The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations
Abstract
:1. Introduction
- Do not stay too long in the sun, even while using a sunscreen product.
- Keep babies and young children out of direct sunlight.
- Over-exposure to the sun is a serious health threat.
- Reducing the quantity of sunscreen product applied will significantly lower the level of protection.
- Apply (generously) sunscreen products before sun exposure.
- Reapply frequently to maintain protection, and especially after perspiration, swimming or towelling.
- (1)
- Ensure uniformity of skin application, resistance to water, sweat and lately, the abrasive action of the sand;
- (2)
- Be photo-stable, in order to preserve the UV filters from the decomposition induced by solar irradiation and, consequently, the loss of efficacy during the period of the consumer exposure;
- (3)
- Reach the right synergic combination targeted for normal skin, sensitive skin and babies consumers, able to avoid all skin sensitization or photosensitization;
2. Efficacy and Evaluation of UVB Sunburn Protection Factor (SPF)
3. Evaluation of the Sun Product Water Resistance
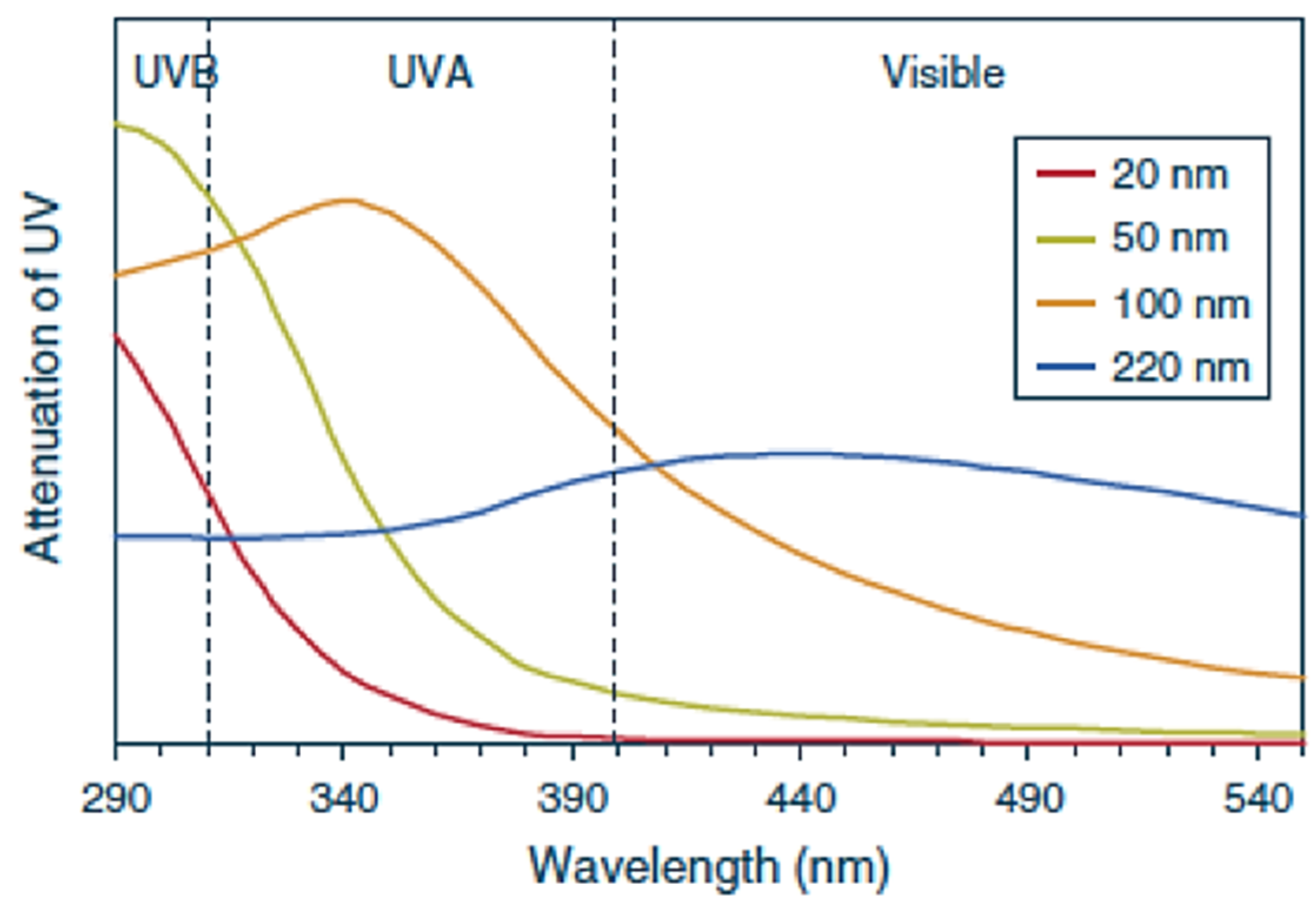
4. In Vitro Determination of UVA Photoprotection
