Electrochemical Characterisation of Bio-Bottle-Voltaic (BBV) Systems Operated with Algae and Built with Recycled Materials
Abstract
:1. Introduction
2. Materials and Methods
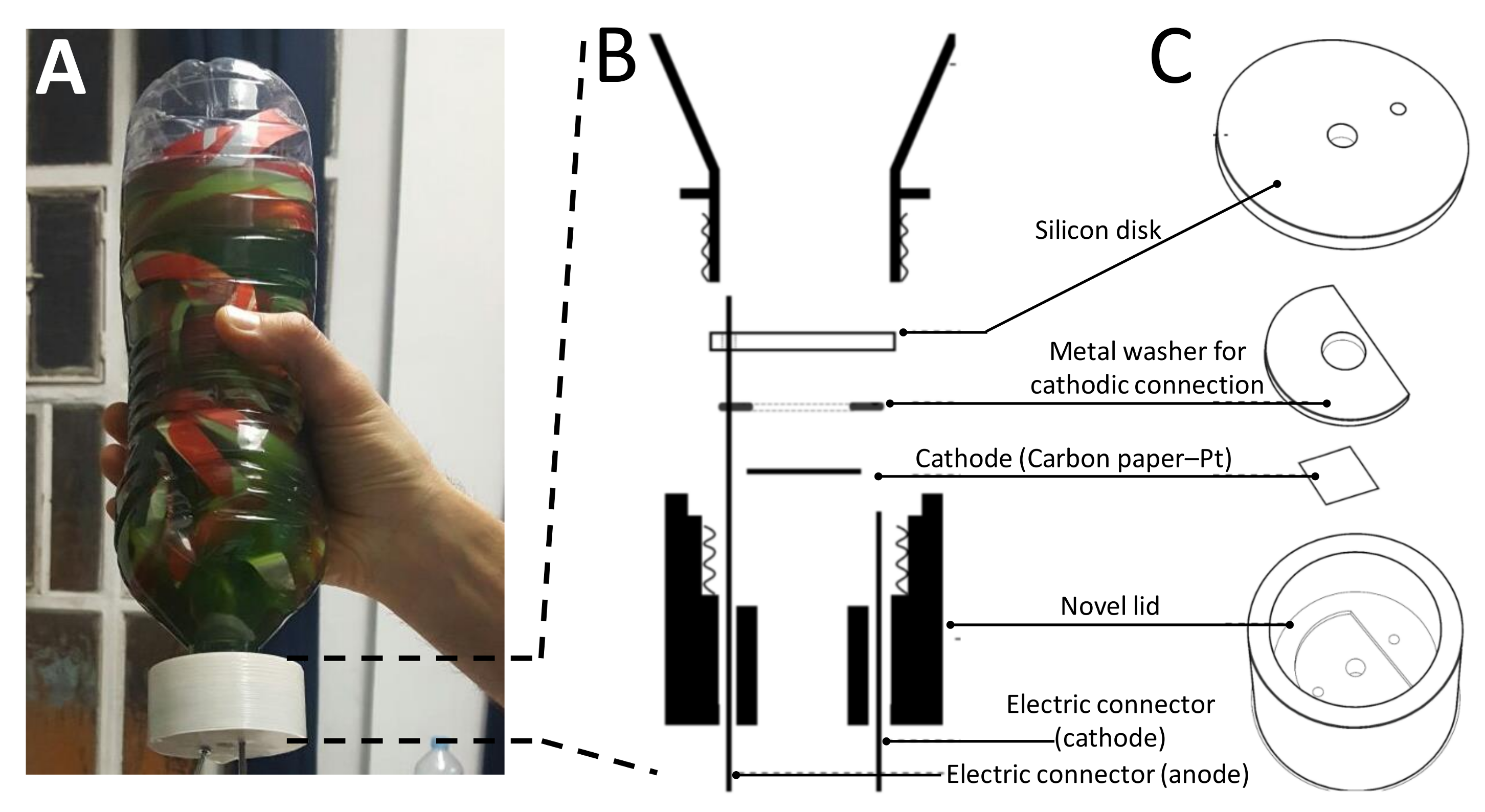
2.1. Building the BBV System
2.2. Culture Conditions and Biofilm Growth
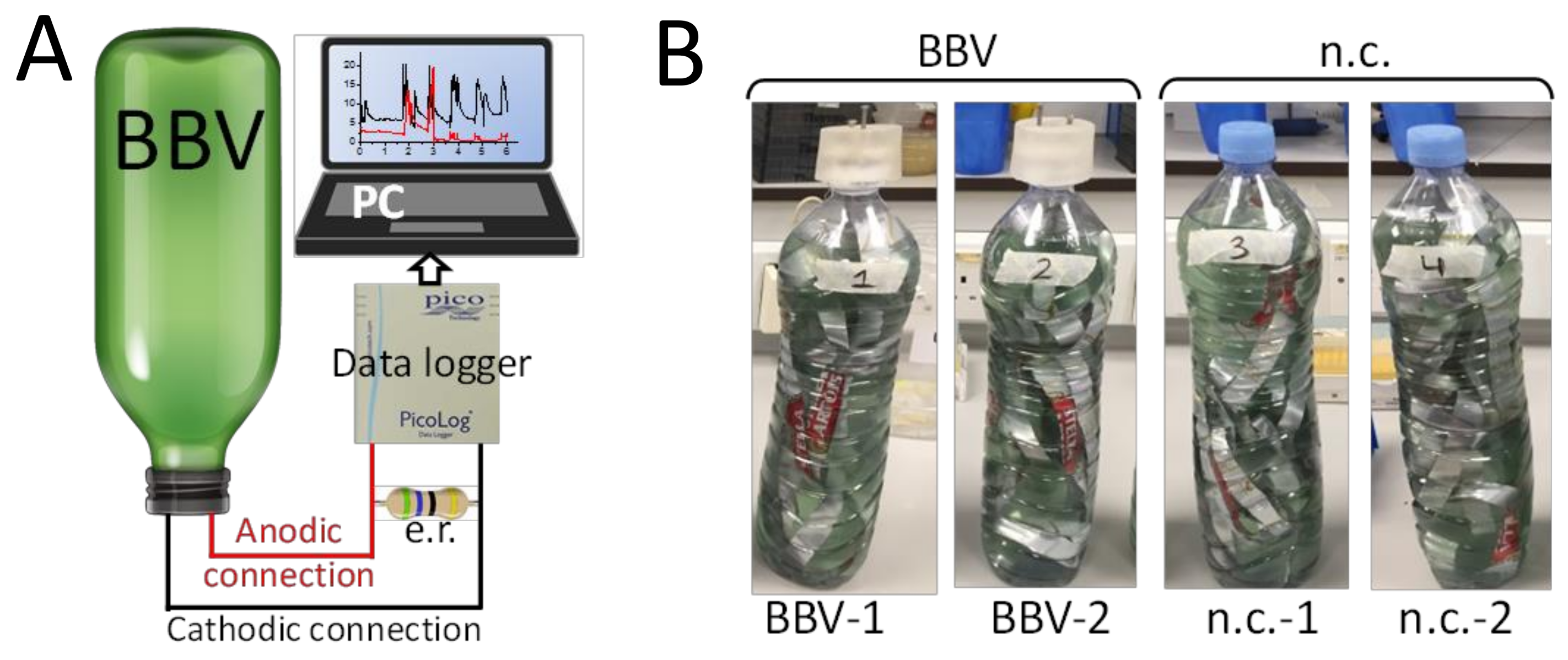
2.3. BPV Operation and Measurements
2.4. Characterization of Algal Biofilm on the Anodic Surface and Algal Chlorophyll Content
2.5. Statistical Analysis
3. Results
3.1. The Electrochemical Setup Used to Run the BBV Systems
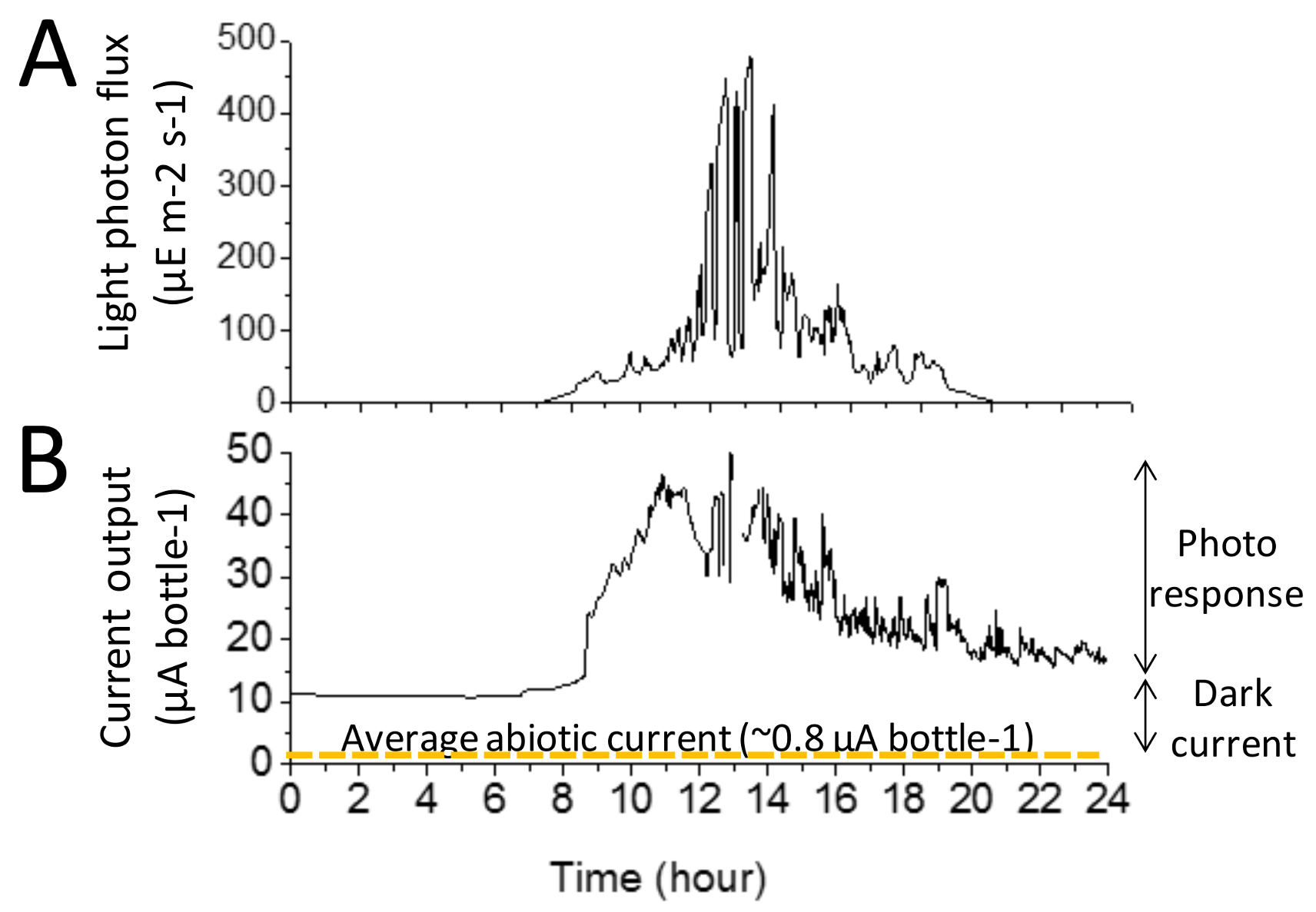
3.2. Illustrative Electrical Output of the BBV Systems
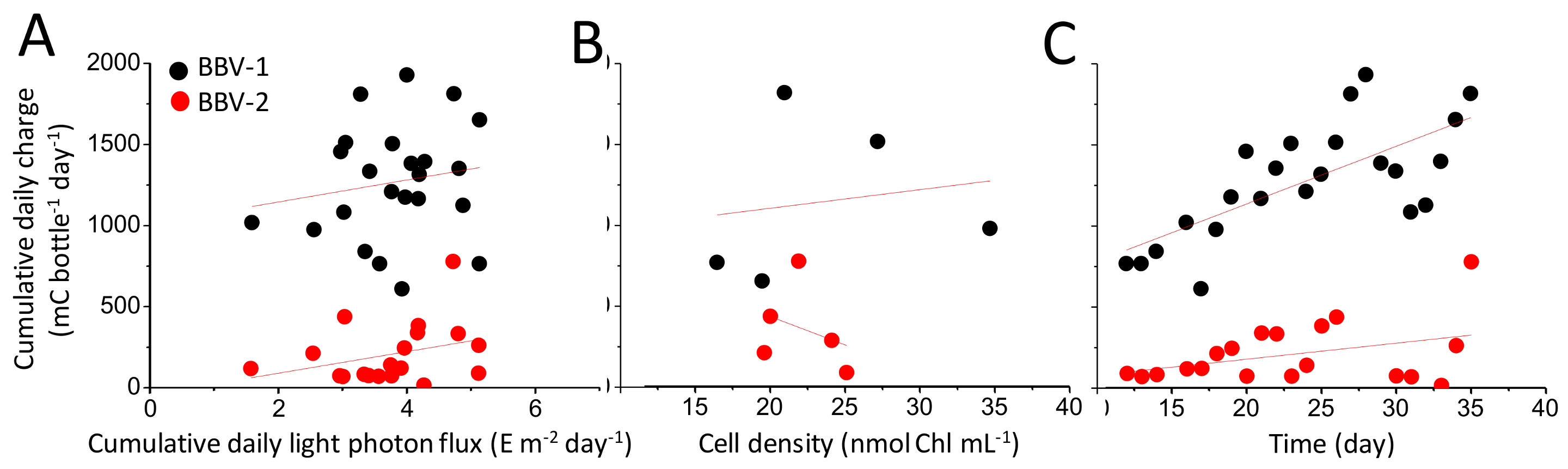
3.3. Characterisation of the Electrical Output
3.4. Biomass Accumulation in the BBV Systems
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- EIA. International Energy Outlook 2013; EIA: Washington, DC, USA, 2013.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: A Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Call, T.P.; Carey, T.; Bombelli, P.; Lea-Smith, D.J.; Hooper, P.; Howe, C.J.; Torrisi, F. Platinum-Free, Graphene Based Anodes and Air Cathodes for Single Chamber Microbial Fuel Cells. J. Mater. Chem. A. 2017, 5, 23872–23886. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.J.; Bombelli, P.; Bradley, R.W.; Thorne, R.; Wenzel, T.; Howe, C.J. Biophotovoltaics: Oxygenic Photosynthetic Organisms in the World of Bioelectrochemical Systems. Environ. Sci. Technol. 2015, 8, 1092–1109. [Google Scholar] [CrossRef]
- Guiry, M.D. How Many Species of Algae are There? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Forti, G.; Furia, A.; Bombelli, P.; Finazzi, G. In Vivo Changes of the Oxidation-Reduction State of NADP and of the ATP/ADP Cellular Ratio Linked to the Photosynthetic Activity in Chlamydomonas reinhardtii. Plant Physiol. 2003, 132, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Lea-Smith, D.J.; Bombelli, P.; Vasudevan, R.; Howe, C.J. Photosynthetic, Respiratory and Extracellular Electron Transport Pathways in Cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, P.; Müller, T.; Herling, T.W.; Howe, C.J.; Knowles, T.P. A High Power-Density, Mediator-Free, Microfluidic Biophotovoltaic Device for Cyanobacterial Cells. Adv. Energy Mater. 2014, 5, 1401299. [Google Scholar] [CrossRef] [PubMed]
- Saar, K.L.; Bombelli, P.; Lea-Smith, D.J.; Call, T.; Aro, E.M.; Müller, T.; Howe, C.J.; Knowles, T.P. Enhancing power-density biological photovoltaics by decoupling storage and power delivery. Nat. Energy 2018, 3, 75–81. [Google Scholar] [CrossRef]
- McCormick, A.J.; Bombelli, P.; Lea-Smith, D.J.; Bradley, R.W.; Scott, A.M.; Fisher, A.C.; Smith, A.G.; Howe, C.J. Hydrogen Production through Oxygenic Photosynthesis Using the Cyanobacterium Synechocystis sp. PCC 6803 in a Bio-Photoelectrolysis Cell (BPE) System. Energy Environ. Sci. 2013, 6, 2682–2690. [Google Scholar] [CrossRef]
- Bombelli, P.; Zarrouati, M.; Thorne, R.J.; Schneider, K.; Rowden, S.J.; Ali, A.; Yunus, K.; Cameron, P.J.; Fisher, A.C.; Wilson, D.I.; et al. Surface Morphology and Surface Energy of Anode Materials Influence Power Outputs in a Multi-Channel Mediatorless Bio-Photovoltaic (BPV) System. Phys. Chem. Chem. Phys. 2012, 14, 12221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Bombelli, P.; Sokol, K.P.; Fantuzzi, A.; Rutherford, A.W.; Howe, C.J.; Reisner, E. Photoelectrochemistry of Photosystem II in Vitro vs in Vivo. J. Am. Chem. Soc. 2017, 140, 6–9. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.J.; Bombelli, P.; Scott, A.M.; Philips, A.J.; Smith, A.G.; Fisher, A.C.; Howe, C.J. Photosynthetic Biofilms in Pure Culture Harness Solar Energy in a Mediatorless Bio-Photovoltaic Cell (BPV) System. Energy Environ. Sci. 2011, 4, 4699. [Google Scholar] [CrossRef]
- Laohavisit, A.; Anderson, A.; Bombelli, P.; Jacobs, M.; Howe, C.J.; Davies, J.M.; Smith, A.G. Enhancing Plasma Membrane NADPH Oxidase Activity Increases Current Output by Diatoms in Biophotovoltaic Devices. Algal Res. 2015, 12, 91–98. [Google Scholar] [CrossRef]
- Anderson, A.; Laohavisit, A.; Blaby, I.K.; Bombelli, P.; Howe, C.J.; Merchant, S.S.; Davies, J.M.; Smith, A.G. Exploiting Algal NADPH Oxidase for Biophotovoltaic Energy. Plant Biotechnol. J. 2015, 14, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, P.; Iyer, D.M.R.; Covshoff, S.; McCormick, A.J.; Yunus, K.; Hibberd, J.M.; Fisher, A.C.; Howe, C.J. Comparison of Power Output by Rice (Oryza sativa) and an Associated Weed (Echinochloa glabrescens) in Vascular Plant Bio-Photovoltaic (VP-BPV) Systems. Appl. Microbiol. Biotechnol. 2012, 97, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, P.; Dennis, R.J.; Felder, F.; Cooper, M.B.; Iyer, D.M.R.; Royles, J.; Harrison, S.T.; Smith, A.G.; Harrison, C.J.; Howe, C.J. Electrical