10-Hydroxy-2-Decenoic Acid in Royal Jelly Extract Induced Both Filaggrin and Amino Acid in a Cultured Human Three-Dimensional Epidermis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Measurement of the Amounts of FLG and AQP3 mRNA in the Human Three-Dimensional Epidermis Model Cultured with 10H2DA and 10HDAA
2.3. Measurement of the Amount of Amino Acids in the HumanThree-Dimensional Epidermis Model Cultured with 10H2DA and 10HDAA
2.4. Immunohistochemical Staining of FLG and AQP3 in the Human Three-Dimensional Epidermis Model Cultured with 10H2DA and 10HDAA
2.5. Data Analysis
3. Results
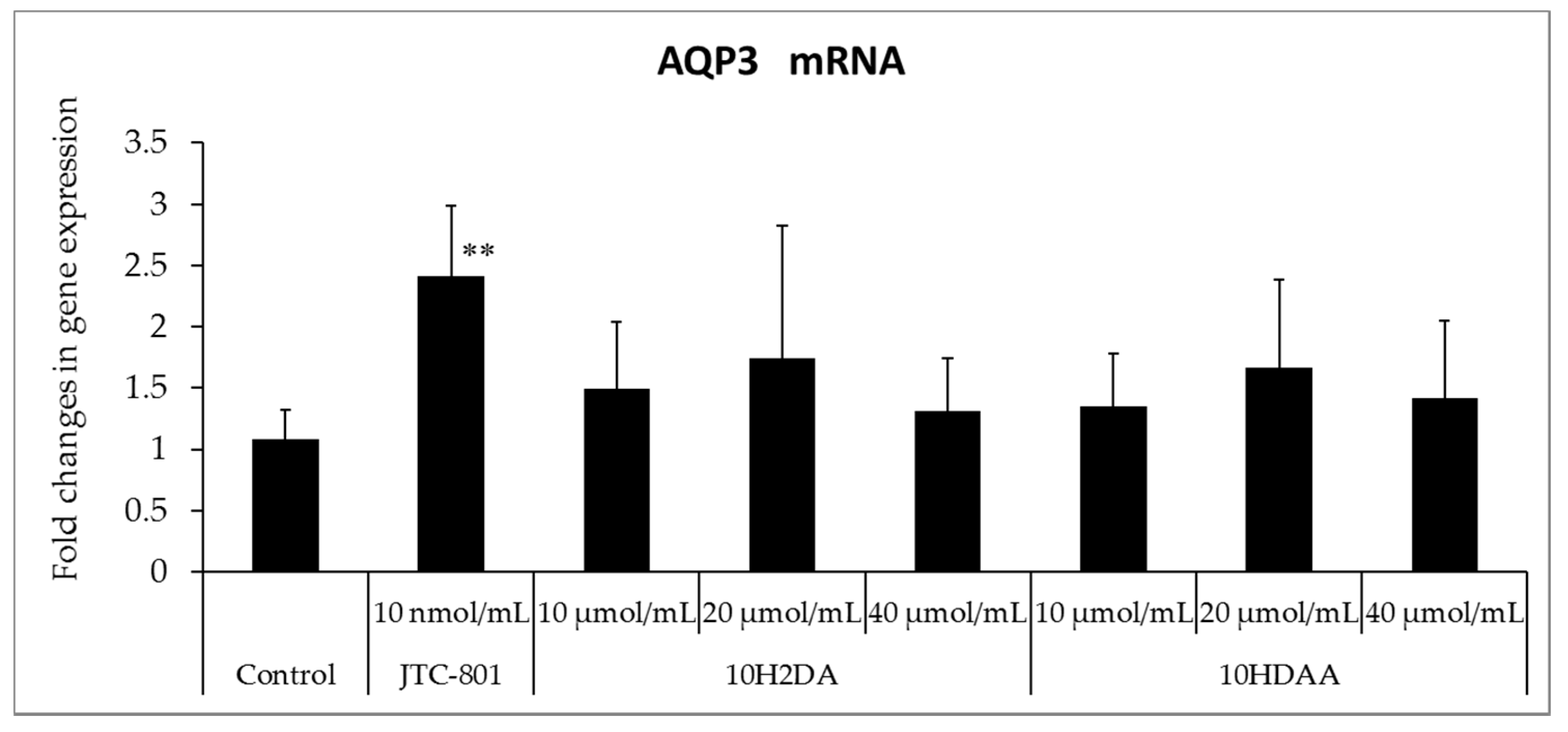
3.1. Effects of 10H2DA and 10HDAA on