5. Further In Vitro/In-Vivo Methods
6. FDA 2011 Final Rule
- -
- the calibration times of the UV source (each 12 months for the FDA, each 18 months for the ISO);
- -
- the reference sunscreen products (P2 for the FDA and P2, P3 or P7 for the ISO);
- -
- progressions of exposure times used.
- 40 min water resistance followed by the SPF value measured after the immersion
- 80 min water resistance followed by the SPF value measured after the immersion
7. Australia Sunscreen Standard AS/NZS 2604:2012
- LOW protection: 4-6-8-10
- MEDIUM protection: 15-20-25
- HIGH protection: 30-40-50
- VERY HIGH protection: 50+
- SPF from 4 to 7: no water resistance properties
- SPF from 8 to 14: maximum 40-min water resistance properties
- SPF from 15 to 29: maximum 2-h water resistance properties
- SPF > 30: maximum 4-h water resistance properties
8. Formulation Solutions
- (a)
- Diethylamino Hydroxybenzoyl Hexyl Benzoate and Ethylhexyl Triazone in 3:1 ratio [24];
- (b)
- Octocrylene and Butyl Methoxydibenzoylmethane in a ratio of more or less 3:1 [25];
- (c)
- Octocrylene + Butyl Methoxydibenzoylmethane + Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine [26];
- (d)
- Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine (Bemotrizinol) + Diethylhexyl Butamido Triazone + Butyl Methoxydibenzoylmethane (based on our experience, unpublished data).
- (1)
- The right stabilizers:
- Among other filters (Octocrylene, Polysilicone-15, Methylbenzylidene Camphor, Bemotrizinol, etc.);
- among antioxidants (Pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, Diethylhexyl Syringylidenemalonate);
- (2)
- Solubility: octocrylene, ethylhexyl and homomenthyl salicylates, to avoid crystal formation;
- (3)
- Avoiding the incompatibility with metal ions, by using chelating agents like Disodium EDTA
- (4)
- Avoiding formaldehyde donors;
- (5)
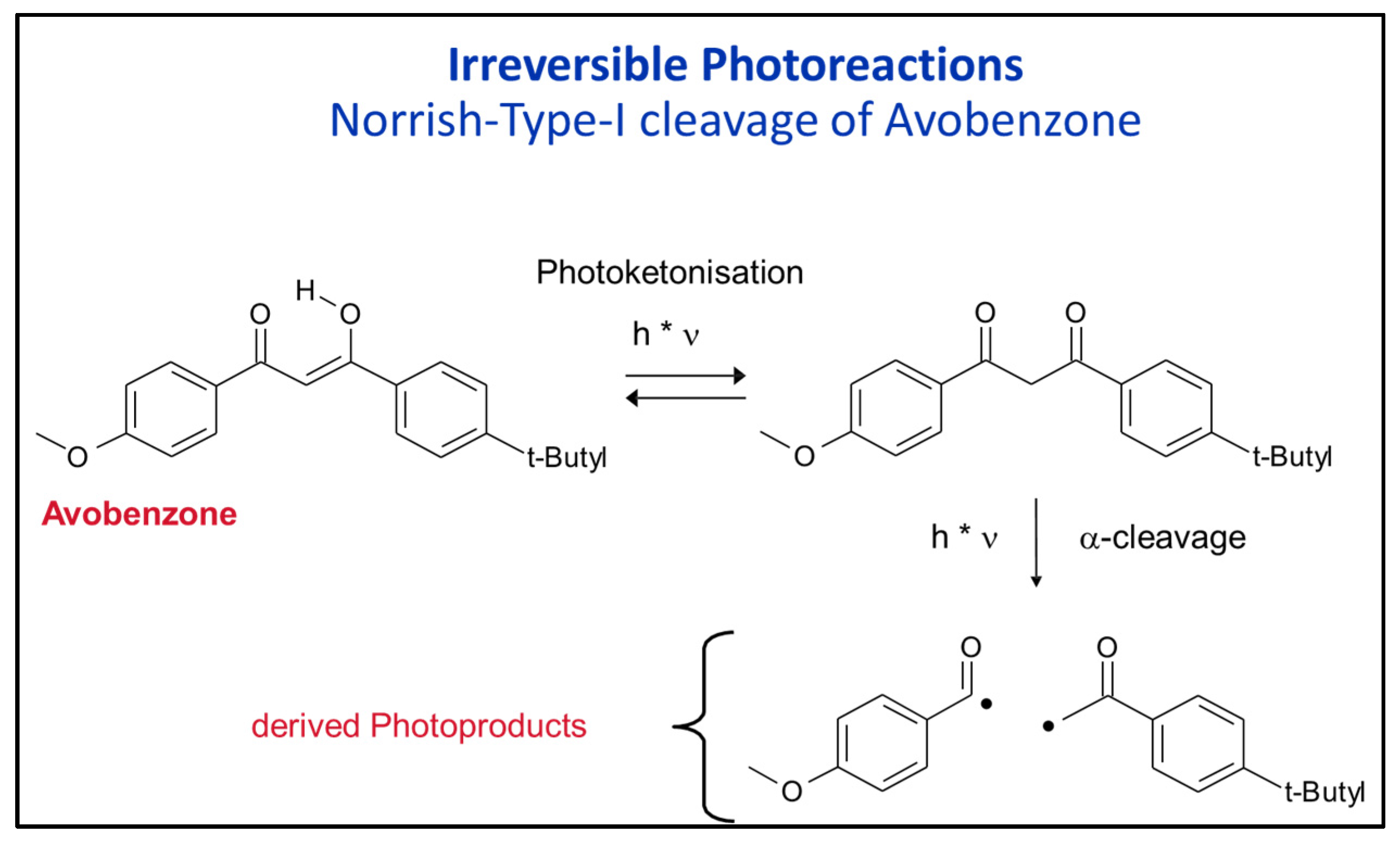
- The combinations of Avobenzone with inorganic UV filters (this is not allowed in the USA) can improve the stability of avobenzone by about 10–15% but the inorganic UV filters must be of the coated type;
- (6)
- Use polar solvents to inhibit Norrish-Type-I cleavage of Avobenzone (Paraffin is bad, Ethanol is good);
- (a)
- The type of final users. The use of Octocrylene, Ethlylhexyl Methoxycinnamate and PABA derivatives is not suggested for consumers with sensitive skin or for baby products [30].
