Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Living Skin Equivalents
2.2. Human Participants
2.3. Tissue Dielectric and pH Measurements
2.4. Temporary Tattoo Sensors and Impedance Measurements
2.5. Headspace Sampling and Gas Chromatography-Mass Spectrometry Analysis of Volatiles
3. Results and Discussion
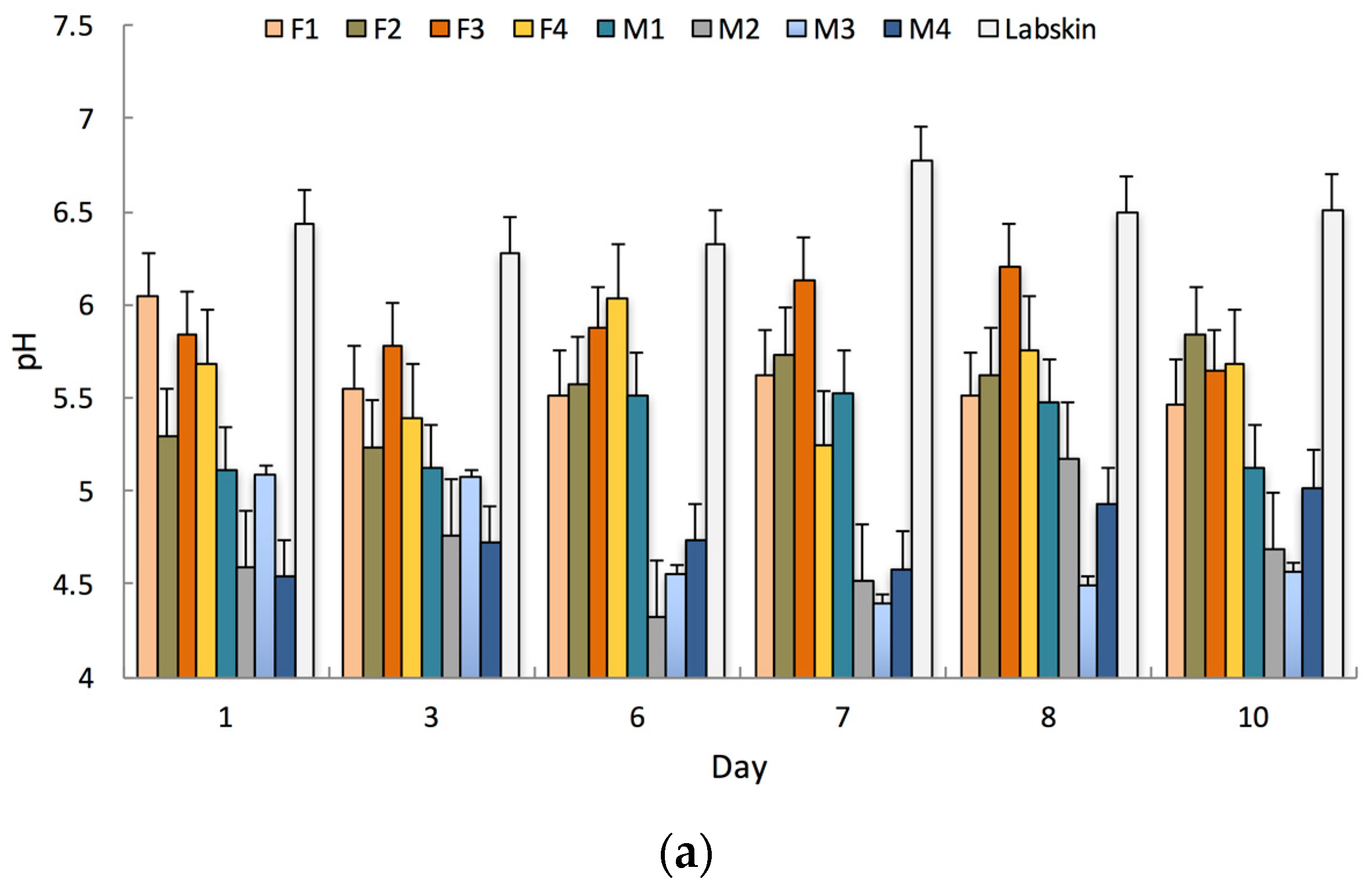
3.1. Biophysical Properties and Temporary Tattoo Sensors
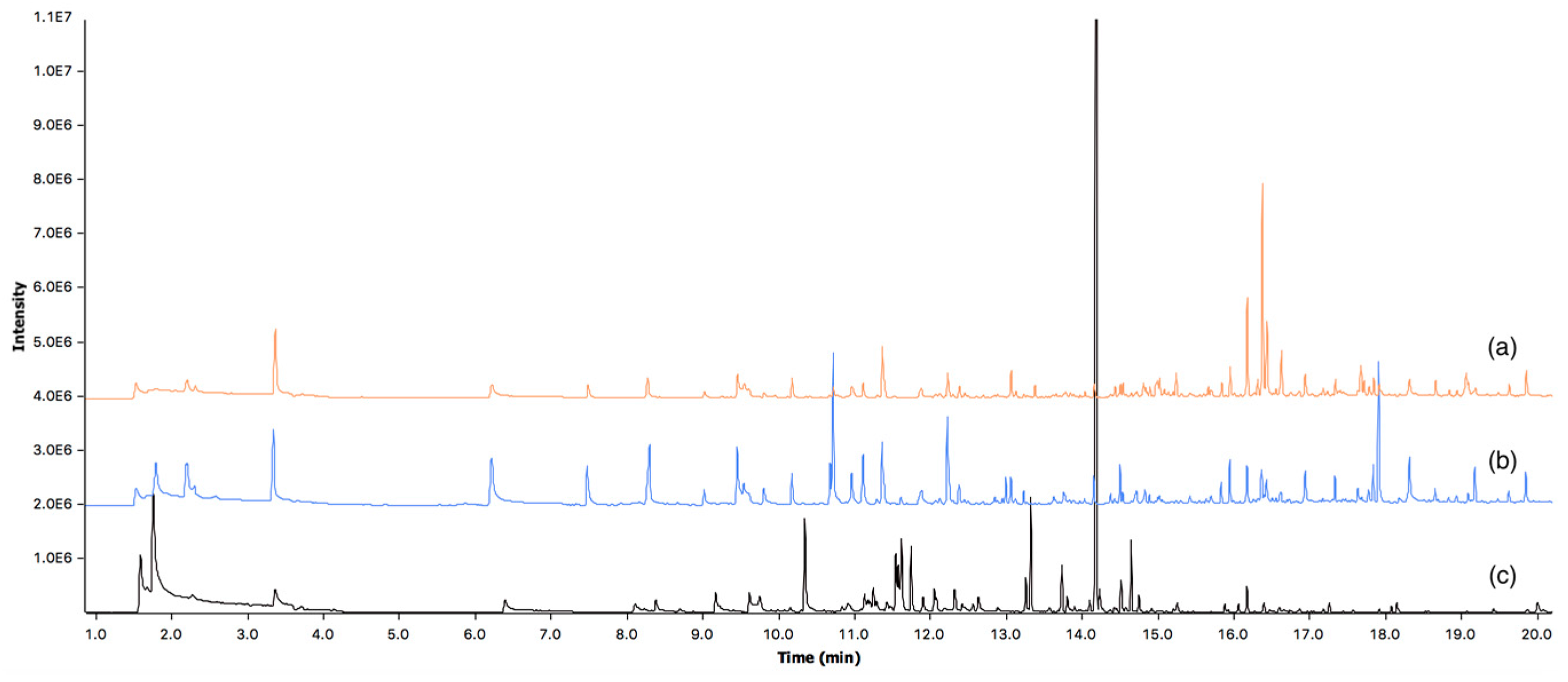
3.2. Volatile Organic Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberts, M.S.; Danick, Y.; Prow, T.W.; Thorling, C.A.; Grice, J.J.; Robertson, T.A.; König, K.; Becker, W. Non-invasive imaging of skin physiology and percutaneous penetration using fluorescence spectral and lifetime imaging with multiphonon and confocal microscopy. Eur. J. Pharm. Biopharm. 2011, 77, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Richters, R.J.H.; Falcone, D.; Uzunbajakave, N.E.; Varghese, B.; Caspers, P.J.; van Erp, P.E.; van de Kerkhof, P.C. Sensitive skin: Assessment of the skin barrier using confocal Raman microspectroscopy. Skin Pharmacol. Physiol. 2017, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Haenssle, H.A. Non-invasive tools for the diagnosis of cutaneous melanoma. Skin Res. Technol. 2017, 23, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Nufer, K.L.; Tomihra, S.; Prow, T.W. Non-invasive nanoparticle imaging technologies for cosmetic and skin care products. Cosmetics 2015, 2, 196–210. [Google Scholar] [CrossRef]
- Bielfeldt, S.; Buttgereit, P.; Brandt, M.; Springmann, G.; Wilhelm, K.P. Non-invasive evaluation techniques to quantify the effects of cosmetic anti-cellulite products. Skin Res. Technol. 2008, 14, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, V.; Balls, M.; Basketter, D.; Berardesca, E.; Edwards, C.; Elsner, P.; Ennen, J.; Lévêque, J.L.; Lóden, M.; Masson, P.; et al. The potential use of non-invasive methods in the safety assessment of cosmetic products. ATLA 1999, 27, 515–537. [Google Scholar] [PubMed]
- Antonov, D.; Schliemann, S.; Elsner, P. Methods for the assessment of barrier function. In Skin Barrier Function; Agner, T., Ed.; Karger: Basel, Switzerland, 2016. [Google Scholar] [CrossRef]
- Hammcock, M.L.; Chortos, A.; Tee, B.C.; Tok, J.B.; Bao, Z. The evolution of electronic skin (e-skin): A brief history, design considerations and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardimci, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2014, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, K.; Morrin, A. Screen-printed tattoo sensor towards the non-invasive assessment of the skin barrier. Electroanalysis 2017, 29, 188–196. [Google Scholar] [CrossRef]