mRNA Expression of FLG and AQP3 in the Cultured Human Three-Dimensional Epidermis Model
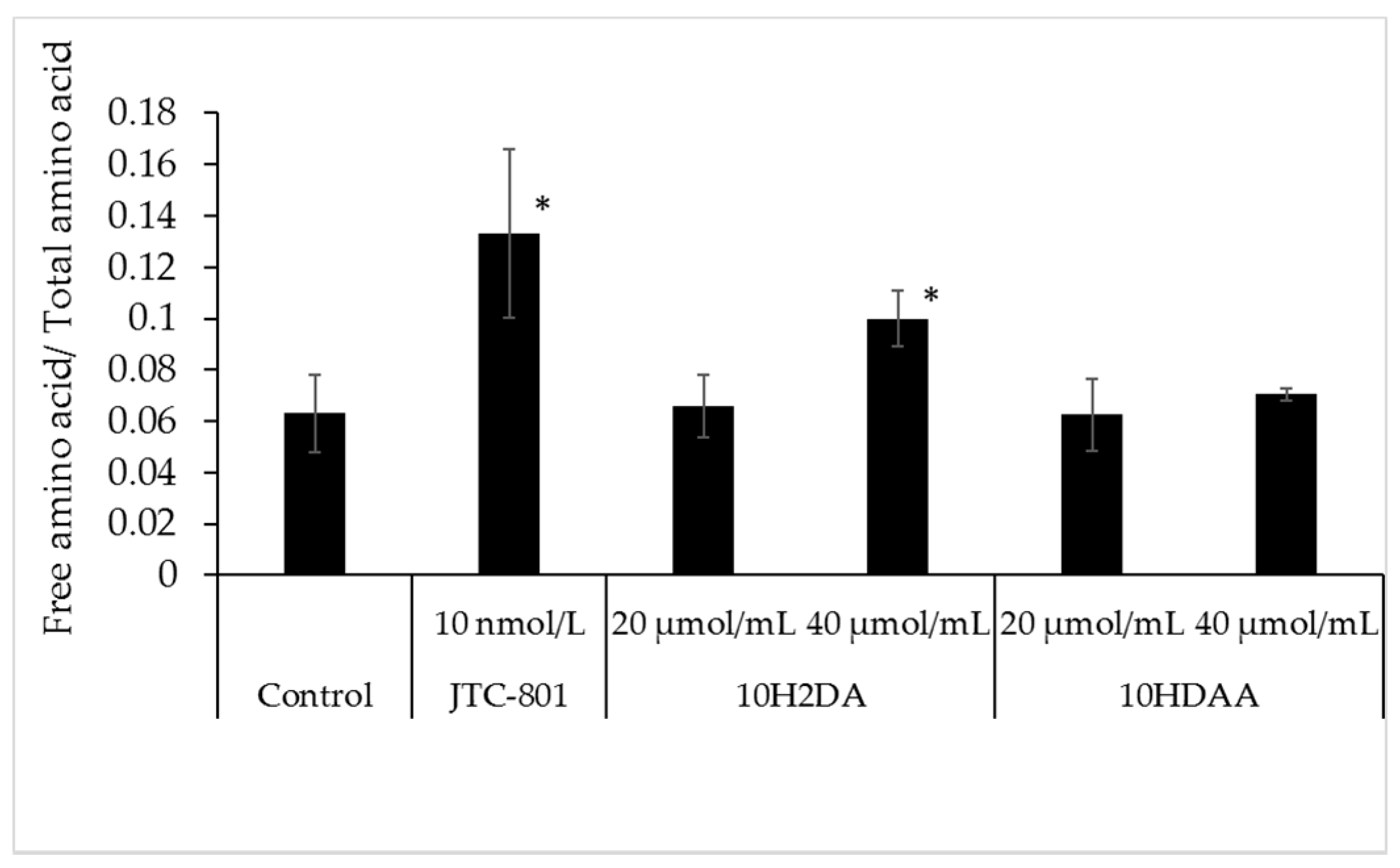
3.2. Effect of 10H2DA and 10HDAA on the Amino Acid Level in the Three-Dimensional Skin Model
