The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy
Abstract
:1. Introduction
2. Use of Microalgae and Cyanobacteria for Health Applications
3. Uses of Microalgae and Cyanobacteria for Cosmetic Applications
4. The Application of Marine Microalgae in Thalassotherapy
5. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vourela, H.; Yli-Kauhaluoma, H. Exploring Marine Resources for Bioactive Compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisti, Y. Microalgae as sustainable cell factories. Environ. Eng. Manag. J. 2006, 5, 261–274. [Google Scholar]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Tamagnini, P.; Axelsson, R.; Lindberg, P.; Oxelfelt, F.; Wünschiers, R.; Lindblad, P. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, T. Introduction. In Biotechnological Applications of Microalgae: Biodiesel and Value-Added Products; Bux, F., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–5. ISBN 978-1-46-651529-1. [Google Scholar]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, A.; Probert, I.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting Marine Plankton. Mar. Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manirafasha, E.; Ndikubwimana, T.; Zen, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ngo, D.-H.; Ta, Q.V.; Kim, S.-K. Marine organisms as a therapeutic source against Herpes simplex virus infection. Eur. J. Pharm. Sci. 2011, 44, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll extraction from microalgae: A review on the process engineering aspects. Int. J. Chem. Eng. 2010. [Google Scholar] [CrossRef]
- Bin, S.; Wang, Z.P.; Liu, X.Y.; Yu, J.X.; Lan, J.J.; Wang, J.M.; Ma, L.F.; Chen, Z.Y. Breeding of a Chlorella strain with high yield of polysaccharide and its effect on growth and immunoregulation of Litopenaeus vannamei. J. Nucl. Agric. Sci. 2013, 27, 168–172. [Google Scholar]
- Guzman, S.; Gato, A.; Calleja, J.M. Anti-Inflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2001, 15, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.J.; Morais, R.M.; Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.E.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Klok, A.J.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. Edible oils from microalgae insights in TAG accumulation. Trends Biotechnol. 2014, 32, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Moore, A. Blooming prospects? Humans have eaten seaweed for millennia; now microalgae are to be served up in a variety of novel health supplements, medicaments and preparations. EMBO Rep. 2001, 2, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I. The Evolution and Versatility of Microalgal Biotechnology: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Sharma, T.; Gour, R.S.; Kant, A.; Chauhan, R.S. Lipid content in Scenedesmus species correlates with multiple genes of fatty acid and triacylglycerol biosynthetic pathways. Algal Res. 2015, 12, 341–349. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Cosmética termal: Valor añadido en los centros termales. In Proceedings of the 1st International Congress on Water Healing Spa and Quality of Life; Failde, J.M., Formella, A., Fraiz, J.A., Gómez, M., Pérez, F., Rodríguez, V., Eds.; University of Vigo: Pontevedra, Spain, 2015; pp. 99–105, ISBN 978-8-48-158704-3. [Google Scholar]