- Ackmann, J.J.; Seitz, M. Methods of complex impedance measurements in biologic tissue. Crit. Rev. Biomed. Eng. 1984, 11, 281–311. [Google Scholar] [PubMed]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Caroprese, A.; Gabbanini, S.; Beltramini, C.; Lucchi, E.; Valgimigli, L. HS-SPME-GC-MS analysis of body odor to test the efficacy of foot deodorant formulations. Skin Res. Technol. 2009, 15, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Smith, D. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444626202. [Google Scholar]
- Prada, P.A.; Furton, K.G. Human scent detection: A review of its developments and forensic applications. Rev. Cien. Forenses 2008, 1, 81–87. [Google Scholar]
- Dormont, L.; Bessière, J.-M.; McKey, D.; Couhet, A. New methods for field collection of human skin volatiles and perspectives for their application in the chemical ecology of human-pathogen-vector interactions. J. Expt. Biol. 2013, 216, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Abaffy, T.; Möller, M.G.; Riemer, D.D.; Millikowski, C.; DeFazio, R.A. Comparative analysis of volatile metabolomics signals from melanoma and benign skin: A pilot study. Metabolomics 2013, 9, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.; Capuano, R.; Strand, T.; Ek, A.-C.; Lindgren, M.; Paolesse, R.; Di Natale, C.; Lundström, I. Volatile emissions from compressed tissue. PLoS ONE 2013, 8, e69271. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Jacobs, M.R.; Kirby, B.; Morrin, A. Probing skin physiology through the volatile footprint: Discriminating emissions before and after acute barrier disruption. Exp. Dermatol. 2017, 26, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid-Phase Microextraction; Elsevier: London, UK, 2012; ISBN 978-0-12-416017-0. [Google Scholar]
- Dormont, L.; Bessière, J.-M.; Couhet, A. Human skin volatiles: A review. J. Chem. Ecol. 2013, 39, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Birgersson, U.; Birgersson, E.; Nicander, I.; Ollmar, S. A methodology for extracting the electrical properties of human skin. Physiol. Meas. 2013, 34, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Wenig, P.; Odermatt, J. OpenChrom: A cross-platform open source software for the mass spectrometric analysis of chromatographic data. BMC Bioinform. 2010, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hosseini, M.; Vainio, S.; Taïeb, A.; Cario-André, M.; Rezvani, H.R. Skin equivalents: Skin from reconstructions as models to study skin development and diseases. Br. J. Dermaotl. 2015, 173, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Matousek, J.L.; Campbell, K.L. A comparative review of cutaneous pH. Vet. Dermatol. 