Amino Carbonylation of Epidermal Basement Membrane Inhibits Epidermal Cell Function and Is Suppressed by Methylparaben
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Effects of Glyceraldehyde on the Relative Fluorescence Intensity Associated with Amino Carbonylation of Epidermal BM
2.3. Culture of Epidermal Keratinocytes on Amino-Carbonylated BM
2.4. Measurement of Transepidermal Water Loss (TEWL) in the 3D Human Epidermis Model
2.5. Measurement of FLG, TGase1, SPTLC2, βGCase, and aSMase mRNA Levels
2.6. Immunohistochemical Analysis of FLG and TGase1 Expression
2.7. Determination of Effects of Glyceraldehyde on the Relative Fluorescence Intensity Associated with Amino Carbonylation of Collagen
2.8. Measurement of ATP Content
2.9. Measurement of FLG mRNA Levels in Cultured HaCaT
2.10. Data Analysis
3. Results
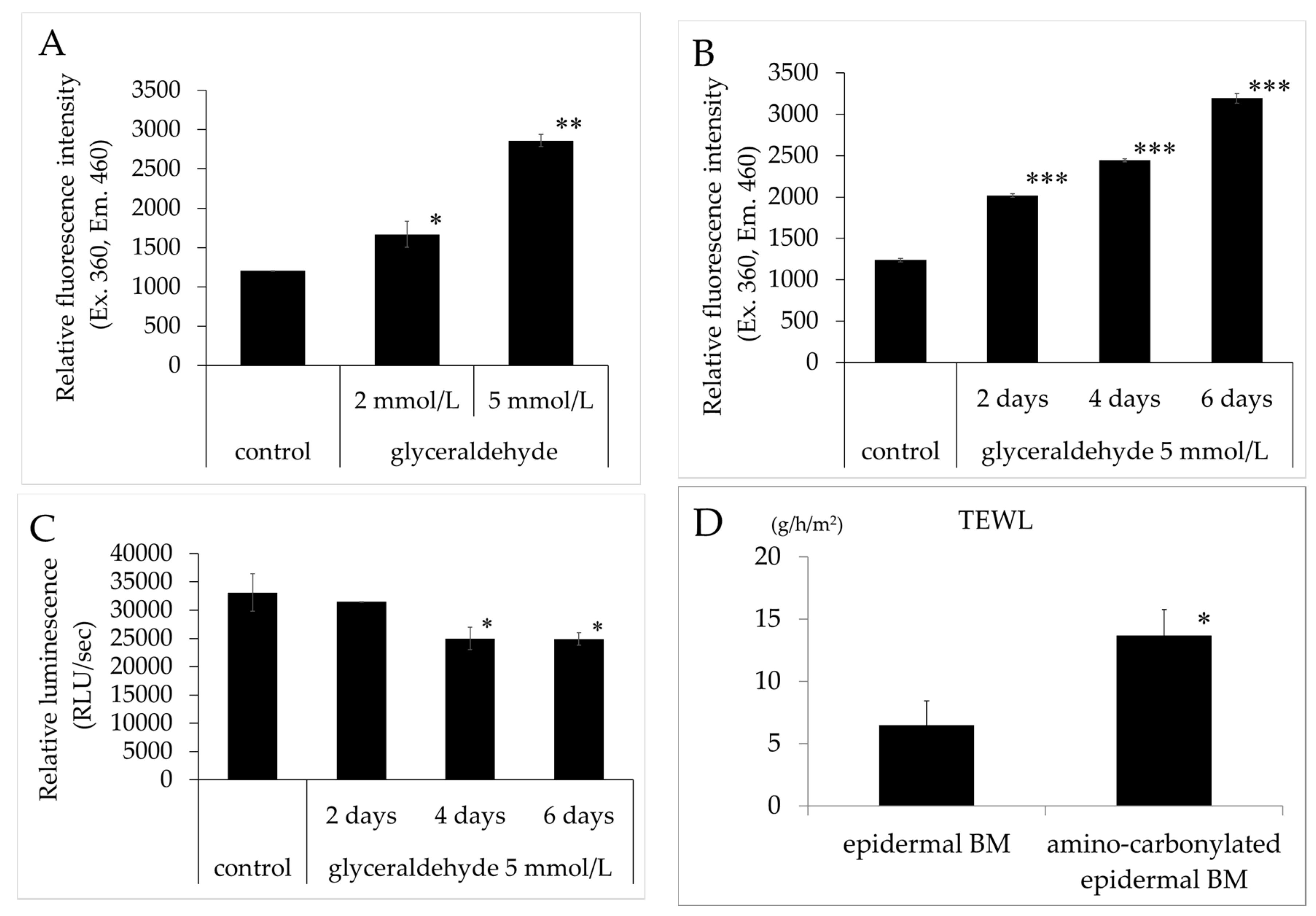
3.1. Effects of Glyceraldehyde on the Relative Fluorescence Intensity of Amino-carbonylated BM and Effect of Amino Carbonylation of the BM on TEWL
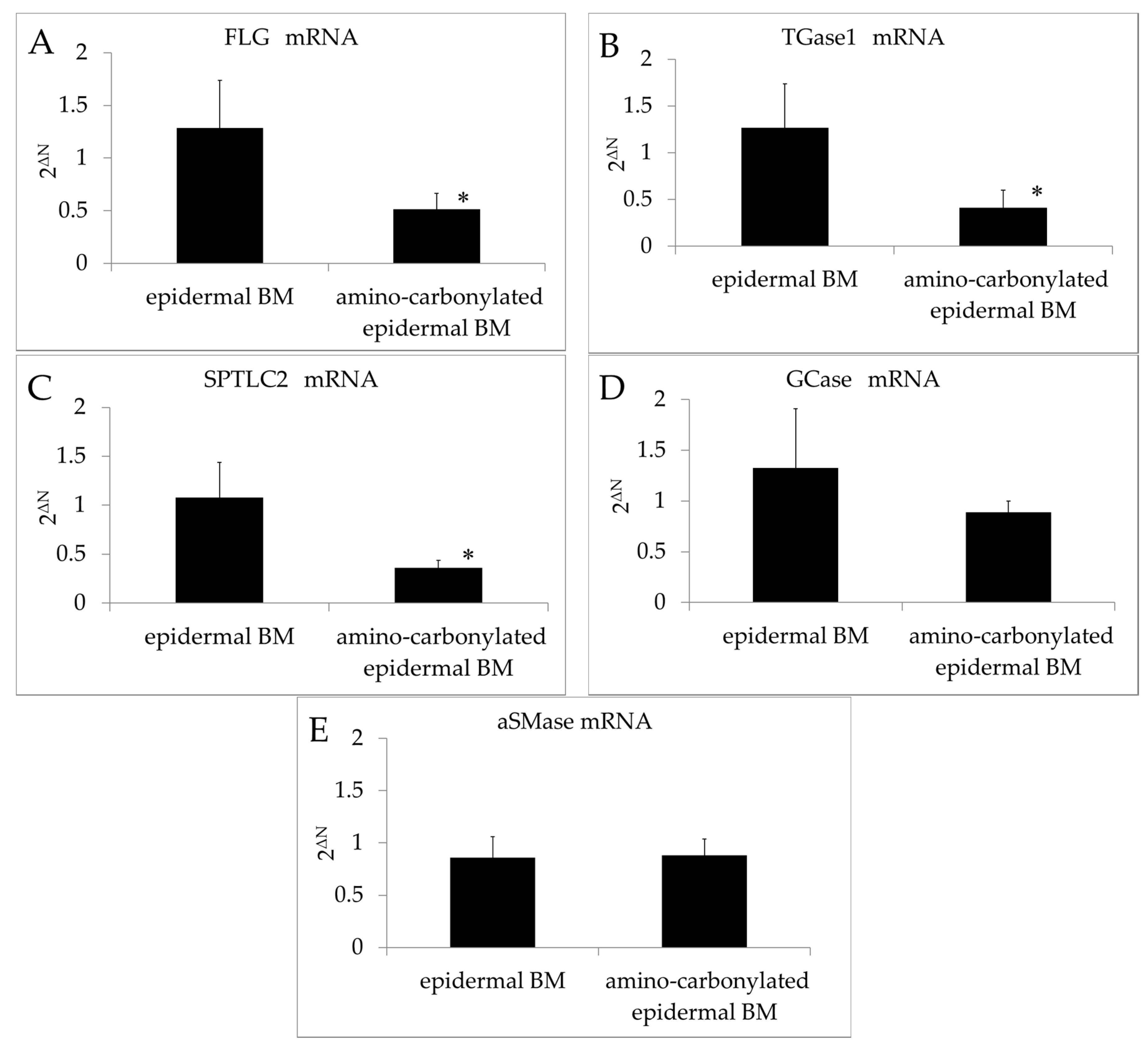
3.2. Effect of Amino Carbonylation of BM on the mRNA Expressions of FLG, TGase1, SPTLC2, GCase, and aSMase
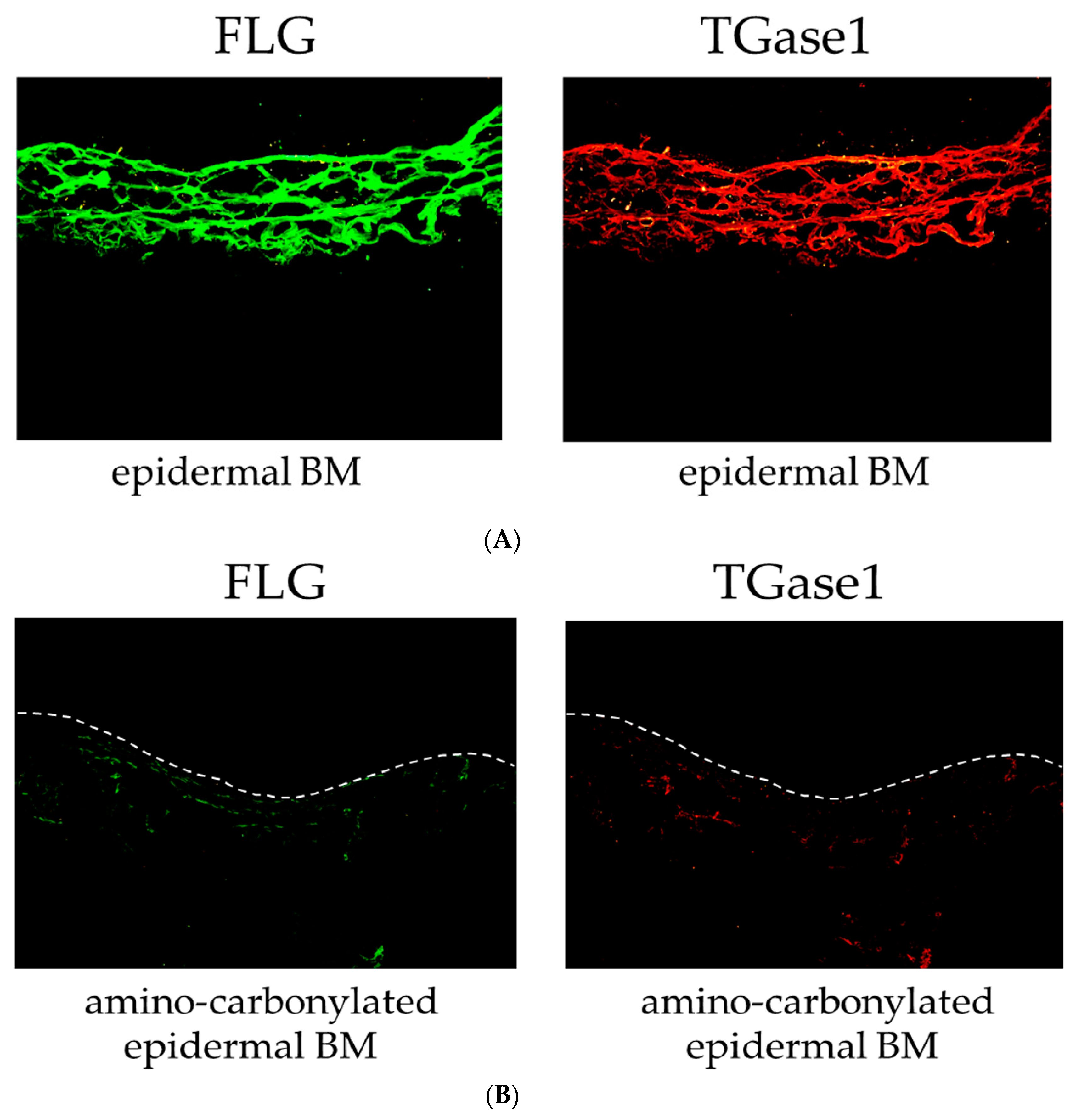
3.3. Effect of Amino Carbonylation of the BM on FLG and TGase1 Protein Levels
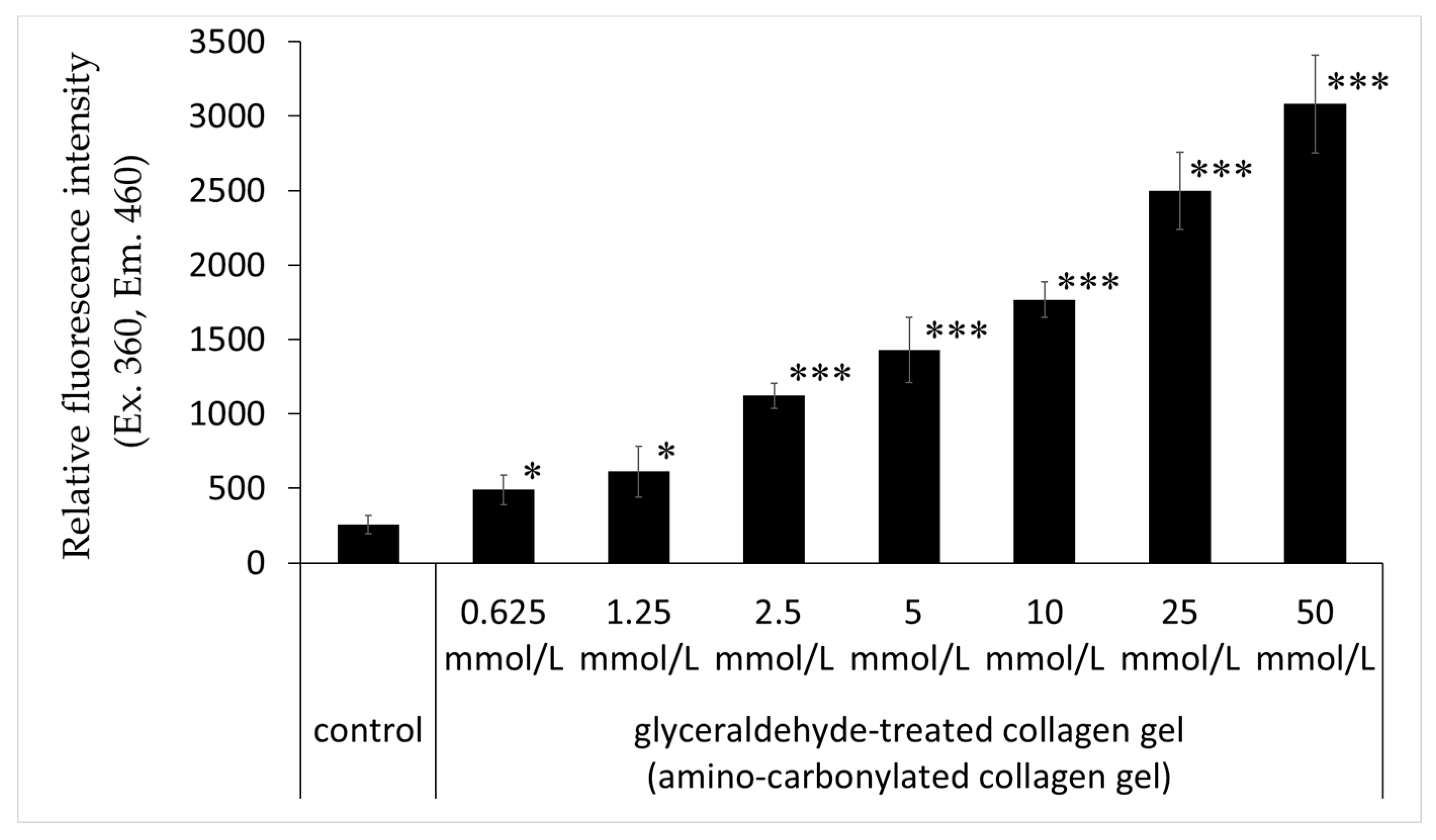
3.4. Effects of Glyceraldehyde on the Relative Fluorescence Intensity of Collagen Gel
3.5. Effect of Methylparaben on the Decrease in ATP and FLG mRNA Levels Caused by Amino Carbonylation of Collagen
