A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessment Framework and Common Inventory Database
2.2. Resource Depletion Impact Assessment Methodologies
3. Results
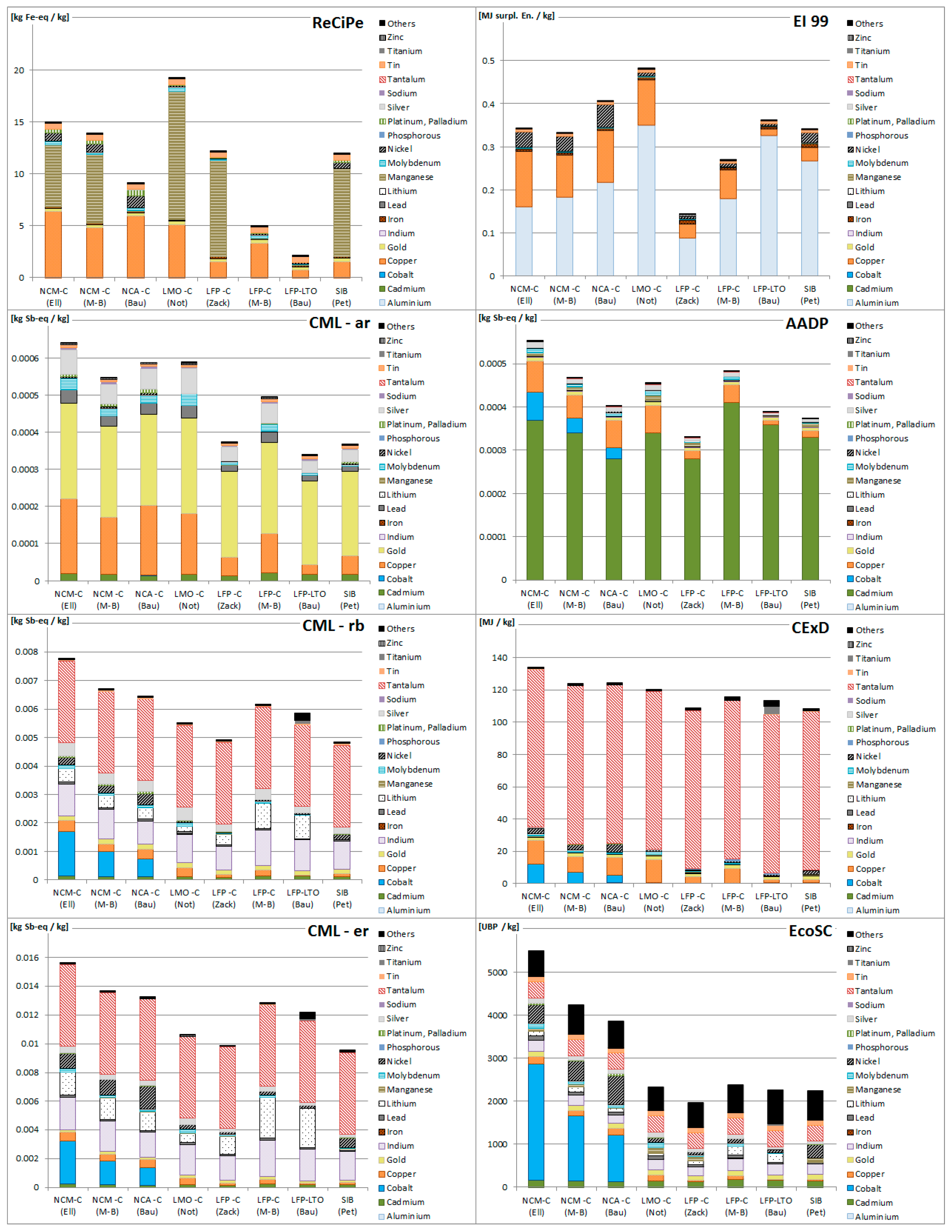
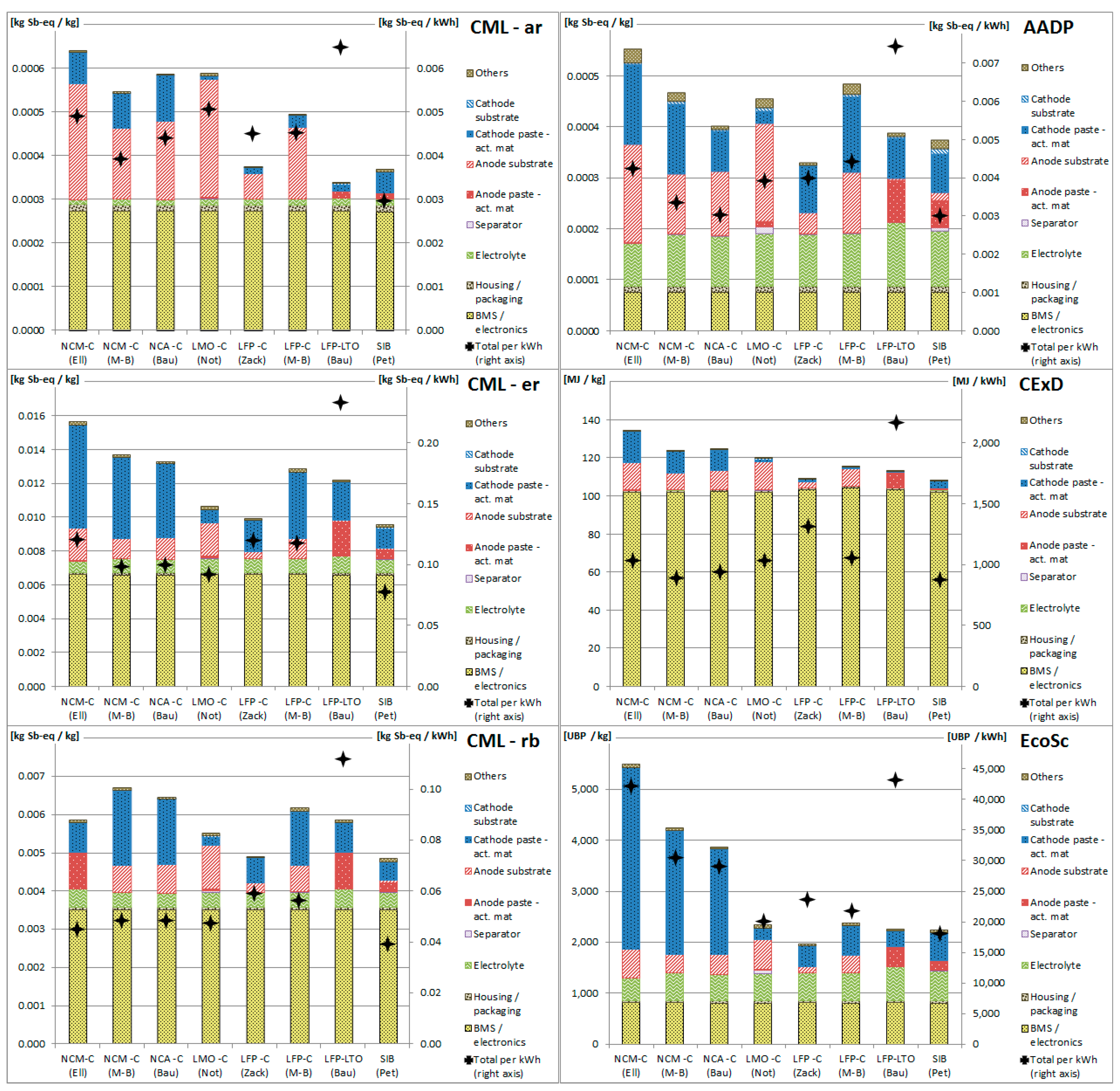
3.1. Resource Depletion Impacts of Battery Production
3.2. Resource Depletion Impacts of Battery Components
3.3. Applicability of Methodologies for Battery Assessment
3.4. Allocation of Resource Depletion to Co-Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AADP | Anthropogenic stock extended Abiotic Depletion Potential (impact assessment methodology) |
| BMS | Battery Management System |
| CExD | Cumulative Exergy Demand (impact assessment methodology) |
| CML | Name of LCIA methodology (derived from ‘Centrum voor Milieukunde, Leiden University’) |
| EcoSc | Ecological Scarcity (impact assessment methodology) |
| EI99 | Eco Indicator 99 (impact assessment methodology) |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LCIA | Life Cycle Impact Assessment |
| ILCD | International Reference Life Cycle Data System |
| LIB | Lithium-Ion Battery |
| RDP | Resource Depletion Potential |