Output of Bryophyte Microbial Fuel Cell Systems Is Sufficient to Power a Radio or an Environmental Sensor. R. Soc. Open Sci. 2016, 3, 160249. [Google Scholar] [CrossRef] [PubMed]
- BIO [BOTTLE] VOLTAIC—IAAC Blog. Available online: www.iaacblog.com/programs/bio-bottle-voltaic-bbv/ (accessed on 2 January 2018).
- World Ocean’s Day (London, UK, 2017). Available online: https://www.zsl.org/blogs/reducing-plastic-pollution-with-plants (accessed on 2 January 2018).
- National Science Week (Canberra, Australia, 2017). Available online: https://www.scienceweek.net.au/ (accessed on 2 January 2018).
- Kropat, J.; Hong-Hermesdorf, A.; Casero, D.; Ent, P.; Castruita, M.; Pellegrini, M.; Merchant, S.S.; Malasarn, D. A Revised Mineral Nutrient Supplement Increases Biomass and Growth Rate in Chlamydomonas reinhardtii. Plant J. 2011, 66, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Soper, Daniel. Calculator: Analysis of Variance (ANOVA)—One-Way ANOVA from Summary Data. Free Analysis of Variance (ANOVA) Calculator—One-Way ANOVA from Summary Data—Free Statistics Calculators. 10 December 2017. Available online: www.danielsoper.com/statcalc3/calc.aspx?id=43 (accessed on 2 January 2018).