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yamagishi, S.; Inagaki, Y.; Amano, S.; Koga, K.; Abe, R.; Takeuchi, M.; Ohno, S.; Yoshimura, A.; Makita, Z. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J. 2002, 16, 1928–1930. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Shimizu, T.; Sugawara, H.; Watanabe, H.; Nakamura, H.; Choei, H.; Sasaki, N.; Yamagishi, S.; Takeuchi, M.; Shimizu, H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J. Investig. Dermatol. 2004, 122, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, K.E.; Salmela, P.I.; Linnaluoto, M.K.; Ikäheimo, M.J.; Ahola, K.; Ryhänen, L.J. Diminished arterial elasticity in diabetes: Association with fluorescent advanced glycosylation end products in collagen. Cardiovasc. Res. 1993, 27, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Asselineau, D. An in vitro approach to the chronological aging of skin by glycation of the collagen: The biological effect of glycation on the reconstructed skin model. Ann. N. Y. Acad. Sci. 2005, 1043, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Técher, M.P.; Asselineau, D. Reconstructed skin modified by glycation of the dermal equivalent as a model for skin aging and its potential use to evaluate anti-glycation molecules. Exp. Gerontol. 2008, 43, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Mizutari, K.; Ono, T.; Ikeda, K.; Kayashima, K.; Horiuchi, S. Photo-enhanced modification of human skin elastin in actinic elastosis by N(epsilon)-(carboxymethyl)lysine, one of the glycoxidation products of the Maillard reaction. J. Investig. Dermatol. 