| ReCiPe | Name of LCIA methodology |
| -rb | reserve base (approach for estimating global reserves; CML methodology) |
| -er | economic reserve (approach for estimating global reserves; CML methodology) |
| -ar | absolute reserve (approach for estimating global reserves; CML methodology) |
Battery chemistries
| LFP | Lithium-Iron-Phosphate LiFePO4 (cathode material) |
| LTO | Lithium-Titanate Li4Ti5O12 (anode material) |
| NCM333 | Lithium-Nickel-Cobalt-Manganese-Oxide LiNi0.33Co0.33Mn033O2 (cathode material) |
| NCM424 | Lithium-Nickel-Cobalt-Manganese-Oxide LiNi0.4Co0.2Mn0.4O2 (cathode material) |
| NCA | Lithium-Nickel-Cobalt-Aluminium-Oxide LiNi0.8Co0.15Al0.05O2 (cathode material) |
| NMMT | Sodium-Nickel-Manganese-Magnesium-Titanium-Oxide Na1.1Ni0.3Mn0.5Mg0.05Ti0.05O2 (cathode material) |
| LMO | Lithium-Manganese-Oxide LiMnO2 (cathode material) |
References
- Strategen Consulting LLC. DOE Global Energy Storage Database; Sandia National Laboratories: Livermore, CA, USA, 2016.
- Kihm, A.; Trommer, S. The new car market for electric vehicles and the potential for fuel substitution. Energy Policy 2014, 73, 147–157. [Google Scholar] [CrossRef]