- Borowitzka, M.A. Microalgae as source of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1999, 7, 3–15. [Google Scholar] [CrossRef]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Jain, R.; Raghukumar, S.; Tharanathan, R.; Bhosle, N.B. Extracellular polysaccharide production by thraustochytrid protists. Mar. Biotechnol. 2005, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Chandra, R.; Parra, R.; Iqbal, H.M.N. Phycobiliproteins: A Novel Green Tool from Marine Origen Blue-Green Algae and Red Algae. Protein Pept. Lett. 2017, 24, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Morelli, E.; Scarano, G. Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalgae Phaeodactylum tricornutum. Plant Sci. 2004, 167, 289–296. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Juárez, A.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; Ríos de Molina, M.C. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Saf. 2009, 72, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of Marine Phytoplankton Produce Bioactive Pigments and Lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Suter, P.M. Vitamin A, Nutrition, and Health Values of Algae: Spirulina, Chlorella, and Dunaliella. J. Pharm. Nutr. Sci. 2011, 1, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Sayo, T.; Sugiyama, Y.; Inoue, S. Lutein, a nonprovitamin A, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci. Biotechnol. Biochem. 2013, 6, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Bermejo, R.; Gabriel Acién, F.; Ibáñez, M.J.; Fernéndez, J.M.; Molina, E.; Alvarez-Pez, J.M. Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. J. Chromatogr. B 2003, 790, 317–325. [Google Scholar] [CrossRef]
- Arad, S.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Technol. 1992, 3, 92–97. [Google Scholar] [CrossRef]
- Matsunaga, T.; Burgess, J.G.; Yamada, N.; Komatsu, K.; Yoshida, S.; Wachi, Y. An ultraviolet (UV-A) absorbing biopterin glucoside from the marine planktonic cyanobacterium Oscillatoria sp. Appl. Microbiol. Biotechnol. 1993, 39, 250–253. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rani, B.; Chauhan, A.K.; Maheshwari, R. Lycopene’s antioxidant activity in cosmetics meadow. IRJP 2013, 3, 46–47. [Google Scholar]
- Hashtroudi, M.S.; Shariatmadari, Z.; Riahi, H.; Ghassempour, A. Analysis of Anabaena vaginicola and Nostoc calcicola from Northern Iran, as rich sources of major carotenoids. Food Chem. 2013, 136, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.M. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C.W. Preliminary Characterization, Antioxidant Properties and Production of Chrysolaminarin from Marine Diatom Odontella aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef] [PubMed]
- Shivanand, P.; Mugeraya, G. Halophilic bacteria and their compatible solutes—Osmoregulation and potential applications. Curr. Sci. 2011, 100, 1516–1521. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, J. Phytohormones in microalgae a new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Michelet, J.F.; Olive, C.; Rieux, E.; Fagot, D.; Simonetti, L.; Galey, J.B.; Dalko-Csiba, M.; Bernard, B.A.; Pereira, R. The anti-ageing potential of a new jasmonic acid derivative (LR2412): In vitro evaluation using reconstructed epidermis episkin™. Exp. Dermatol. 2012, 21, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.A.; Airs, R.L. Distribution and Abundance of MAAs in 33 Species of Microalgae across 13 Classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H.M.N. High value compounds from microalgae with industrial exploitability—A review. Front. Biosci. 2017, 9, 319–342. [Google Scholar]