- (b)
- The market: Europe, Japan, USA, etc., similarly to what happens for test methods, also the number of available sunscreen ingredients is different according to the different legislations. In USA (where sunscreen products are sold as OTC and not as cosmetic products) the available number of UV filters is extremely limited in comparison to Europe. Also their maximum allowed percentages are in many cases different (Ethlylhexyl Methoxycinnamate allowed at 10% in Europe, at 20% in Japan);
- (c)
- If a water resistant formula is requested, the use of hydrosoluble UV filters (Phenylbenzimidazol Sulfonic Acid) could be a bad idea;
- -
- Preparing a fully anhydrous formulation with the addition of film forming polymers;
- -
- Creating w/o emulsions;
- -
- Obtaining a meta-stable o/w emulsion with a low level of emulsifiers, adding, also in this case, good film forming polymers;
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Bonfigli, A. Misura del Fattore di Protezione Solare. In Manuale del Cosmetologo, 2nd ed.; Tecniche Nuove: Milano, Italy, 2007; ISBN 978-88-481-2952-7. [Google Scholar]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Australian Cancer Council. Available online: http://www.cancer.org.au/preventing-cancer/sun-protection/ (accessed on 13 April 2017).
- European Commission. Commission Recommendation of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto. Off. J. Eur. Union 2006, 265, 39–43. [Google Scholar]
- World Health Organisation. Available online: http://www.who.int/uv/sun_protection/en/ (accessed on 13 April 2017).
- US Food and Drug Administration. Sunscreen Drug Products for Over-the-Counter HumanUse. Tentative Final Monograph 58. Fed. Regist. 1993, 58, 28194–28302. [Google Scholar]
- Deutsches Institut für Normung (DIN). Experimental Evaluation of Erythema Protection of External Sunscreen Products for the Human Skin; DIN 67501; DIN: Berlin, Germany, 1993. [Google Scholar]
- Standard Association of Australia. Sunscreen Products, Evaluation and Classification; Standards Australia: Sydney, Australia, 1986. [Google Scholar]
- Japan Cosmetic Industry Association (JCIA). Standard SPF Test Method; Japan Cosmetic Industry Association (JCIA): Tokyo, Japan, 1991. [Google Scholar]
- South African Bureau of Standards. Standard Specifications, Sunscreen Products; SABS: Pretoria, South Africa, 1992. [Google Scholar]
- COLIPA. Sun Protection Factor Test Method; COLIPA: Bruxelles, Belgium, 1994. [Google Scholar]
- CTFA South Africa; COLIPA; JCIA. International Sun Protection Factor (SPF) Test Method; COLIPA: Bruxelles, Belgium, 2006. [Google Scholar]
- International Organization for Standardization (ISO). In Vivo Determination of the Sun Protection Factor (SPF); ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). Labelling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed. Regist. 2011, 76, 35620–35665. [Google Scholar]
- COLIPA. Guidelines for Evaluating Sun Product Water Resistance; COLIPA: Bruxelles, Belgium, 2005. [Google Scholar]
- COLIPA. Recommendation N°16; COLIPA: Bruxelles, Belgium, 2006. [Google Scholar]
- COLIPA. Method for the In Vitro Determination of UVA Protection Provided by Sunscreen Products; COLIPA: Bruxelles, Belgium, 2007. [Google Scholar]
- COLIPA. In Vitro Method for the Determination of the UVA Protection Factor and “Critical Wavelength” Values of Sunscreen Products—Guidelines; COLIPA: Bruxelles, Belgium, 2009. [Google Scholar]
- COLIPA. In Vitro Method for the Determination of the UVA Protection Factor and “Critical Wavelength” Values of Sunscreen Products—Guidelines; COLIPA: Bruxelles, Belgium, 2011. [Google Scholar]
- International Organization for Standardization (ISO). Determination of Sunscreen UVA Photoprotection In Vitro; ISO: Geneva, Switzerland, 2012; ISO 24443:2012. [Google Scholar]
- Japan Cosmetic Industry Association Technical Bulletin. Measurement Standards for UVA Protection Efficacy; Japan Cosmetic Industry Association: Tokyo, Japan, 1996. [Google Scholar]
- International Organization for Standardization (ISO). In Vivo Determination of Sunscreen UVA Protection; ISO: Geneva, Switzerland, 2011; ISO:24442:2011. [Google Scholar]
- Australian/New Zealand Standard—AS/NZS. Sunscreen Products—Evaluation and Classification; Standards Australia: Sydney, Australia, 2012. [Google Scholar]
- Floesser-Mueller, H. Three Lines of Defense: A Successful Strategy for Efficient Prevention of Photoageing; Lecture at IFSCC 2007; IFSCC: Amsterdam, the Netherland, 2007. [Google Scholar]
- Niculae, G.; Badea, N.; Meghea, A.; Oprea, O.; Lacatusu, I. Coencapsulation of Butyl-Methoxydibenzoylmethane and Octocrylene into Lipid Nanocarriers: UV Performance, Photostability and in vitro Release. Photochem. Photobiol. 2013, 89, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Gabard, B. Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a New UV Broadband Filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef]
- Cawthray, J.F. UVA Chemical Filters: A Systematic Study. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, February 2008. [Google Scholar]
- Afonso, S.; Horita, K.; e Silva, J.S.; Almeida, I.F.; Amaral, M.H.; Lobao, P.A.; Lobo, J.S. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B: Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Deckner, G. Avobenzone: A Globally Approved UVA Absorber. Available online: https://knowledge.ulprospector.com (accessed on 27 March 2015).
