; 7(1):2. https://doi.org/10.3390/biology7010002
Chicago/Turabian StyleButler, Thomas O., Gordon J. McDougall, Raymond Campbell, Michele S. Stanley, and John G. Day. 2018. "Media Screening for Obtaining Haematococcus pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids" Biology 7, no. 1: 2. https://doi.org/10.3390/biology7010002