2002, 13, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.B.; Bojar, R.A.; Jeremy, A.H.T.; Ingham, E.; Holland, K.T. Microbial colonization of an in vitro model of a tissue engineered human skin equivalent—A novel approach. FEMS Microbiol. Lett. 2008, 279, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mayrovitz, H.N.; Carson, S.; Luis, M. Male-female differences in forearm skin tissue dielectric constant. Clin. Physiol. Funct. Imaging 2010, 30, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamamoto, Y. Electrical properties of the epidermal stratum corneum. Med. Biol. Eng. 1976, 14, 151–158. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Orazem, M.E.; Bunge, A.L. Characterization of damaged skin by impedance spectroscopy: Mechanical damage. Pharm. Res. 2013, 30, 2036–2049. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Orazem, M.E.; Bunge, A.L. Characterization of damaged skin by impedance spectroscopy: Chemical damage by dimethyl sulfoxide. Pharm. Res. 2013, 30, 2607–2624. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Buommino, E.; De Gregoria, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Moore, M. Bioelectrical impedance assessment of wound healing. J. Diabetes Sci. Technol. 2012, 6, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Patel, R.A.; Shinn, A.H.; Choi, S.Y.; Byun, H.J.; Park, K.C.; Youn, S.W. Evaluation of gender difference in skin type and pH. J. Detmatol. Sci. 2006, 41, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.; Oysocki, C.J.; Leyden, J.J.; Spielman, A.I.; Sun, X.; Preti, G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Gallagher, M.; Ozdener, M.H.; Wysocki, C.J.; Goldsmith, B.R.; Isamah, A.; Faranda, A.; Fakharzadeh, S.S.; Herlyn, A.T.; Johnson, C.; et al. Volatile biomarkers from human melanoma cells. J. Chromatogr. B 2013, 931, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.M.; Ramirez, C.F.; Schoon, A.A.; Furton, K.G. The frequency of occurrence and discriminatory power of compounds found in human scent across a population determined by SPME-GC/MS. J. Chromatogr. B 2007, 846, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Sánchez, E.Y.; Reyes, J.G.; Young, M.E. Volatile organic compounds produced by human skin cells. Biol. Res. 2007, 40, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Santonico, M.; Pennazza, G.; Martinellli, E.; Paolesse, R.; D’Amico, A.; Di Natale, C. A sensor array and GC study about VOCs and cancer cells. Sens. Actuators B 2010, 146, 483–488. [Google Scholar] [CrossRef]
- Penn, D.J.; Oberzaucher, E.; Grammer, K.; Fischer, G.; Soini, H.A.; Wiesler, D.; Novotny, M.V.; Dixon, S.J.; Xu, Y.; Brereton, R.G. Invididual and gender fingerprints in human body odour. J. R. Soc. Interface 2007, 4, 331–340. [Google Scholar] [CrossRef] [PubMed]



| Compound | CAS | F1, Day: | F2, Day: | F3, Day: | F4, Day: | ||||||||||||||||||||
| 1 | 3 | 6 | 7 | 8 | 10 | 1 | 3 | 6 | 7 | 8 | 10 | 1 | 3 | 6 | 7 | 8 | 10 | 1 | 3 | 6 | 7 | 8 | 10 | ||
| Hexane | 110-54-3 | × | × | × | × | × | × | ||||||||||||||||||