1997, 108, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Oyobikawa, M.; Tada, A.; Maeda, T.; Takiwaki, H.; Itoh, M.; Kanto, H. Melanin and facial skin fluorescence as markers of yellowish discoloration with aging. Skin Res. Technol. 2009, 15, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Dinischiotu, A. RAGE and TGF-β1 Cross-Talk Regulate Extracellular Matrix Turnover and Cytokine Synthesis in AGEs Exposed Fibroblast Cells. PLoS ONE 2016, 11, e0152376. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.; Zhang, X.; Zhang, X.; Kapurniotu, A.; Bernhagen, J.; Teichberg, S.; Basgen, J.; Wagle, D.; Shih, D.; Terlecky, I.; et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature 1996, 382, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Litchfield, J.E.; Baynes, J.W. AGE-breakers cleave model compounds, but do not break Maillard crosslinks in skin and tail collagen from diabetic rats. Arch. Biochem. Biophys. 2003, 412, 42–46. [Google Scholar] [CrossRef]
- Rahbar, S.; Figarola, J.L. Novel inhibitors of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Anisuzzaman; Hatta, T; Miyoshi, T; Matsubayashi, M; Islam, M.K; Alim, M.A; Anas, M.A; Hasan, M.M; Matsumoto, Y; Yamamoto, Y; et al. Longistatin in tick saliva blocks advanced glycation end-product receptor activation. J. Clin. Investig. 2014, 124, 4429–4444. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Modulation of HMGB1 translocation and RAGE/NFκB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp. Dermatol. 