- Lucchetta, M.C.; Monaco, G.; Valenzi, V.I.; Fraioli, A. The historical-scientific bases of thalassotherapy: State of the art. Clin. Ter. 2013, 158, 533–541. [Google Scholar]
- Gomes, C.; Carretero, I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and pelotherapy: Historical evolution, classification and glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Legido, J.L.; Mourelle, L.; Torres, J.; Martín, M.C.; Fernández, C.; Gómez, C.P. Caracterización termofísica de mezclas de arcilla, aguas mineromedicinales y microalgas. In Proceedings of the 3rd Iberoamerican Congress of Peloids, Sao Miguel, Azores, Portugal, 1–7 October 2013; Nunes, J.C., Baptista, J.B., Gomes, C.F., Eds.; Instituto de Innovaçao Tecnológica dos Açores: Azores, Portugal, October 2003; pp. 119–123. [Google Scholar]
- Casás, L.M.; Legido, J.L.; Pozo, M.; Mourelle, L.; Plantier, F.; Bessieres, D. Specific heat of mixtures of bentonitic clay with seawater or distilled water for their use in thermotherapy. Thermochim. Acta 2011, 524, 68–73. [Google Scholar] [CrossRef]
- Casás, L.M.; Pozo, M.; Gómez, C.P.; Pozo, E.; Bessieres, D.; Plantier, F.; Legido, J.L. Thermal behavior of mixtures of bentonitic clay and saline solutions. Appl. Clay Sci. 2013, 72, 18–25. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L.; Legido, N. Innovación en el uso de microalgas en termalismo. Bol. Soc. Esp. Hidrol. Méd. 2016, 31, 53–64. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Mancera-Andrade, E.; Robledo-Padilla, F.; Iqbal, H.M.N.; Parra-Saldivar, R.A. Chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res. 2017, 26, 312–322. [Google Scholar] [CrossRef]
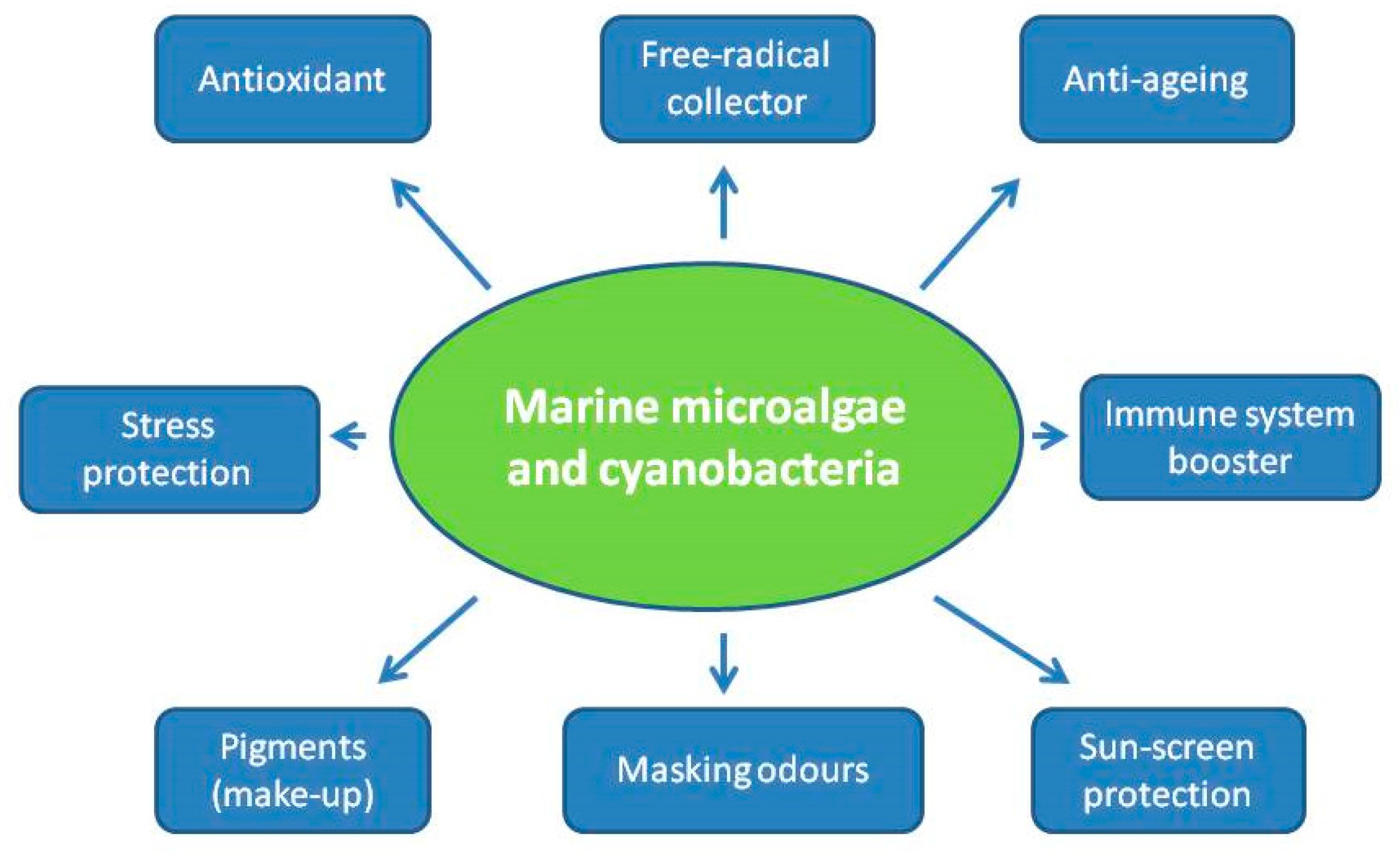

| Bioactive Compounds | Microalgae/Cyanobacteria | Potential Activities and Uses in Cosmetics | References |
|---|---|---|---|
| Polysaccharides | Chlorella | Moisturizing and thickener agent | Jain et al., 2005 |
| Methanolic extracts of exopolysaccharides | Arthrospira platensis | Antioxidant | Raposo et al. 2015 |
| Chrysolaminarin | Odontella aurita | Antioxidant | Xia et al., 2014 |
| Sulfated polysacharides | Porphyridium and Rhodella reticulata | Antioxidant | Raposo et al., 2015 |
| ß-1,3-Glucan | Chlorella Skeletonema Porphyridium Nostoc flegelliforme | Free-radical collector Immune system booster Anti-inflammatory | Spolaore et al., 2006 Koller et al., 2014 Bin et al., 2013 Hamed, 2016 |
| ß-carotenes | Dunaliella salina | Antioxidant | Hamed, 2016 |
| Asthaxanthin | Haematococcus pluvialis | Antioxidant Sunscreen protection | Hamed, 2016 Koller et al., 2014 |
| Phycocyanobilin phycoerythrobilin | Spirulina Porphyridium | Antioxidant Pigment for eye-liner and lipsticks | Hamed, 2016 |
| β-Cryptoxanthin | Dunaliella salina | Anti-inflammatory Promote Hialuronan synthesis | Tang and Suter, 2011 |
| Chlorophyll | Chlorella sp. | To mask odors in dentifrices and deodorants | Hosikian et al., 2010 |