| Hexanal | 66-25-1 | ||||||||||||||||||||||||
| 1-Nonene | 124-11-8 | × | × | × | × | ||||||||||||||||||||
| Heptanal | 111-71-7 | × | × | × | × | ||||||||||||||||||||
| Benzaldehyde | 100-52-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||
| Octanal | 124-13-0 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| 2-ethyl-1-hexanol | 104-76-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Benzyl alcohol | 100-51-6 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||
| 1-Octanol | 111-87-5 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||
| Nonanal | 124-19-6 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Decanal | 112-31-2 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Nonanoic acid | 112-05-5 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Undecanal | 112-44-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||||
| Dodecanal | 112-54-9 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||
| Geranyl acetone | 689-67-8 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Undecanoic acid | 112-37-8 | × | × | × | × | × | × | × | × | × | |||||||||||||||
| α-Isomethyl ionone | 127-51-5 | × | × | × | × | × | × | × | × | × | × | × | × | ||||||||||||
| β-Ionone | 14901-07-6 | × | × | × | × | × | × | × | × | × | × | ||||||||||||||
| Pentadecane | 629-62-9 | × | × | × | × | × | × | ||||||||||||||||||
| Lilial | 80-54-6 | × | × | × | × | × | × | × | × | × | × | ||||||||||||||
| Dodecanoic acid | 143-07-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||
| Hedione | 24851-98-7 | × | × | × | × | × | × | × | × | ||||||||||||||||
| Octyl ether | 629-82-3 | ||||||||||||||||||||||||
| Tetradecanoic acid | 544-63-8 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||||
| Octyl octanoate | 2306-88-9 | × | × | × | × | × | × | × | × | × | × | × | |||||||||||||
| Isopropyl palmitate | 142-91-6 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||||||
| Oleic acid | 112-80-1 | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||||||||
| Compound | CAS | M1, Day: | M2, Day: | M3, Day: | M4, Day: | ||||||||||||||||||||
| 1 | 3 | 6 | 7 | 8 | 10 | 1 | 3 | 6 | 7 | 8 | 10 | 1 | 3 | 6 | 7 | 8 | 10 | 1 | 3 | 6 | 7 | 8 | 10 | ||
| Hexane | 110-54-3 | × | × | × | × | × | × | ||||||||||||||||||
| Hexanal | 66-25-1 | × | × | × | × | ||||||||||||||||||||
| 1-Nonene | 124-11-8 | × | × | × | × | × | × | × | × | × | × | ||||||||||||||
| Heptanal | 111-71-7 | × | × | × | × | ||||||||||||||||||||
| Benzaldehyde | 100-52-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||
| Octanal | 124-13-0 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||
| 2-ethyl-1-hexanol | 104-76-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Benzyl alcohol | 100-51-6 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||
