Groundsel Bush (Baccharis halimifolia) Extract Promotes Adipocyte Differentiation In Vitro and Increases Adiponectin Expression in Mature Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Preparation of Groundsel Bush Extract
2.2. Cell Culture and Treatments
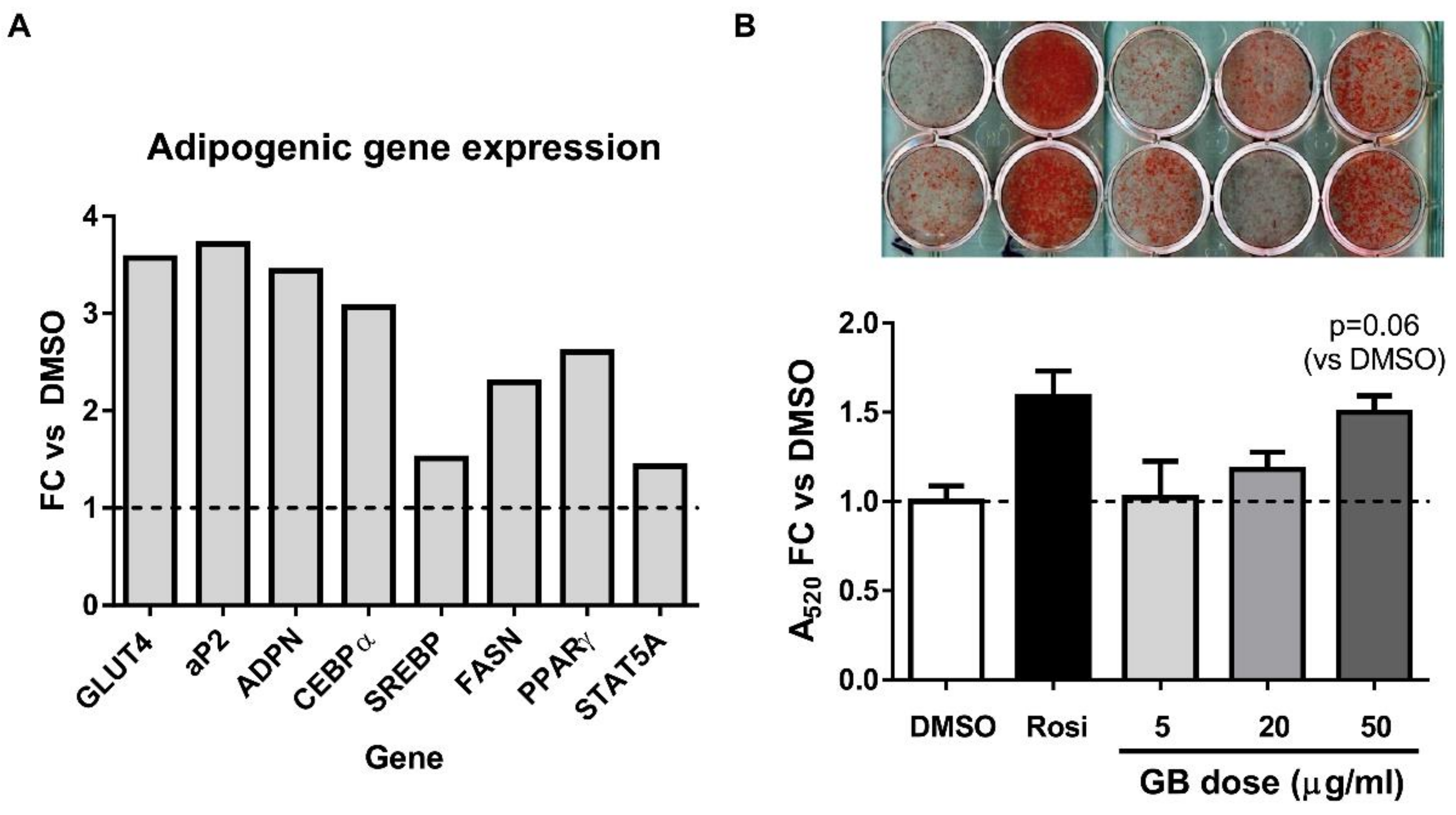
2.3. Lipid Accumulation
2.4. Gene Expression
2.5. Whole-Cell Extract and Tissue Preparation
2.6. Gel Electrophoresis and Immunoblotting
2.7. Statistics
3. Results
3.1. Groundsel Bush Extract Enhances Neutral Lipid Accumulation and Expression of Adipogenic Marker Genes in Differentiating 3T3-L1 Adipocytes
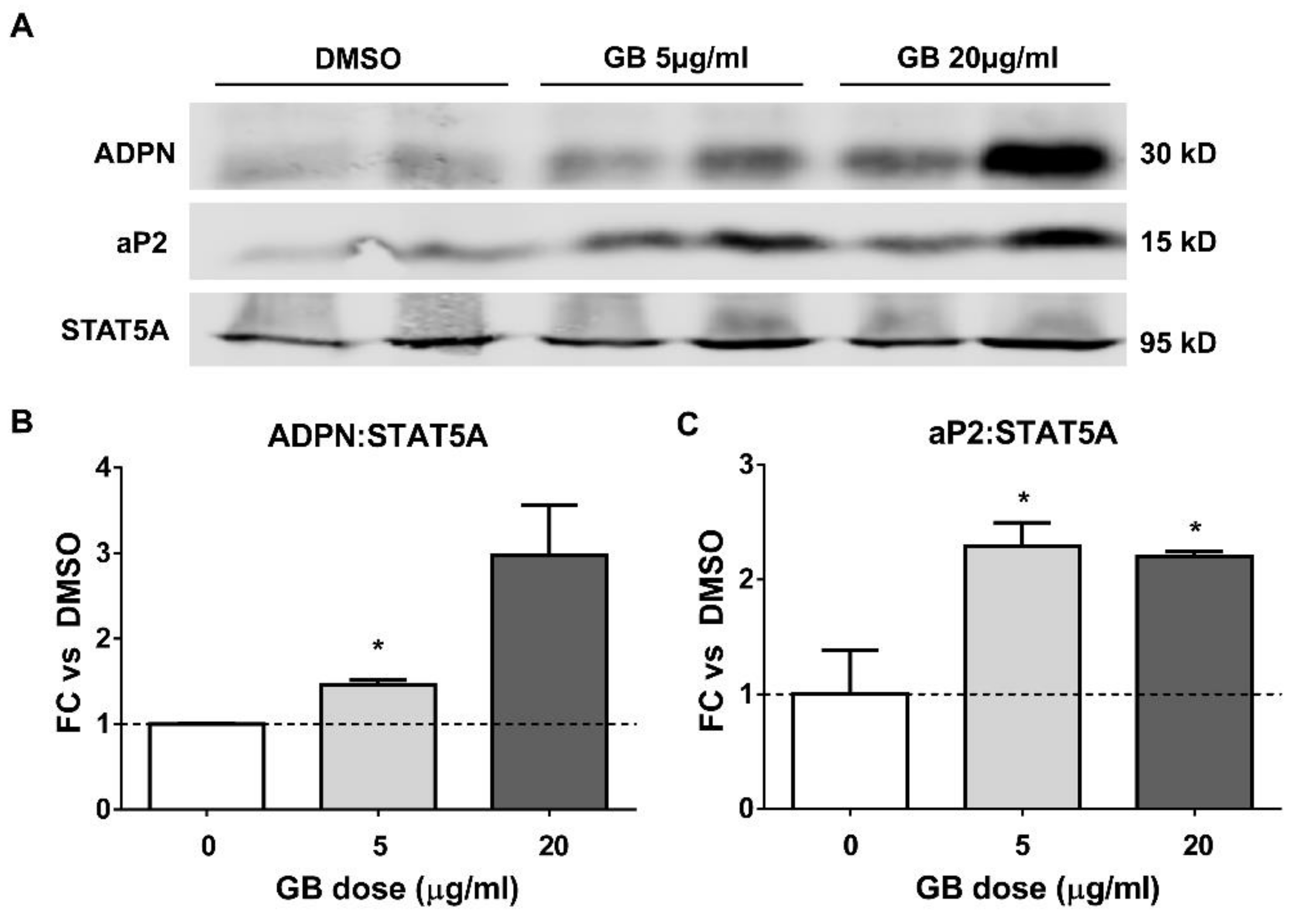
3.2. Groundsel Bush Extract Increases the Protein Expression of Adiponectin and aP2 in Differentiating Adipocytes
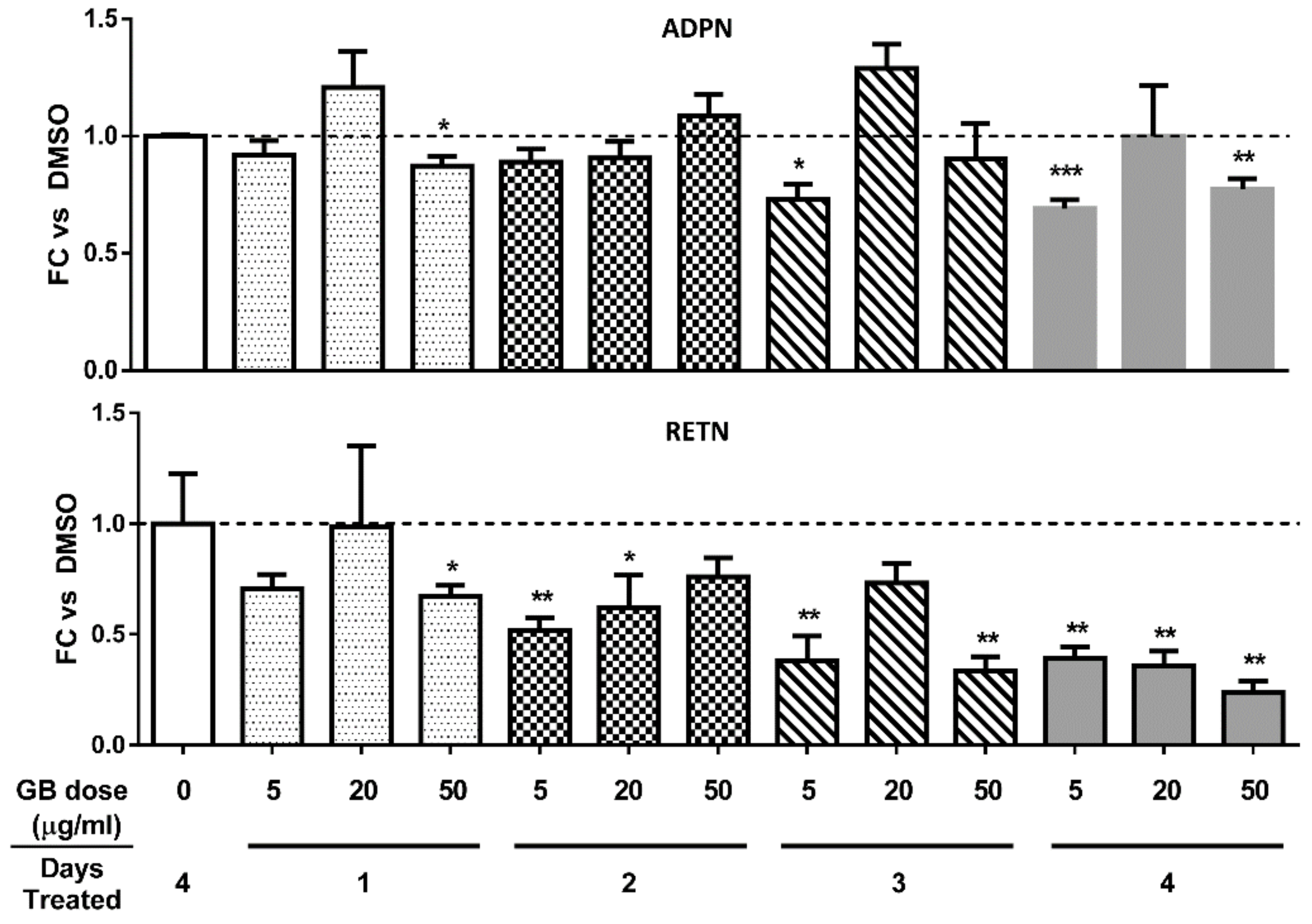
3.3. Groundsel Bush Extract does not Substantially Alter Adiponectin Gene Expression, but Decreases Resistin Gene Expression in a Time-Dependent Manner in Mature 3T3-L1 Adipocytes
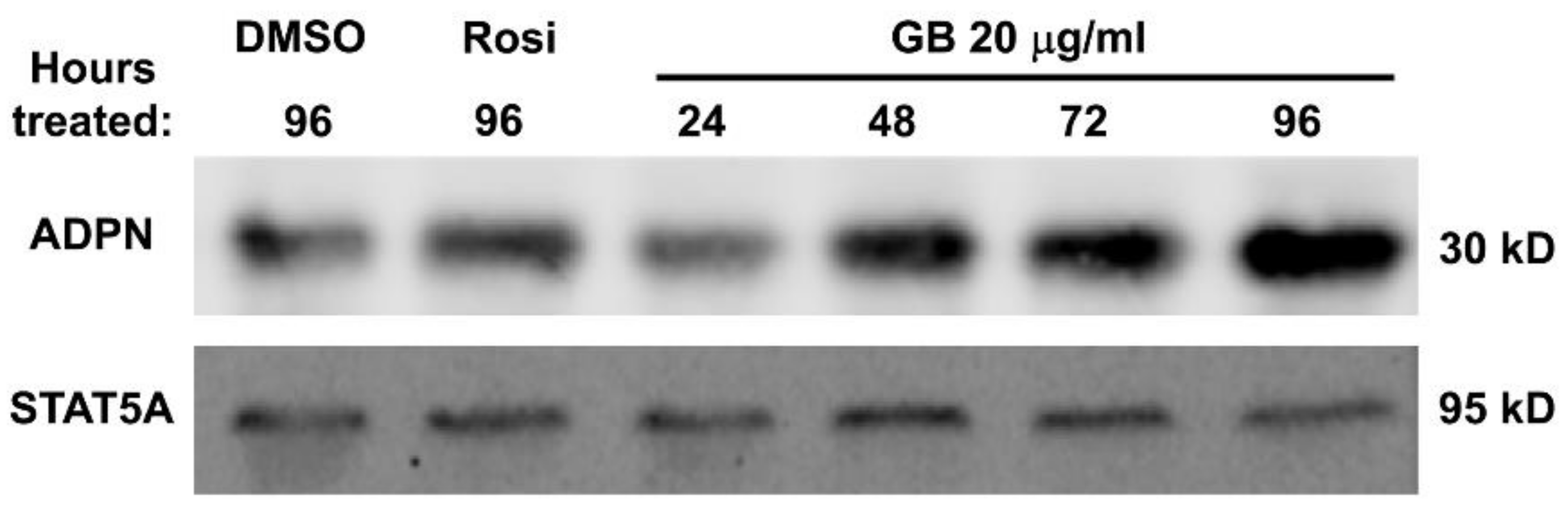
3.4. Groundsel Bush Extract Increases Adiponectin Protein Expression in Mature 3T3-L1 Adipocytes in a Time-Dependent Manner
3.5. Groundsel Bush Extract Attenuates TNF-α-Induced Repression of Adiponectin Expression in 3T3-L1 Adipocytes
