Characterization of Chlorella sorokiniana, UTEX 1230
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
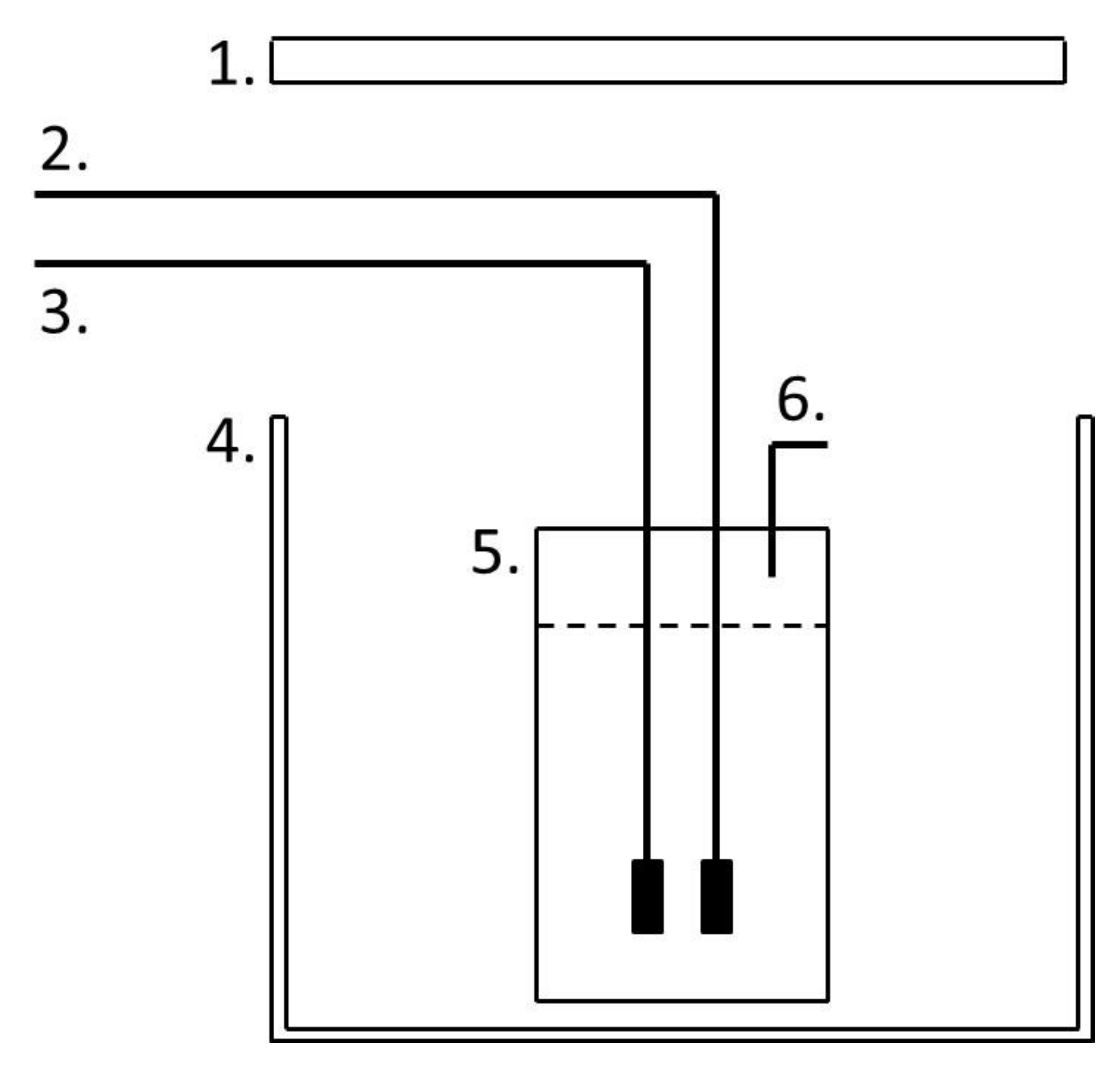
2.1.1. Reactor System
2.1.2. Growth with Different Carbon Sources
2.1.3. Testing the Parameter Space
2.1.4. Nutrient Removal
2.1.5. Fed-Batch, and Concentration Effect Experimentation
2.2. Formulas
2.2.1. Maximal Specific Growth Rate
2.2.2. Final Yield and Productivity
2.2.3. Doubling Time
2.2.4. Substrate Uptake
2.2.5. Photosynthetic Yield on Photosynthetically Active Radiation (PAR)
2.3. Biomass and Lipid Quantification Techniques
2.4. Determining Nutrient and pH Levels
2.5. Data Analysis
3. Results
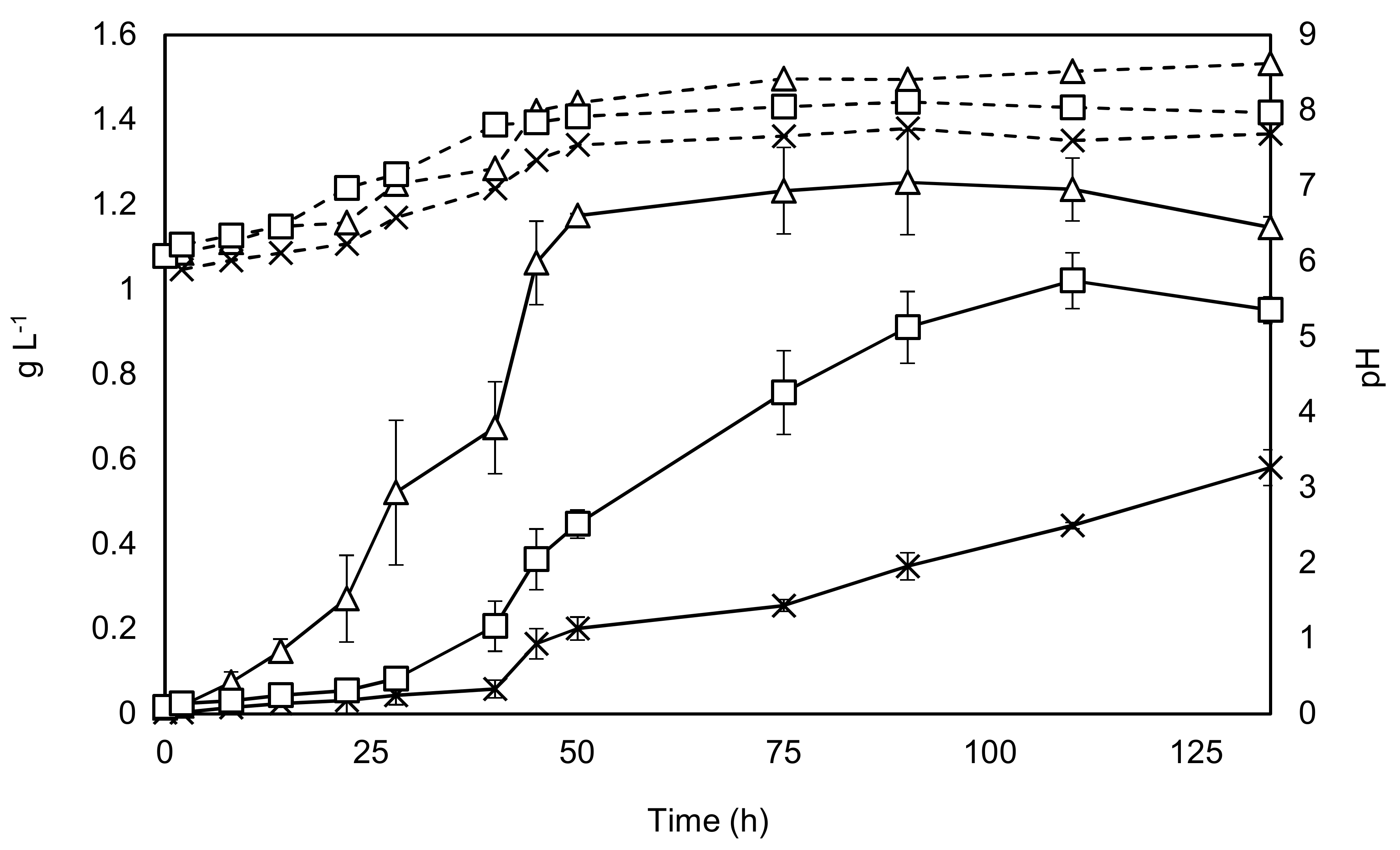
3.1. Growth under Different Carbon Sources
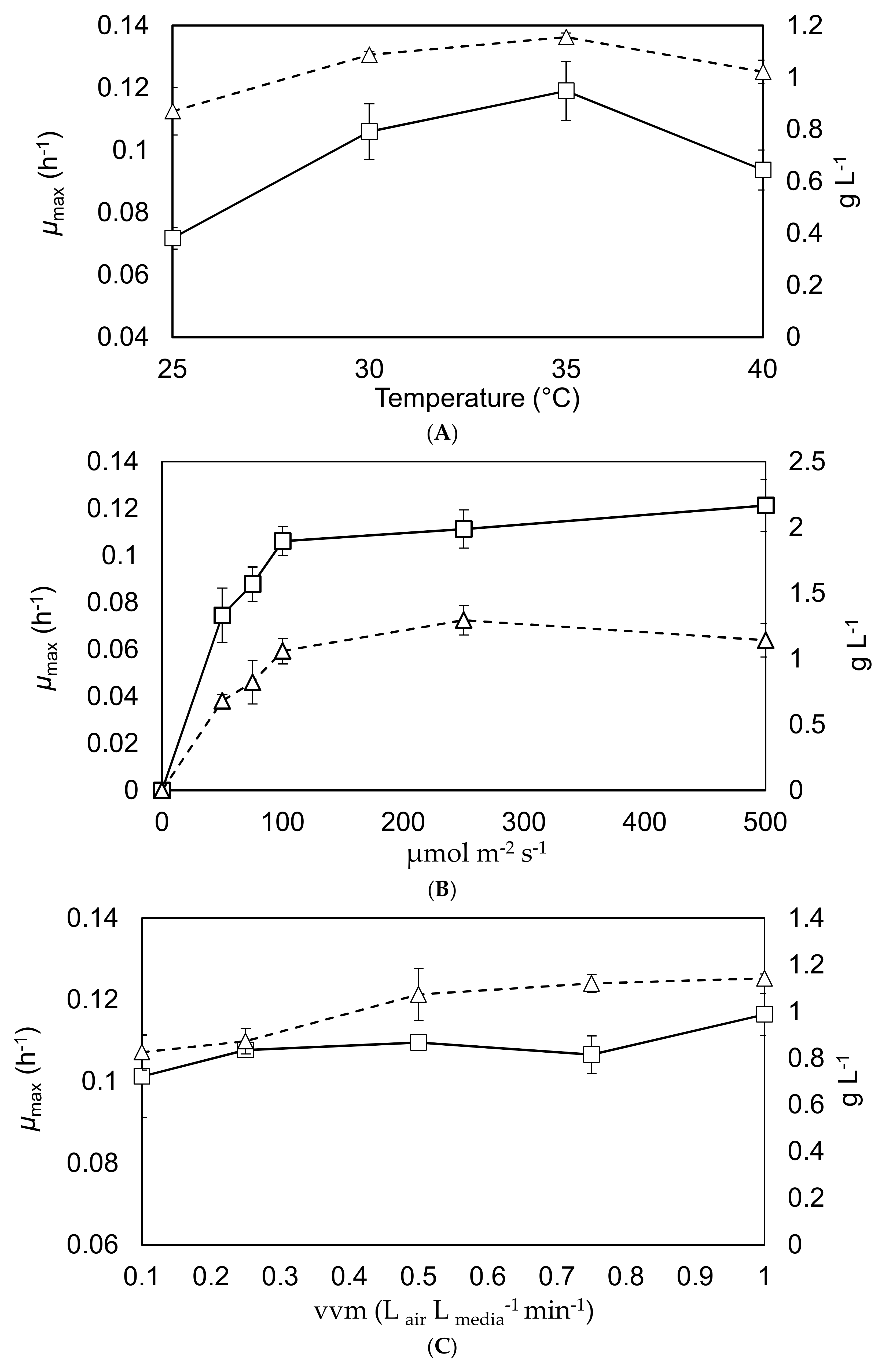
3.2. Testing the Parameter Space
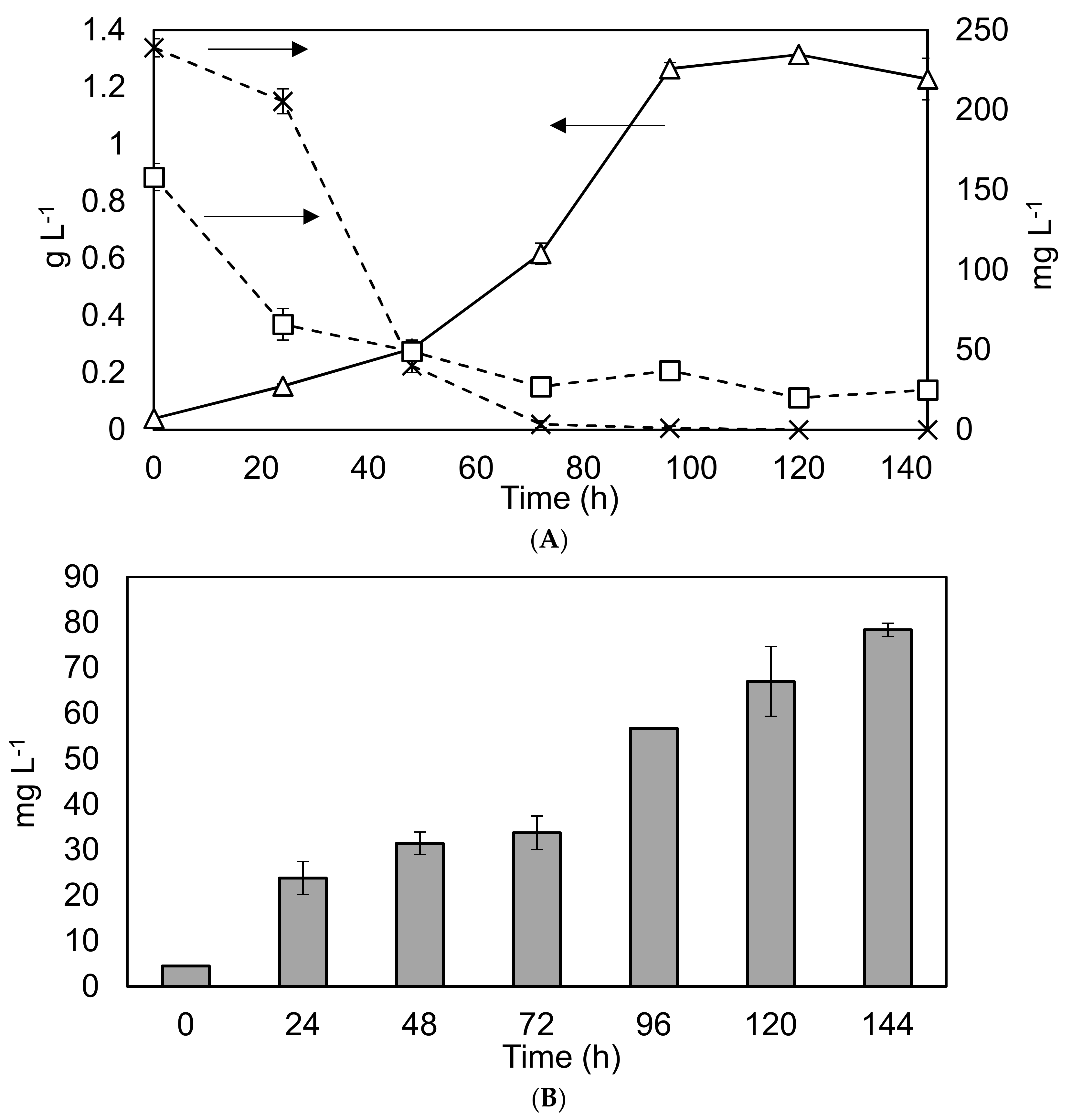
3.3. Nutrient Removal and Lipid Production
3.4. Fed-Batch and Concentration Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Triolein Conversion Graph

References
- Ebenezer, V.; Medlin, L.K.; Ki, J.S. Molecular Detection, Quantification, and Diversity Evaluation of Microalgae. Mar. Biotechnol. 2012, 14, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M. Algal biotechnology products and processes—Matching science and economics. J. Appl. Phycol. 1992, 4, 267–279. [Google Scholar] [CrossRef]