- Free Pearson Calculator. Available online: http://www.socscistatistics.com/tests/pearson/Default2.aspx (accessed on 12 March 2018).
- Sawa, M.; Fantuzzi, A.; Bombelli, P.; Howe, C.J.; Hellgardt, K.; Nixon, P.J. Electricity Generation from Digitally Printed Cyanobacteria. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Blanken, W.; Janssen, M.; Cuaresma, M.; Libor, Z.; Bhaiji, T.; Wijffels, R.H. Biofilm Growth of Chlorella sorokiniana a Rotating Biological Contactor Based Photobioreactor. Biotechnol. Bioeng. 2014, 111, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Calignano, F.; Tommasi, T.; Manfredi, D.; Chiolerio, A. Additive Manufacturing of a Microbial Fuel Cell—A Detailed Study. Sci. Rep. 2015, 5, 17373. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C.W. Biofilm Formation and the Food Industry, a Focus on the Bacterial Outer Surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.O.; Rao, K.K. Photosynthesis—Sixth Edition; Cambridge University Press: New York, NY, USA, 1999. [Google Scholar]
- Weather Station (NW3). Available online: http://nw3weather.co.uk/wx8.php (accessed on 2 January 2018).
- Franco, M.C.; Buffing, M.F.; Janssen, M.; Lobato, C.V.; Wijffels, R.H. Performance of Chlorella sorokiniana under Simulated Extreme Winter Conditions. J. Appl. Phycol. 2011, 24, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Sun, L.; Wu, S.; Liu, C.; Zhao, P.; Xie, X.; Wang, G. Utilization of Glucose and Acetate by Chlorella and the Effect of Multiple Factors on Cell Composition. J. Appl. Phycol. 2016, 29, 23–33. [Google Scholar] [CrossRef]
- Wei, X.; Lee, H.; Choi, S. Biopower Generation in a Microfluidic Bio-Solar Panel. Sens. Actuators B Chem. 2016, 228, 151–155. [Google Scholar] [CrossRef]
- Thorne, R.; Hu, H.; Schneider, K.; Bombelli, P.; Fisher, A.; Peter, L.M.; Dent, A.; Cameron, P.J. Porous ceramic anode materials for photo-microbial fuel cells. J. Mater. Chem. 2011, 21, 18055–18060. [Google Scholar] [CrossRef]
- Bracke, W.; Merken, P.; Puers, R.; Van Hoof, C. Ultra-Low-Power Interface Chip for Autonomous Capacitive Sensor Systems. IEEE Trans. Circuits Syst. I 2007, 54, 130–140. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bateson, P.; Fleet, J.E.H.; Riseley, A.S.; Janeva, E.; Marcella, A.S.; Farinea, C.; Kuptsova, M.; Conde Pueyo, N.; Howe, C.J.; Bombelli, P.; et al. Electrochemical Characterisation of Bio-Bottle-Voltaic (BBV) Systems Operated with Algae and Built with Recycled Materials. Biology 2018, 7, 26. https://doi.org/10.3390/biology7020026
Bateson P, Fleet JEH, Riseley AS, Janeva E, Marcella AS, Farinea C, Kuptsova M, Conde Pueyo N, Howe CJ, Bombelli P, et al. Electrochemical Characterisation of Bio-Bottle-Voltaic (BBV) Systems Operated with Algae and Built with Recycled Materials. Biology. 2018; 7(2):26. https://doi.org/10.3390/biology7020026
Chicago/Turabian StyleBateson, Peter, Jack E. H. Fleet, Anthony S. Riseley, Elena Janeva, Anastasia S. Marcella, Chiara Farinea, Maria Kuptsova, Núria Conde Pueyo, Christopher J. Howe, Paolo Bombelli, and et al. 2018. "Electrochemical Characterisation of Bio-Bottle-Voltaic (BBV) Systems Operated with Algae and Built with Recycled Materials" Biology 7, no. 2: 26. https://doi.org/10.3390/biology7020026