- Simon, B.; Ziemann, S.; Weil, M. Potential metal requirement of active materials in lithium-ion battery cells of electric vehicles and its impact on reserves: Focus on Europe. Resour. Conserv. Recycl. 2015, 104, 300–310. [Google Scholar] [CrossRef]
- Rydh, C.J.; Svärd, B. Impact on global metal flows arising from the use of portable rechargeable batteries. Sci. Total Environ. 2003, 302, 167–184. [Google Scholar] [CrossRef]
- Graedel, T.E. On the Future Availability of the Energy Metals. Annu. Rev. Mater. Res. 2011, 41, 323–335. [Google Scholar] [CrossRef]
- Oliveira, L.; Messagie, M.; Rangaraju, S.; Sanfelix, J.; Hernandez Rivas, M.; Van Mierlo, J. Key issues of lithium-ion batteries—From resource depletion to environmental performance indicators. J. Clean. Prod. 2015, 108, 354–362. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Gaines, L.L.; Nelson, P. Lithium-Ion Batteries: Examining Material Demand and Recycling Issues, Argonne; Argonne National Laboratory: Argonne, IL, USA, 2010.
- Johnson, C.S.; Zhou, D.; Lee, E.; Slater, M.D. Energy Storage Using Sodium-Ion Batteries (SIB); Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2014.