2015, 24, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside Inhibits UVB and advanced glycation end products-induced MMP-1 wxpression by suppressing the MAPK and NF-κB pathways in HaCaT cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Meng, F.H.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.H.; Zhang, H.; Li, C.C. Inhibition of Methylglyoxal-Induced AGEs/RAGE Expression Contributes to Dermal Protection by N-Acetyl-l-Cysteine. Cell Physiol. Biochem. 2017, 41, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Norlén, L. Stratum corneum ceramides and their role in skin barrier function. In Skin Moisturization, 2nd ed.; Rawlings, A.V., Leyden, J.J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 31–60. [Google Scholar]
- Tagami, H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch. Dermatol. Res. 2008, 300, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Kemperman, P.M.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Holleran, W.M.; Feingold, K.R.; Man, M.Q.; Gao, W.N.; Lee, J.M.; Elias, P.M. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J. Lipid Res. 1991, 32, 1151–1158. [Google Scholar] [PubMed]
- Rogers, J.; Harding, C.; Mayo, A.; Banks, J.; Rawlings, A. Stratum corneum lipids: The effect of ageing and the seasons. Arch. Dermatol. Res. 1996, 288, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Yamashita, F.; Ishida-Yamamoto, A.; Yamada, K.; Kinoshita, C.; Fushiki, S.; Ueda, E.; Morishima, Y.; Tabata, K.; Yasuno, H.; et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase). Proc. Natl. Acad. Sci. USA 