| Canthaxanthin | Nannochloropsis salina Nannochloropsis oculata Nannochloropsis gaditana | Tanning cosmetics and cosmeceutics | Koller et al., 2014 |
| Phycocyanin | Porphyridium cruentum Spirulina platensis | Eye-shadows | Bermejo et al., 2003 Arad and Yaron, 1992 |
| Lycopene | Anabaena vaginicola | Antioxidant Anti-ageing Sunscreen | Singh et al., 2012 Hashtroudi et al., 2013 |
| Scytonemin | Marine cyanobacteria | Sunscreen | Takamatsu et al., 2003 |
| Vitamin E (α-Tocopherol) | Dunaliella tertiolecta Tetraselmis suecica | Antioxidant | Hashtroudi et al., 1999 |
| Biopterin glucose | Marine planktonic cyanobacterium | Sunscreen | Matsunaga et al., 1993 |
| Ectoine | Halomonas elongata Halomonas boliviensis Brevibacterium epidermis Chromohalobacter israelensis Chromohalobacter salexigens | Immune protection UV protection Stress protection Moisturizing agent | Kim et al., 2008 Shivanand and Mugeraya, 2011 |
| Phytohormones (auxin, abscisic acid, cytokinin, ethylene, gibberellins) | Broad spectrum of microalgal lineages Nannochloropsis oceanica | Anti-ageing | Lu and Xu, 2015 Michelet et al., 2012 |
| Micosporine-like amino acids | Cyanobacteria | Sunscreen | Llewellyn and Airs, 2010 Singh, 2017 |
| Chlorella vulgaris extracts | Chlorella vulgaris | Collagen repair (anti-ageing) | Koller et al., 2014 |
| Microalgae extracts | Phaeodactylum tricornutum Scenedesmus vacuolatus and Chlorella kessleri | Antioxidant Antioxidant | Morelli et al., 2004 Sabatini et al., 2009 |
| INCI Name | Description |
|---|---|
| ALGAE EXOPOLYSACCHARIDES | Algae Exopolysaccharides are exopolysaccharides released by the fermentation of various species of microalgae of the divisions, Rhodophyta and Chlorophyta. |
| PARACHLORELLA BEIJERINCKII EXOPOLYSACCHARIDES | Parachlorella Beijerinckii Exopolysaccharides are exopolysaccharides produced through the fermentation of the microalgae Parachlorella beijerinckii. |
| APHANOTHECE SACRUM POWDER | Anacardoyl Tripeptide-35 is the product obtained by the reaction of anacardic acid and Tripeptide-35, Cyanobacteriaceae. |
| ARTHROSPIRA EXTRACT | Arthrospira Extract is an extract of the cyanobacterium, Arthrospira maxima. |
| ARTHROSPIRA PLATENSIS CULTURE CONDITIONED MEDIA | Arthrospira Platensis Culture Conditioned Media is the growth media removed from cultures of Arthrospira platensis after several days of growth, Phormidiaceae. |
| CYANOBACTERIUM APONINUM FERMENT | Cyanobacterium Aponinum Ferment is the product obtained by the fermentation of Cyanobacterium aponinum. |
| ODONTELLA AURITA EXTRACT | Odontella Aurita Extract is an extract of the phytoplankton Odontella aurita, Bacilaiophyceae. |
| ODONTELLA AURITA OIL | Odontella Aurita Oil is the oil obtained from the phytoplankton Odontella aurita, Bacilaiophyceae. |
| PARALLELOSTROMBIDIUM SICULUM EXTRACT | Parallelostrombidium Siculum Extract is the extract of the plankton, Parallelostrombidium siculum, Strombidiidae. |
| PLANKTON EXTRACT (CAS No. 91079-57-1) | Plankton Extract is an extract obtained from marine plankton. |
| TETRASELMIS SUECICA EXTRACT | Tetraselmis Suecica Extract is an extract of the plankton Tetraselmis suecica. |
| CHLORELLA ELLIPSOIDEA EXTRACT | Chlorella Ellipsoidea Extract is the extract of the alga, Chlorella ellipsoidea, Chlorellaceae. |