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Ziemann, S.; Weil, M.; Schebek, L. Tracing the fate of lithium––The development of a material flow model. Resour. Conserv. Recycl. 2012, 63, 26–34. [Google Scholar] [CrossRef]
- Weil, M.; Ziemann, S. Recycling of Traction Batteries as a Challenge and Chance for Future Lithium Availability; Lithium-Ion Batter; Elsevier: Amsterdam, The Netherlands, 2014; pp. 509–528. [Google Scholar]
- ISO. ISO 14040—Environmental Management—Life Cycle Assessment—Principles and Framework; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. ISO 14044—Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- EC-JRC. ILCD Handbook: Recommendations for Life Cycle Impact Assessment in the European Context; EC-JRC—Institute for Environment and Sustainability: European Commission—Joint Research Centre, Institute for Environment and Sustainability: Ispra, Italy, 2011. [Google Scholar]
- Brentrup, F.; Küsters, J.; Lammel, J.; Kuhlmann, H. Impact assessment of abiotic resource consumption conceptual considerations. Int. J. Life Cycle Assess. 2002, 7, 301–307. [Google Scholar] [CrossRef]
- Van Oers, L.; Guinée, J. The Abiotic Depletion Potential: Background, Updates, and Future. Resources 2016, 5, 16. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.J.; Zimmermann, B.; Braun, J.; Weil, M. The environmental impact of Li-Ion batteries and the role of key parameters—A review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Peters, J.F.; Simon, B.; Rodriguez-Garcia, G.; Weil, M. Building a Common Base for LCA Benchmarking of Li-Ion Batteries. Available online: https://www.researchgate.net/profile/Jens_Peters3/publication/304659477_Building_a_common_base_for_LCA_benchmarking_of_Li-Ion_batteries/links/5776570e08aead7ba071c426.pdf?origin=publication_detail (accessed on 10 December 2016).
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Majeau-Bettez, G.; Hawkins, T.R.; Strømman, A.H. Life Cycle Environmental Assessment of Lithium-Ion and Nickel Metal Hydride Batteries for Plug-In Hybrid and Battery Electric Vehicles. Environ. Sci. Technol. 2011, 45, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C. Ökobilanz von Lithium-Ionen Batterien; Paul Scherrer Institut, Labor für Energiesystem-Analysen (LEA): Villigen, Switzerland, 2010. [Google Scholar]
- Notter, D.A.; Gauch, M.; Widmer, R.; Wäger, P.; Stamp, A.; Zah, R.; Althaus, H.J. Contribution of Li-Ion Batteries to the Environmental Impact of Electric Vehicles. Environ. Sci. Technol. 2010, 44, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, L.A.-W.; Majeau-Bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life Cycle Assessment of a Lithium-Ion Battery Vehicle Pack: LCA of a Li-Ion Battery Vehicle Pack. J. Ind. Ecol. 2014, 18, 113–124. [Google Scholar] [CrossRef]
- Peters, J.; Buchholz, D.; Passerini, S.; Weil, M. Life cycle assessment of sodium-ion batteries. Energy Environ. Sci. 2016, 9, 1744–1751. [Google Scholar] [CrossRef]
- Winter, S.; Emara, Y.; Ciroth, A.; Su, C.; Srocka, M. openLCA 1.4—Comprehensive User Manual 2015. Available online: http://www.openlca.org/index.php/learning/ (access on 10 December 2016).
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1, 2–7. [Google Scholar] [CrossRef]
- Guinee, J.; Gorrée, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; De Koning, A.; Van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; De Haes, H.; et al. Life Cycle Assessment—An Operational Guide to the ISO Standards; Leiden University: Leiden, The Netherlands, 2001. [Google Scholar]
- Goedkop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; Van Zelm, R. ReCiPe 2008. A life Cycle Impact Assessment Method Which Comprises Harmonised Category Indicators at the Midpoint and the Endpoint level. First Edition Report I: Characterisation. Available online: http://www.leidenuniv.nl/cml/ssp/publications/recipe_characterisation.pdf (accessed on 10 December 2016).