- Pigatto, P.D.; Guzzi, G.; Schena, D.; Guarrera, M.; Foti, C.; Francalanci, S.; Cristaudo, A.; Ayala, F.; Vincenzi, C. Photopatch Tests: An Italian Multicentre Study from 2004 to 2006. Contact Derm. 2008, 59, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Tyner, K.M.; Wokovich, A.M.; Godar, D.E.; Doub, W.H.; Sadrieh, N. The state of nano-sized titanium dioxide [TiO2] may affect sunscreen performance. Int. J. Cosmet. Sci. 2011, 33, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, J.W. Nanotechnology and Skin Delivery: Infinitely Small or Infinite Possibilities? Cosmet. Toilet. 2009, 124, 28. [Google Scholar]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied. Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Zinc Oxide (Nano form); SCCS: Brussels, Belgium, 2012. [Google Scholar]
- Hewitt, J. Chapter 32: Inorganic sunscreens. In The Chemistry and Manufacture of Cosmetics, 3rd ed.; Schlossman, M.L., Ed.; Allured Publishing: Carol Stream, IL, USA, 2002; Volume III, pp. 527–551. ISBN 978-1932633481. [Google Scholar]
- Darvin, M.E.; König, K.; Kellner-Hoefer, M.; Breunig, H.G.; Werncke, W.; Meinke, M.C.; Patzelt, A.; Sterry, W.; Lademann, J. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-Rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacol. Physiol. 2012, 25, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety (SCCS). Opinion on Titanium Dioxide (Nano Form); SCCS: Brussels, Belgium, 2013. [Google Scholar]

| Protection Level | SPF Value |
|---|---|
| Low protection | 6, 10 |
| Medium protection | 15, 20, 25 |
| High protection | 30, 40 |
| Very high protection | 50+ |
| Country | SPF Method | ISSUE DATE |
|---|---|---|
| USA * | FDA | 1978 (‘93) |
| Australia/NZ | AS/NZS | 1983 |
| Germany | DIN | 1984 |
| Japan | JCIA | 1992 |
| South Africa | SABS1557 | 1992 |
| Photo-Type | Fitzpatrick Classification |
|---|---|
| Type 1 | always burns, never tans |
| Type 2 | usually burns, tans with difficulty |
| Type 3 | sometimes burns, sometimes tans |
| Type 4 | burns minimally, always tans |
| Type 5 | rarely burns, tans profusely |
| Type 6 | never burns, deeply tans |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionetti, N.; Rigano, L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics 2017, 4, 15. https://doi.org/10.3390/cosmetics4020015
Lionetti N, Rigano L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics. 2017; 4(2):15. https://doi.org/10.3390/cosmetics4020015
Chicago/Turabian StyleLionetti, Nicola, and Luigi Rigano. 2017. "The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations" Cosmetics 4, no. 2: 15. https://doi.org/10.3390/cosmetics4020015