| CHLORELLA EMERSONII EXTRACT (CAS No. 223749-78-8) | Chlorella Emersonii Extract is an extract of the Alga, Chlorella emersonii, Oocystaceae. |
| CHLORELLA VULGARIS CALLUS CULTURE EXTRACT | Chlorella Vulgaris Callus Culture Extract is the extract of a culture of the callus of the alga, Chlorella vulgaris, Chlorellaceae. |
| PHORMIDIUM UNCINATUM EXTRACT | Phormidium Uncinatum Extract is the extract of the Alga, Phormidium uncinatum. |
| PHORMIDIUM FERMENT | Phormidium Ferment is the product obtained through fermentation by the microorganism, Phormidium. |
| APHANIZOMENON FLOS-AQUAE EXTRACT | Aphanizomenon Flos-Aquae Extract is the extract of the alga, Aphanizomenon flos-aquae, Nostocaceae. |
| ALPHA-GLUCAN OLIGOSACCHARIDE | Alpha-Glucan Oligosaccharide is a glucose oligomer exhibiting a degree of polymerization ranging from 2–10. It is prepared by the action of a Leuconostoc mesenteroides glucosyl transferase on sucrose. |
| NOSTOC FLAGELLIFORME EXTRACT | Nostoc Flagelliforme extract is an extract of the Alga, Nostoc flagelliforme, Nostocaceae. |
| DUNALIELLA BARDAWIL EXTRACT | Dunaliella Bardawil Extract is an extract of the Alga, Dunaliella bardawil, Dunaliellaceae. |
| DUNALIELLA SALINA/HAEMATOCOCCUS PLUVIALIS EXTRACT | Dunaliella Salina/Haematococcus Pluvialis Extract is the extract of the alga, Dunaliella salina and Haematococcus pluvialis. |
| PORPHYRIDIUM CRUENTUM EXTRACT (CAS No. 223751-77-7) | Porphyridium Cruentum Extract is an extract of the Alga, Porphyridium cruentum, Porphyridaceae. |
| THALASSIOSIRA PSEUDONANA EXTRACT | Thalassiosira Pseudonana Extract is the extract of the alga, Thalassiosira pseudonana. |
| NANNOCHLOROPSIS OCULATA POWDER | Nannochloropsis Oculata Powder is the powder obtained from the dried, ground alga, Nannochloropsis oculata. |
| SKELETONEMA COSTATUM EXTRACT | Skeletonema Costatum Extract is an extract of Skeletonema costatum. |
| DUNALIELLA SALINA/ISOCHRYSIS GALBANA/NANNOCHLOROPSIS GADITANA/PHAEODACTYLUM TRICORNUTUM/TETRASELMIS CHUII EXTRACT | Dunaliella Salina/Isochrysis Galbana/Nannochloropsis Gaditana/Phaeodactylum Tricornutum/Tetraselmis Chuii Extract is the extract of the whole plants of Dunaliella salina, Isochrysis galbana, Nannochloropsis gaditana, Phaeodactylum tricornutum and Tetraselmis chuii. |
| PSEUDANABAENACEAE FERMENT FILTRATE | Pseudanabaenaceae Ferment Filtrate is a filtrate of the product obtained by the fermentation of a growth media by the microorganism, Pseudanabaenaceae. |
| RHODELLA VIOLACEA EXTRACT | Rhodella Violacea Extract is the extract of the alga, Rhodella violacea, Rhodellaceae. |
| HALOMONAS ANTICARIENSIS FERMENT EXTRACT FILTRATE | Halomonas Anticariensis Ferment Extract Filtrate is a filtrate of an extract of the product obtained by the fermentation of the bacteria, Halomonas anticariensis. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. https://doi.org/10.3390/cosmetics4040046
Mourelle ML, Gómez CP, Legido JL. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics. 2017; 4(4):46. https://doi.org/10.3390/cosmetics4040046
Chicago/Turabian StyleMourelle, M. Lourdes, Carmen P. Gómez, and José L. Legido. 2017. "The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy" Cosmetics 4, no. 4: 46. https://doi.org/10.3390/cosmetics4040046





