Contact Allergy to Castor Oil, but Not to Castor Wax
Abstract
:1. Introduction
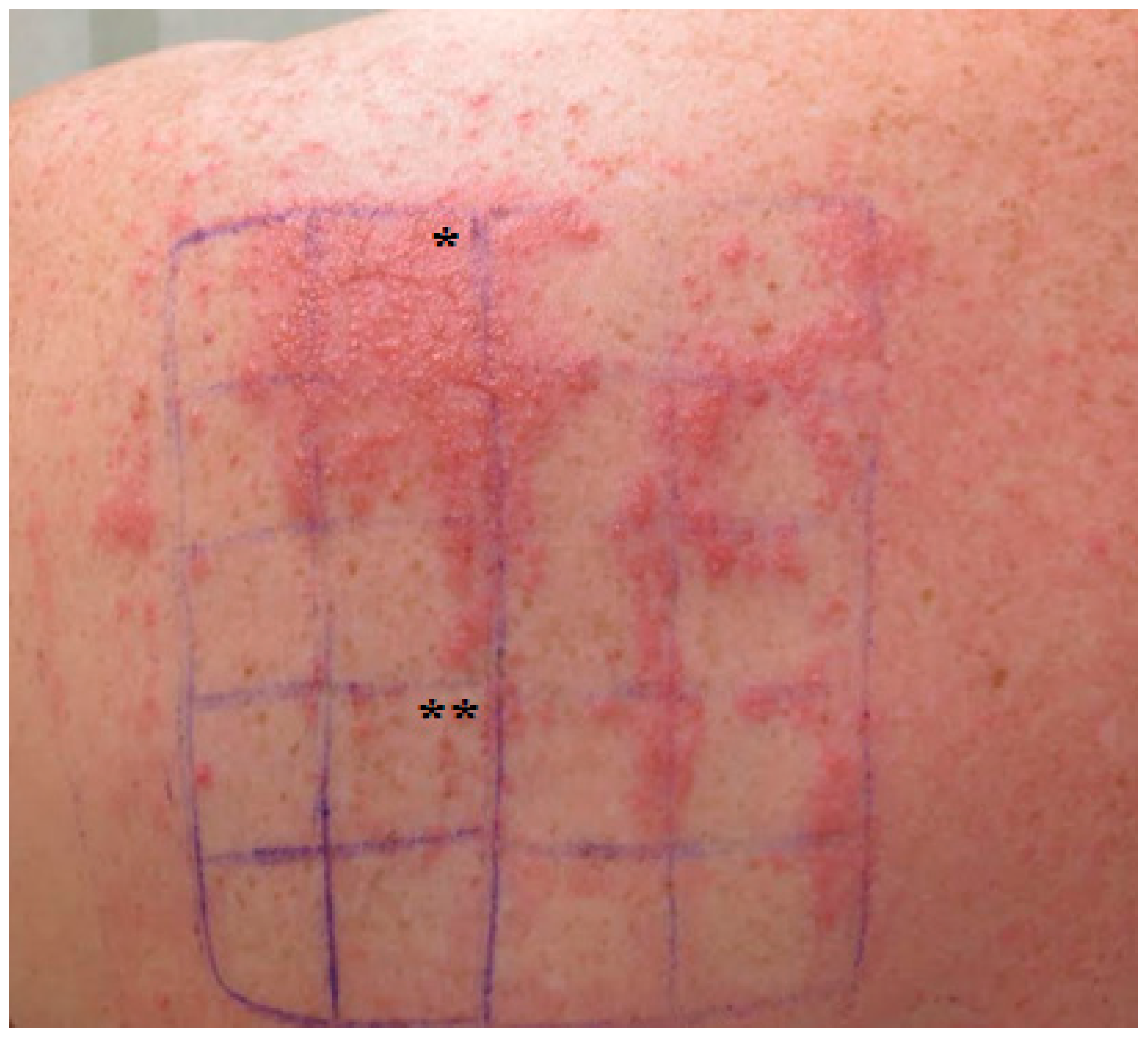
2. Case Reports
2.1. Patient 1
2.2. Patient 2
3. Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Fiero, G.W.; Lockie, L.D. Hydrogenated castor oil in ointments—Part II Cosmetics. J. Am. Pharm. Assoc. 1938, 27, 402–404. [Google Scholar]
- Hydrogenated Castor Oil. Available online: http://www.castoroil.in/castor/castor_seed/castor_oil/hco/hco_hydrogenated_castor_oil_castoroil.html (accessed on 30 December 2016).
- Kulkarni, M.G.; Sawant, S.B. Some physical properties of castor oil esters and hydrogenated castor oil esters. Eur. J. Lipid Sci. Technol. 2003, 105, 214–218. [Google Scholar] [CrossRef]
- Shaw, D.W. Allergic contact dermatitis from 12-hydroxystearic acid and hydrogenated castor oil. Dermatitis 2009, 20, E16–E20. [Google Scholar] [PubMed]
- Sánchez-Guerrero, I.M.; Huertas, A.J.; López, M.P.; Carreño, A.; Ramírez, M.; Pajarón, M. Angioedema-like allergic contact dermatitis to castor oil. Contact Dermat. 2010, 62, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.B.; Noble, A.L.; Roberts, M.E.; Lear, J.T.; English, J.S. Allergic contact dermatitis from oleyl alcohol in lipstick cross-reacting with ricinoleic acid in castor oil and lanolin. Contact Dermat. 1997, 37, 41–42. [Google Scholar] [CrossRef]
- Sasseville, D.; Desjardins, M.; Almutawa, F. Allergic contact dermatitis caused by glycyrrhetinic acid and castor oil. Contact Dermat. 2011, 64, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—Recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.; Moreau, L.; Sasseville, D. Emergent and unusual allergens in Cosmetics. Dermatitis 2010, 21, 127–137. [Google Scholar] [PubMed]
- Fernie, C. News review: Lipid technology 5/2014. Lipid Technol. 2014, 26, 99–102. [Google Scholar] [CrossRef]
- Taghipour, K.; Tatnall, F.; Orton, D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermat. 2008, 58, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Kalavala, M.; Hughes, T.M.; Stone, N.M. Allergic contact dermatitis to polytheylene glycol-7 hydrogenated castor oil. Contact Dermat. 2007, 56, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Di Berardino, L.; Della Torre, F. Side effects to castor oil. Allergy 2003, 58, 826. [Google Scholar] [CrossRef] [PubMed]
- Final report on the safety assessment of Ricinus communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate. Int. J. Toxicol. 2007, 26 (Suppl. 3), 31–77.
- Patch Testing. Test Concentrations and Vehicles for 3700 Chemicals, 2nd ed.; De Groot, A.C. (Ed.) Elsevier: Amsterdam, The Netherlands, 1994.
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verheyden, M.; Rombouts, S.; Lambert, J.; Aerts, O. Contact Allergy to Castor Oil, but Not to Castor Wax. Cosmetics 2017, 4, 5. https://doi.org/10.3390/cosmetics4010005
Verheyden M, Rombouts S, Lambert J, Aerts O. Contact Allergy to Castor Oil, but Not to Castor Wax. Cosmetics. 2017; 4(1):5. https://doi.org/10.3390/cosmetics4010005
Chicago/Turabian StyleVerheyden, Michel, Sven Rombouts, Julien Lambert, and Olivier Aerts. 2017. "Contact Allergy to Castor Oil, but Not to Castor Wax" Cosmetics 4, no. 1: 5. https://doi.org/10.3390/cosmetics4010005