- Wu, H.L.; Hseu, R.S.; Lin, L.P. Identification of Chlorella spp. isolates using ribosomal DNA sequences. Bot. Bull. Acad. Sin. 2001, 42, 115–121. [Google Scholar]
- Furnas, M.J. Insitu growth-rates of marine-phytoplankton—Approaches to measurement, community and species growth-rates. J. Plankton Res. 1990, 12, 1117–1151. [Google Scholar] [CrossRef]
- Sorokin, C.; Myers, J. A high-temperature strain of Chlorella. Science 1953, 117, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.F. Response of the alga Chlorella sorokiniana to 60 Co gamma radiation. Nature 1972, 236, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E. Upper limits of temperature for growth in Chlorella (Chlorophyceae). Plant Syst. Evolut. 1985, 151, 67–71. [Google Scholar] [CrossRef]
- Dorr, R.; Huss, V.A.R. Characterization of nuclear-DNA in 12 species of Chlorella (Chlorococcales, Chlorophyta) by DNA reassociation. Biosystems 1990, 24, 145–155. [Google Scholar] [CrossRef]
- Kessler, E.; Huss, V.A.R. Comparative physiology and biochemistry and taxonomic assignment of the Chlorella (Chlorophyceae) strains of the culture collection of the university of texas at ausin. J. Phycol. 1992, 28, 550–553. [Google Scholar] [CrossRef]