- Goedkoop, M.; Spriensma, R. The Eco-Indicator 99: A Damage Oriented Method for Life Cycle Impact Assessment—Methodology Report; PRé Consultants: Amersfoord, The Netherlands, 2001. [Google Scholar]
- Schneider, L.; Berger, M.; Finkbeiner, M. The anthropogenic stock extended abiotic depletion potential (AADP) as a new parameterisation to model the depletion of abiotic resources. Int. J. Life Cycle Assess. 2011, 16, 929–936. [Google Scholar] [CrossRef]
- Schneider, L.; Berger, M.; Finkbeiner, M. Abiotic resource depletion in LCA—Background and update of the anthropogenic stock extended abiotic depletion potential (AADP) model. Int. J. Life Cycle Assess. 2015, 20, 709–721. [Google Scholar] [CrossRef]
- Bösch, M.E.; Hellweg, S.; Huijbregts, M.A.J.; Frischknecht, R. Applying cumulative exergy demand (CExD) indicators to the ecoinvent database. Int. J. Life Cycle Assess. 2007, 12, 181–190. [Google Scholar] [CrossRef]
- Frischknecht, R.; Büsser Knöpfel, S. Swiss Eco-Factors 2013 according to the Ecological Scarcity Method. Methodological Fundamentals and Their Application in Switzerland; Federal Office for the Environment (FOEN): Bern, Switzerland, 2013. [Google Scholar]
- Tarascon, J.-M.; Masquelier, C.; Croguennec, L.; Patoux, S. A Promising New Prototype of Battery; CNRS Press Releases: Paris, France, 2015. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Althaus, H.-J.; Doka, G.; Heck, T.; Hellweg, S.; Hischier, R.; Nemecek, T.; Rebitzer, G.; Spielmann, M.; Wernet, G. Ecoinvent Report No. 1—Overview and methodology. In Sachbilanzen von Energiesystemen: Grundlagen für den ökologischen Vergleich von Energiesystemen und den Einbezug von Energiesystemen in Ökobilanzen für die Schweiz; Frischknecht, R., Jungbluth, N., Eds.; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
| Battery Component | LFP-C (M-B) [21] | LFP-C (Zack) [20] | LFP-LTO (Bau) [22] | LMO-C (Not) [23] | NCA-C (Bau) [22] | NCM424-C (M-B) [21] | NCM333-C (Ell) [24] | SIB (Pet) [25] | |
|---|---|---|---|---|---|---|---|---|---|
| Energy Density (Wh/kg) | 109.3 | 82.9 | 52.4 | 116.1 | 133.1 | 139.1 | 130.4 | 124.0 | |
| Anode | Share | 20.2% | 18.8% | 25.0% | 31.0% | 26.3% | 22.0% | 29.0% | 33.8% |
| Active Material | Type | Graphite | Graphite | Li4Ti5O12 | Graphite | Graphite | Graphite | Graphite | Hard carbon |
| Share | 9.4% | 13.4% | 20.3% | 15.0% | 14.8% | 11.1% | 12.0% | 28.0% | |
| Binder | Type * | CMC-SBR | CMC-SBR | CMC-SBR | CMC-SBR | CMC-SBR | CMC-SBR | CMC-SBR | CMC-SBR |
| Share | 0.5% | 1.7% | 0.5% | 0.5% | 0.3% | 0.6% | 0.5% | 1.2% | |
| Current Collector | Type | Cu | Cu | Al | Cu | Cu | Cu | Cu | Al |
| Share | 10.3% | 3.7% | 4.2% | 15.5% | 11.2% | 10.3% | 16.5% | 4.7% | |
| Cathode | Share | 35.2% | 39.5% | 25.6% | 25.3% | 27.8% | 33.3% | 31.9% | 25.1% |
| Active material | Type | LiFePO4 | LiFePO4 | LiFePO4 | LiMnO2 | LiNi0.8Co0.15 Al0.05O2 | LiNi0.4Co0.2 Mn0.4O2 | Li(NiCoMn)0.33O2 | Na1.1Ni0.3Mn0.5 Mg0.05Ti0.05O2 |