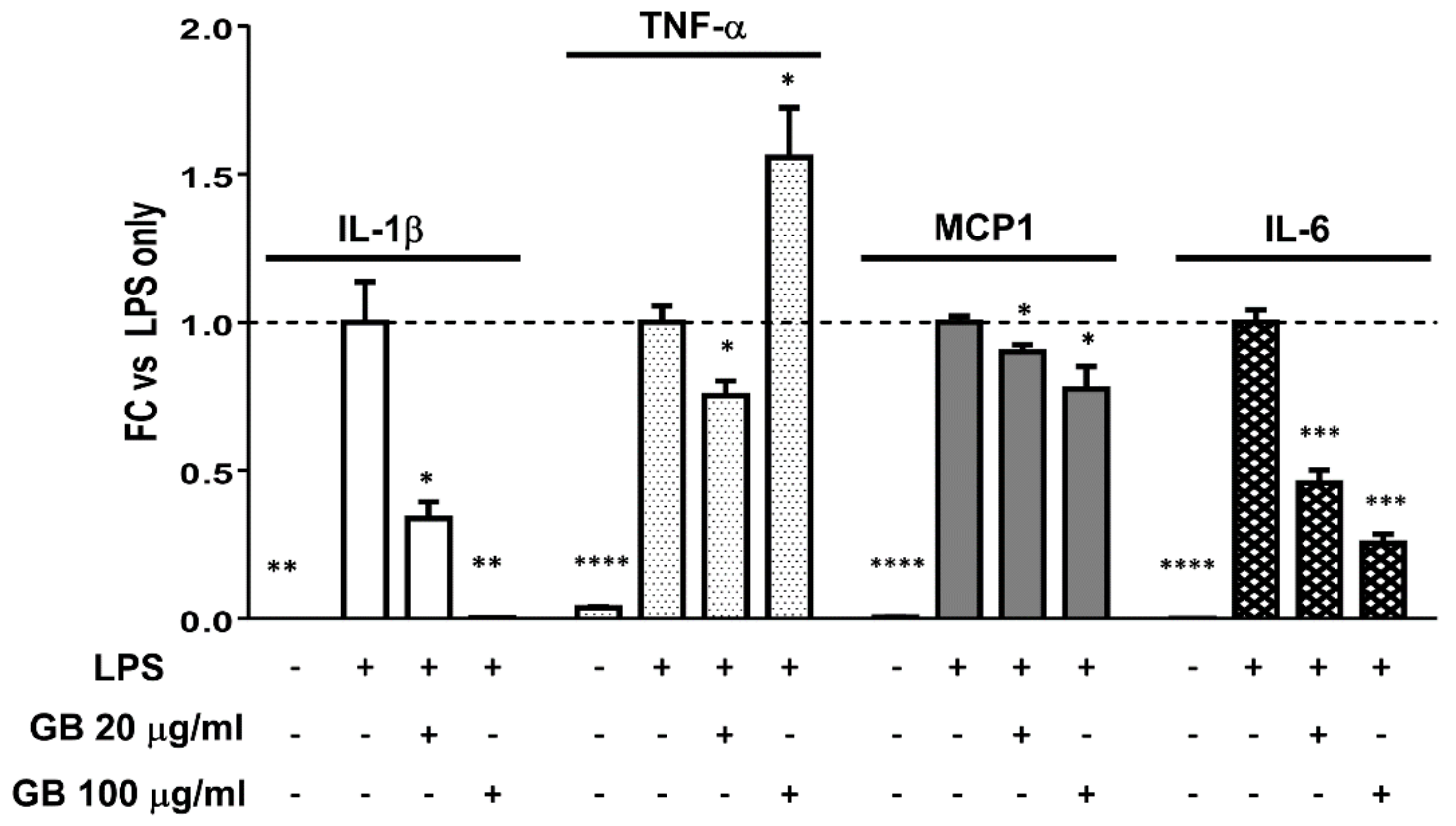
3.6. Groundsel Bush Extract Attenuates LPS-Induced Expression of IL-1β, IL-6, and MCP1, but not TNF-α, in RAW Macrophages
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the Asia-pacific region: A systematic review. BMC Public Health 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome. Endocrinol. Nutr. 2013, 60, 39–43. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mullican, S.E.; DiSpirito, J.R.; Peed, L.C.; Lazar, M.A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPAR. Proc. Natl. Acad. Sci. USA 2013, 110, 18656–18661. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Gregg, B. Metformin; a review of its history and future: From lilac to longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; Burris, T.P.; Sanchez-Infantes, D.; Wang, Y.; Ribnicky, D.M.; Stephens, J.M. Artemisia extracts activate PPARγ, promote adipogenesis, and enhance insulin sensitivity in adipose tissue of obese mice. Nutrition 2014, 30, S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; Fuller, S.; Fedorcenco, V.; Beyl, R.; Burris, T.P.; Mynatt, R.; Ribnicky, D.M.; Stephens, J.M. Artemisia scoparia enhances adipocyte development and endocrine function in vitro and enhances insulin action in vivo. PLoS ONE 2014, 9, e98897. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, A.; Cheng, D.M.; Ruiz, C.; Ribnicky, D.; Allain, L.; Brassieur, C.R.; Turnipseed, D.P.; Cefalu, W.T.; Floyd, Z.E. Screening native botanicals for bioactivity: An interdisciplinary approach. Nutrition 2014, 30. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Li, T.; Manson, J.A.E.; Meigs, J.B.; Hu, F.B. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, J.L.; Sun, Q.; Olenczuk, D.; Qi, L.; Zhu, Y.; Hu, F.B.; Mantzoros, C.S. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. Eur. J. Endocrinol. 2010, 162, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chumanevich, A.; Fletcher, E.; Larsen, B.; Lattwein, K.; Kaur, K.; Fayad, R. Adiponectin deficiency: Role in chronic inflammation induced colon cancer. Biochim. Biophys. Acta 2012, 1822, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Van Stijn, C.M.W.; Kim, J.; Barish, G.D.; Tietge, U.J.F.; Tangirala, R.K. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PLoS ONE 2014, 9, e86404. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Pu, H.; Wei, Q.; Duan, M.; Zhang, C.; Jiang, T.; Shou, X.; Zhang, J.; Yang, Y. Adiponectin improves NF-κB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids Health Dis. 2016, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Ayina, C.N.A.; Noubiap, J.J.N.; Etoundi Ngoa, L.S.; Boudou, P.; Gautier, J.F.; Mengnjo, M.K.; Mbanya, J.C.; Sobngwi, E. Association of serum leptin and adiponectin with anthropomorphic indices of obesity, blood lipids and insulin resistance in a Sub-Saharan African population. Lipids Health Dis. 2016, 15, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qatanani, M.; Szwergold, N.R.; Greaves, D.R.; Ahima, R.S.; Lazar, M.A. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J. Clin. Investig. 2009, 119, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Drolet, R.; Noël, S.; Paris, G.; Tchernof, A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism 2012, 61, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Morinaga, H.; Talukdar, S.; Bae, E.J.; Olefsky, J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012, 61, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sorisky, A.; Molgat, A.S.D.; Gagnon, A. Macrophage-induced adipose tissue dysfunction and the preadipocyte: Should I stay (and differentiate) or should I go? Adv. Nutr. 2013, 4, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.W. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Alligier, M.; Gabert, L.; Meugnier, E.; Lambert-Porcheron, S.; Chanseaume, E.; Pilleul, F.; Debard, C.; Sauvinet, V.; Morio, B.; Vidal-Puig, A.; et al. Visceral fat accumulation during lipid overfeeding is elated to subcutaneous adipose tissue characteristics in healthy men. J. Clin. Endocrinol. Metab. 2013, 98, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, R.; Momiyama, Y.; Kato, R.; Taniguchi, H.; Ogura, M.; Ayaori, M.; Nakamura, H.; Ohsuzu, F. Associations Between Serum Resistin Levels and Insulin Resistance, Inflammation, and Coronary Artery Disease. J. Am. Coll. Cardiol. 2005, 46, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Hu, C.Z.; Meng, X.; Wang, D.F.; Zhang, J. Expression of TNF-alpha protein in omental and subcutaneous adipose tissue in obesity. Diabetes Res. Clin. Pract. 2008, 79, 214–219. [Google Scholar] [CrossRef]
- Hector, J.; Schwarzloh, B.; Goehring, J.; Strate, T.G.; Hess, U.F.; Deuretzbacher, G.; Hansen-Algenstaedt, N.; Beil, F.-U.; Algenstaedt, P. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm. Metab. Res. 2007, 39, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Zanni, M.V.; Johnsen, S.; Rasheed, S.; Makimura, H.; Lee, H.; Khor, V.K.; Ahima, R.S.; Grinspoon, S.K. TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E146–E150. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Ishimine, H.; Kinoshita, K.; Watanabe-Susaki, K.; Kato, H.; Doi, K.; Kuno, S.; Kurisaki, A.; Yoshimura, K. Characterization of Human Adipose Tissue-Resident Hematopoietic Cell Populations Reveals a Novel Macrophage Subpopulation with CD34 Expression and Mesenchymal Multipotency. Stem Cells Dev. 2013, 22, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Lê, K.A.; Mahurkar, S.; Alderete, T.L.; Hasson, R.E.; Adam, T.C.; Kim, J.S.; Beale, E.; Xie, C.; Greenberg, A.S.; Allayee, H.; et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes 2011, 60, 2802–2809. [Google Scholar] [CrossRef] [PubMed]

| Gene Name and Symbol | Forward Primer, 5′-3′ | Reverse Primer, 5′-3′ |
|---|---|---|
| adiponectin (adpn) | AAAAGGGCTCAGGATGCTACTG | TGGGCAGGATTAAGAGGAACA |
| resistin (retn) | CCTTTTCTTCCTTGTCCCTGA | TGTCCAGCAATTTAAGCCAATG |
| interleukin-1 (il1b) | GACCTGTTCTTTGAAGTTGACG | CTCTTGTTGATGTGCTGCTG |
| tumor necrosis factor-alpha (tnf) | AGACCCTCACACTCAGATCA | TCTTTGAGATCCATGCCGTTG |