- Ramanna, L.; Guldhe, A.; Rawat, I.; Bux, F. The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour. Technol. 2014, 168, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Trejo, A.; Huss, V.A.R.; Hernandez, J.P.; Bashan, Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour. Technol. 2008, 99, 4980–4989. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Kuijpers, T.C.; Veldhoen, B.; Ternbach, M.B.; Tramper, J.; Mur, L.R.; Wijffels, R.H. Specific growth rate of Chlamydomonas reinhardtii and Chlorella sorokiniana under medium duration light/dark cycles: 13–87 s. J. Biotechnol. 1999, 70, 323–333. [Google Scholar] [CrossRef]
- Wan, M.X.; Wang, R.M.; Xia, J.L.; Rosenberg, J.N.; Nie, Z.Y.; Kobayashi, N.; Oyler, G.A.; Betenbaugh, M.J. Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol. Bioeng. 2012, 109, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Ding, S.Y.; Hoe, C.H.; Low, C.S. Mixotrophic growth of Chlorella sorokiniana in outdoor enclosed photobioreactor. J. Appl. Phycol. 1996, 8, 163–169. [Google Scholar] [CrossRef]
- Lane, C.D.; Coury, D.A.; Allnutt, F.C.T. Composition and Potential Products from Auxenochlorella protothecoides, Chlorella sorokiniana and Chlorella vulgaris. Ind. Biotechnol. 2017, 13, 270–276. [Google Scholar] [CrossRef]
- Béchet, Q.; Muñoz, R.; Shilton, A.; Guieysse, B. Outdoor cultivation of temperature-tolerant Chlorella sorokiniana in a column photobioreactor under low power-input. Biotechnol. Bioeng. 