| 1-Octanol | 111-87-5 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||||||
| Nonanal | 124-19-6 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Decanal | 112-31-2 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Nonanoic acid | 112-05-5 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||
| Undecanal | 112-44-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||||
| Dodecanal | 112-54-9 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||
| Geranyl acetone | 689-67-8 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Undecanoic acid | 112-37-8 | × | × | × | × | ||||||||||||||||||||
| α-Isomethyl ionone | 127-51-5 | × | × | × | × | × | × | × | × | × | |||||||||||||||
| β-Ionone | 14901-07-6 | ||||||||||||||||||||||||
| Pentadecane | 629-62-9 | × | × | × | × | × | × | ||||||||||||||||||
| Lilial | 80-54-6 | × | × | × | |||||||||||||||||||||
| Dodecanoic acid | 143-07-7 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Hedione | 24851-98-7 | ||||||||||||||||||||||||
| Octyl ether | 629-82-3 | × | × | ||||||||||||||||||||||
| Tetradecanoic acid | 544-63-8 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Octyl octanoate | 2306-88-9 | × | × | ||||||||||||||||||||||
| Isopropyl palmitate | 142-91-6 | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||||||
| Oleic acid | 112-80-1 | × | × | × | × | × | × | × | × | × | |||||||||||||||
| Compound | CAS | Day | |||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 7 | 8 | 10 | ||
| Ethylbenzene | 100-41-4 | × | |||||
| Styrene | 100-42-5 | × | × | × | × | × | × |
| Camphene | 79-92-5 | × | × | ||||
| Decane | 124-18-5 | × | |||||
| Octanal | 124-13-0 | × | |||||
| 2-Ethyl-1-hexanol | 104-76-7 | × | × | × | × | × | × |
| Undecane | 1120-21-4 | × | × | ||||
| Nonanal | 124-19-6 | × | |||||
| 1-Nonanol | 143-08-8 | × | × | × | × | × | × |
| Camphor | 76-22-2 | × | |||||
| 2,2,4-trimethyl-1,3-pentanediol | 144-19-4 | × | × | × | |||
| Isoborneol | 124-76-5 | × | |||||
| Dodecane | 112-40-3 | × | × | × | × | × | × |
| Isobornyl acrylate | 5888-33-5 | × | × | × | × | × | × |
| 2,6-Diisopropylnaphthalene | 24157-81-1 | × | |||||
| n-Hexadecanoic acid | 57-10-3 | × | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duffy, E.; Guzman, K.D.; Wallace, R.; Murphy, R.; Morrin, A. Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications. Cosmetics 2017, 4, 44. https://doi.org/10.3390/cosmetics4040044
Duffy E, Guzman KD, Wallace R, Murphy R, Morrin A. Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications. Cosmetics. 2017; 4(4):44. https://doi.org/10.3390/cosmetics4040044
Chicago/Turabian StyleDuffy, Emer, Keana De Guzman, Robert Wallace, Ronan Murphy, and Aoife Morrin. 2017. "Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications" Cosmetics 4, no. 4: 44. https://doi.org/10.3390/cosmetics4040044