1998, 95, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Casadio, R.; Bergamini, C.M. Transglutaminases: Nature’s biological glues. Biochem. J. 2002, 368, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Stachowitz, S.; Alessandrini, F.; Abeck, D.; Ring, J.; Behrendt, H. Permeability barrier disruption increases the level of serine palmitoyltransferase in human epidermis. J. Investig. Dermatol. 2002, 119, 1048–1052. [Google Scholar] [PubMed]
- Doering, T.; Brade, H.; Sandhoff, K. Sphingolipid metabolism during epidermal barrier development in mice. J. Lipid Res. 2002, 43, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, S.; Hara, M.; Nishio, H.; Otsuka, F.; Suzuki, A.; Uchida, Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J. Investig. Dermatol. 2002, 119, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Carlson, E.C.; Monnier, V.M. Differential effects of type 2 (non-insulin-dependent) diabetes mellitus on pentosidine formation in skin and glomerular basement membrane. Diabetologia 1993, 36, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Haitoglou, C.S.; Tsilibary, E.C.; Brownlee, M.; Charonis, A.S. Altered cellular interactions between endothelial cells and nonenzymatically glucosylated laminin/type IV collagen. J. Biol. Chem. 1992, 267, 12404–12407. [Google Scholar] [PubMed]
- Alikhani, Z.; Alikhani, M.; Boyd, C.M.; Nagao, K.; Trackman, P.C.; Graves, D.T. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J. Biol. Chem. 2005, 280, 12087–12095. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Hayase, F. Isolation and identification of the 3-hydroxy-5-hydroxymethyl-pyridinium compound as a novel advanced glycation end product on glyceraldehyde-related Maillard reaction. Biosci. Biotechnol. Biochem. 2003, 67, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. ACTION I Investigator Group. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol. 2014, 24, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Avtaar Singh, S.S.; Sutherland, F.W.; Di Domenico, F.; Chello, M.; Spadaccio, C. Pharmacologic approaches against advanced glycation end products (AGEs) in diabetic cardiovascular disease. Res. Cardiovasc. Med. 2015, 4, e26949. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, H.; Gu, L.; Zeng, H.; Maeda, K. Amino Carbonylation of Epidermal Basement Membrane Inhibits Epidermal Cell Function and Is Suppressed by Methylparaben. Cosmetics 2017, 4, 38. https://doi.org/10.3390/cosmetics4040038
Morimoto H, Gu L, Zeng H, Maeda K. Amino Carbonylation of Epidermal Basement Membrane Inhibits Epidermal Cell Function and Is Suppressed by Methylparaben. Cosmetics. 2017; 4(4):38. https://doi.org/10.3390/cosmetics4040038
Chicago/Turabian StyleMorimoto, Haruka, Lihao Gu, Haifeng Zeng, and Kazuhisa Maeda. 2017. "Amino Carbonylation of Epidermal Basement Membrane Inhibits Epidermal Cell Function and Is Suppressed by Methylparaben" Cosmetics 4, no. 4: 38. https://doi.org/10.3390/cosmetics4040038