| monocyte chemoattractant protein 1 (ccl2) | GCAGAGAGCCAGACGGGAGGA | TGGGGCGTTAACTGCATCTGG |
| interleukin-6 (il6) | TCCTCTCTGCAAGAGACTTCCATCC | AAGCCTCCGACTTGTGAAGTGGT |
| cyclophilin B (ppib) | AGCAAGTTCCATCGTGTCATC | CCGTAGTGCTTCAGCTTGA |
| ubiquitin B (ubb) | CCAGTGGGCAGTGATGG | GCTTACCATGCAACAAAACCT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudreau, A.; Fuller, S.; Ribnicky, D.M.; Richard, A.J.; Stephens, J.M. Groundsel Bush (Baccharis halimifolia) Extract Promotes Adipocyte Differentiation In Vitro and Increases Adiponectin Expression in Mature Adipocytes. Biology 2018, 7, 22. https://doi.org/10.3390/biology7020022
Boudreau A, Fuller S, Ribnicky DM, Richard AJ, Stephens JM. Groundsel Bush (Baccharis halimifolia) Extract Promotes Adipocyte Differentiation In Vitro and Increases Adiponectin Expression in Mature Adipocytes. Biology. 2018; 7(2):22. https://doi.org/10.3390/biology7020022
Chicago/Turabian StyleBoudreau, Anik, Scott Fuller, David M. Ribnicky, Allison J. Richard, and Jacqueline M. Stephens. 2018. "Groundsel Bush (Baccharis halimifolia) Extract Promotes Adipocyte Differentiation In Vitro and Increases Adiponectin Expression in Mature Adipocytes" Biology 7, no. 2: 22. https://doi.org/10.3390/biology7020022