| Share | 28.3% | 35.7% | 19.4% | 15.6% | 21.9% | 26.6% | 26.7% | 21.9% | |
| Binder | Type * | TFE-PE | TFE-PE | TFE-PE | TFE-PE | TFE-PE | TFE-PE | TFE-PE | TFE-PE |
| Share | 2.5% | 2.2% | 0.8% | 0.2% | 0.4% | 2.3% | 1.7% | 0.9% | |
| Current Collector | Type | Al | Al | Al | Al | Al | Al | Al | Al |
| Share | 4.5% | 1.5% | 5.5% | 9.4% | 5.5% | 4.5% | 3.5% | 2.3% | |
| Electrolyte | Type * | LiPF6/EC | LiPF6/EC | LiPF6/EC | LiPF6/EC | LiPF6/EC | LiPF6/EC | LiPF6/EC | NaPF6/EC |
| Share | 14.9% | 14.7% | 18.0% | 13.8% | 14.0% | 14.9% | 11.9% | 13.6% | |
| Separator | Type | PP/PE | PP/PE | PP/PE | PVF | PP/PE | PP/PE | PP | PP/PE |
| Share | 4.1% | 1.4% | 5.8% | 4.2% | 6.3% | 4.1% | 1.6% | 2.0% | |
| Package | Share * | 20.9% | 20.9% | 20.9% | 20.9% | 20.9% | 20.9% | 20.9% | 20.9% |
| Module | Type * | PP | PP | PP | PP | PP | PP | PP | PP |
| Share * | 18.6% | 18.6% | 18.6% | 18.6% | 18.6% | 18.6% | 18.6% | 18.6% | |
| Cell | Type * | Pouch Al-PET | Pouch Al-PET | Pouch Al-PET | Pouch Al-PET | Pouch Al-PET | Pouch Al-PET | Pouch Al-PET | Pouch Al-PET |
| Share * | 2.3% | 2.3% | 2.3% | 2.3% | 2.3% | 2.3% | 2.3% | 2.3% | |
| BMS | Share * | 4.7% | 4.7% | 4.7% | 4.7% | 4.7% | 4.7% | 4.7% | 4.7% |
| Method. | ReCiPe | EI99 | CML-ar | CML-rb | CML-er | AADP | CExD | EcoSc |
|---|---|---|---|---|---|---|---|---|
| Orig. unit | (kg Fe-eq) | (MJ) | (kg Sb-eq) | (kg Sb-eq) | (kg Sb-eq) | (kg Sb-eq) | (MJ) | (UBP) |
| Al | 0.09 | 46.67 | 0.02 | 15.24 | 5.88 | 0.01 | 2.27 | 3.55 |
| Co | 1.01 | -- | 299.12 | 15,405.80 | 13,459.71 | 37,818.18 | 76.39 | 5263.16 |
| Cu | 42.69 | 719.61 | 26,043.70 | 1506.01 | 1083.86 | 19,672.73 | 35.85 | 131.58 |
| Fe | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Li | -- | -- | 218.6 | 8001.2 | 12,042.9 | 88.0 | 0 | 473.7 |
| Mn | 76.61 | 6.14 | 48.43 | 14.12 | 159.57 | 2752.73 | 1.76 | 103.95 |
| Mb | 207.51 | 803.92 | 338,766.08 | 42,783.94 | 40,104.64 | 224,363.64 | 574.29 | 7631.58 |
| Ni | 12.53 | 465.69 | 1244.95 | 2513.73 | 4638.59 | 1101.82 | 24.04 | 842.11 |
| Sn | 1271.31 | 11,764.71 | 309,449.27 | 69,436.23 | 21,364.01 | 55,272.73 | 250.00 | 30,263.16 |
| Zn | 2.25 | 80.20 | 10,259.19 | 2195.85 | 2214.67 | 3127.27 | 2.69 | 605.26 |
| Coverage * | 20 | 12 | 49 | 49 | 49 | 30 + 34 ** | 31 | 48 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, J.F.; Weil, M. A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries. Resources 2016, 5, 46. https://doi.org/10.3390/resources5040046
Peters JF, Weil M. A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries. Resources. 2016; 5(4):46. https://doi.org/10.3390/resources5040046
Chicago/Turabian StylePeters, Jens F., and Marcel Weil. 2016. "A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries" Resources 5, no. 4: 46. https://doi.org/10.3390/resources5040046