2012, 110, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lizzul, A.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Liu, K.; Nasr, L.K.; Byers, N.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Bouwer, E.J. Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl. Microbiol. Biotechnol. 2015, 99, 6139–6154. [Google Scholar] [CrossRef] [PubMed]
- Belkoura, M.; Benider, A.; Dauta, A. Effects of temperature, light intensity and growth phase on the biochemical composition of Chlorella sorokiniana Shihira & Krauss. Ann. Limnol. Int. J. Limnol. 1997, 33, 3–11. [Google Scholar]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dasgupta, C.N.; Nayak, B.; Lindblad, P.; Das, D. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour. Technol. 2011, 102, 4945–4953. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Sato, A.; Watanabe, K.; Hirata, A.; Takeshita, T.; Ota, S.; Sato, N.; Zachleder, V.; Tsuzuki, M.; Kawano, S. Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour. Technol. 2013, 129, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, R.; Hotta, M.; Masuda, Y.; Chihara, M.; Karube, I. Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J. Appl. Phycol. 2000, 12, 263–267. [Google Scholar] [CrossRef]
- Dawson, H.N.; Burlingame, R.; Cannons, A.C. Stable Transformation of Chlorella: Rescue of Nitrate Reductase-Deficient Mutants with the Nitrate Reductase Gene. Curr. Microbiol. 1997, 35, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Bioprocess Engineering Principles; Elsevier Academic Press: Cambridge, UK, 1995; p. 439. [Google Scholar]
- Franco, M.C.; Buffing, M.F.; Janssen, M.; Lobato, C.V.; Wijffels, R.H. Performance of Chlorella sorokiniana under simulated extreme winter conditions. J. Appl. Phycol. 2012, 24, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, K.E.; Guckert, J.B.; Williams, S.A.; Callis, P.R. Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J. Microbiol. Methods 1987, 6, 333–345. [Google Scholar] [CrossRef]
- Wan, M.; Liu, P.; Xia, J.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.; Qiu, G. The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Vonlanthen, S. Analysis and Manipulation of Storage Lipids in Microalgae. Ph.D. Thesis, University College, London, UK, 2013. [Google Scholar]
- Sorokin, C.; Krauss, R.W. The Effects of Light Intensity on the Growth Rates of Green Algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, C.; Krauss, R.W. Maximum growth rates of chlorella in steady-state and in synchronized cultures. Proc. Natl. Acad. Sci. USA 1959, 45, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.G.A.; Sevilla, J.M.F.; Pérez, J.A.S.; Grima, E.M.; Chisti, Y. Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: Assessment of design and performance. Chem. Eng. Sci. 2001, 56, 2721–2732. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Kobayashi, N.; Barnes, A.; Noel, E.A.; Betenbaugh, M.J.; Oyler, G.A. Comparative Analyses of Three Chlorella Species in Response to Light and Sugar Reveal Distinctive Lipid Accumulation Patterns in the Microalga C. sorokiniana. PLoS ONE 2014, 9, e92460. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, D.J.; Hipkins, M.F.; Boney, A.D. The effect of osmotic and ionic stress on the primary processes of photosynthesis in Dunaliella tertiolecta. J. Exp. Bot. 1984, 35, 18–27. [Google Scholar] [CrossRef]

| Carbon Source | (h−1) | (g·L−1) | (g·L−1·d−1) | (h−1) |
|---|---|---|---|---|
| Sium acetate | 0.21 * | 1.25 * | 0.6 * | 3.3 * |
| + Carbon dioxide | 0.102 | 1.01 ^ | 0.22 ^ | 6.8 |
| − Carbon dioxide | 0.107 | 0.58 | 0.1 | 6.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. https://doi.org/10.3390/biology7020025
Lizzul AM, Lekuona-Amundarain A, Purton S, Campos LC. Characterization of Chlorella sorokiniana, UTEX 1230. Biology. 2018; 7(2):25. https://doi.org/10.3390/biology7020025
Chicago/Turabian StyleLizzul, Alessandro Marco, Aitor Lekuona-Amundarain, Saul Purton, and Luiza Cintra Campos. 2018. "Characterization of Chlorella sorokiniana, UTEX 1230" Biology 7, no. 2: 25. https://doi.org/10.3390/biology7020025