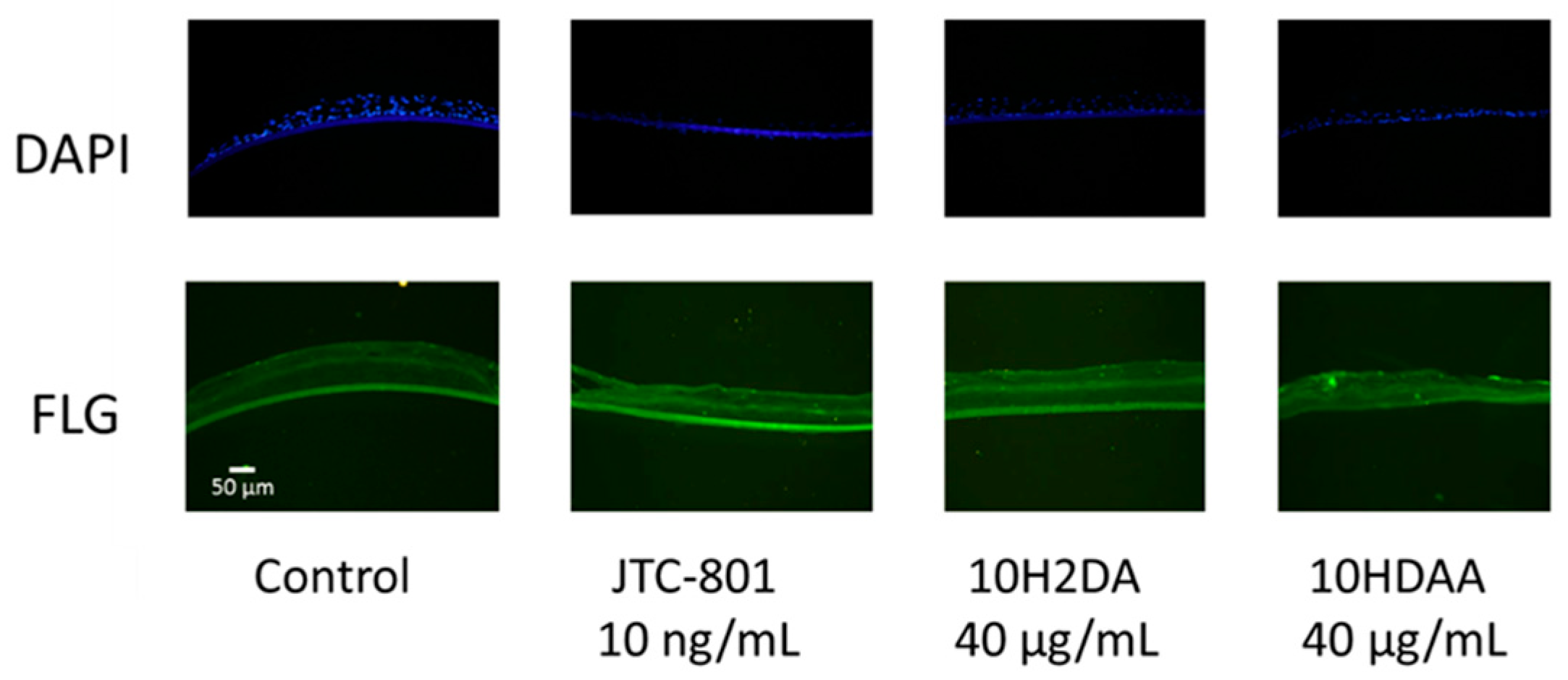
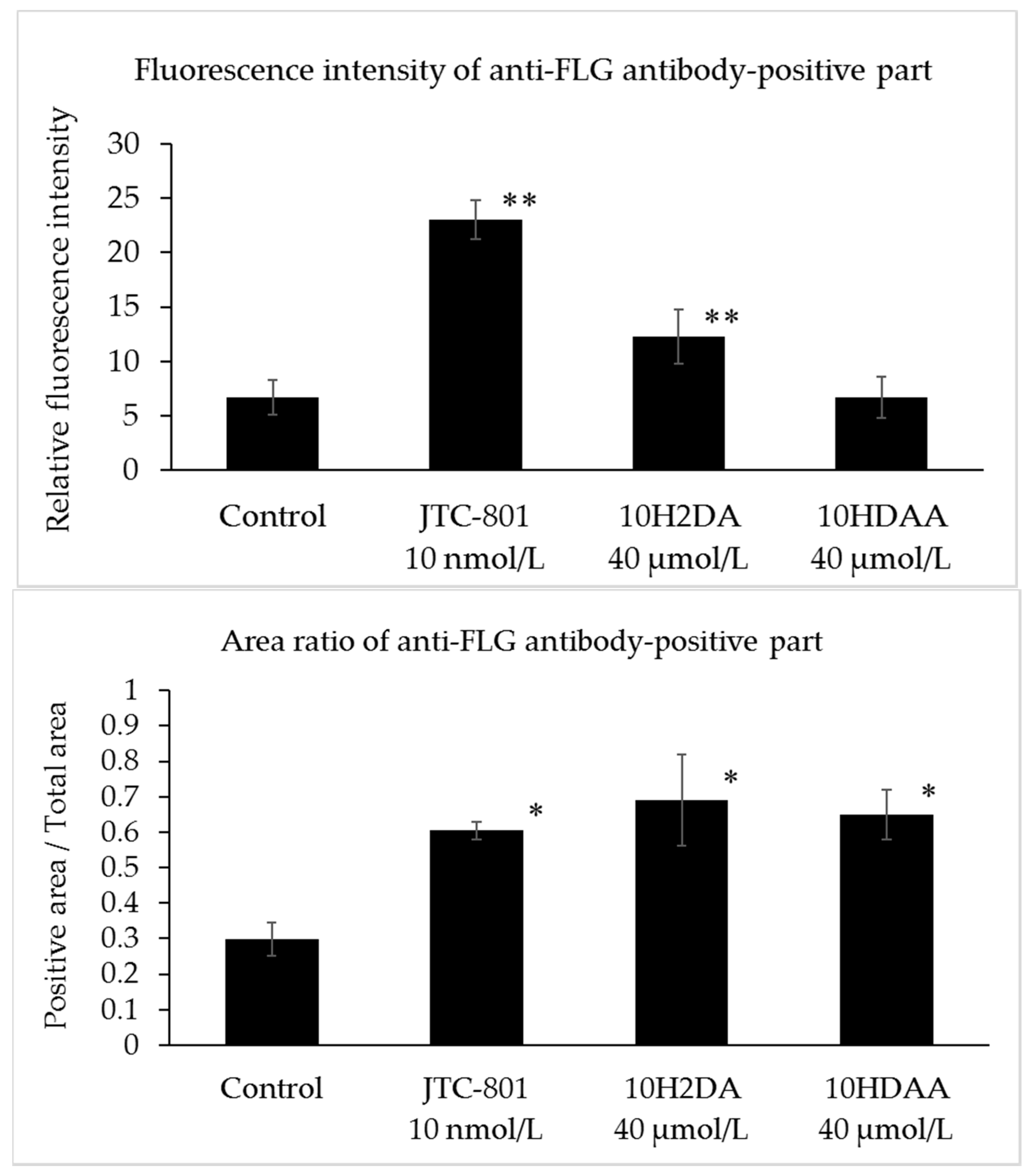
3.3. Immunohistochemical Staining of FLG in the Three-Dimensional Skin Model Treated with 10H2DA or 10HDAA
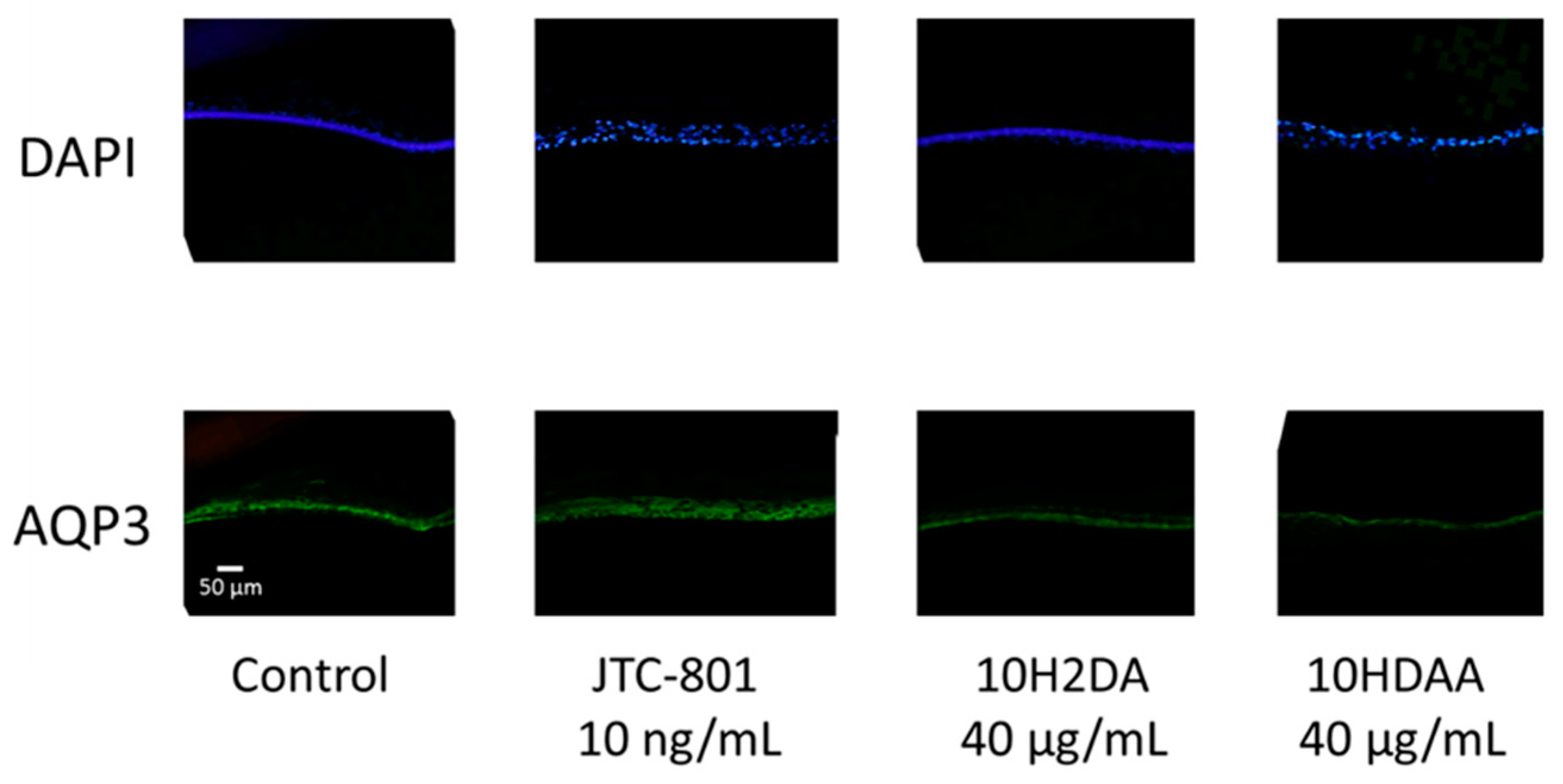
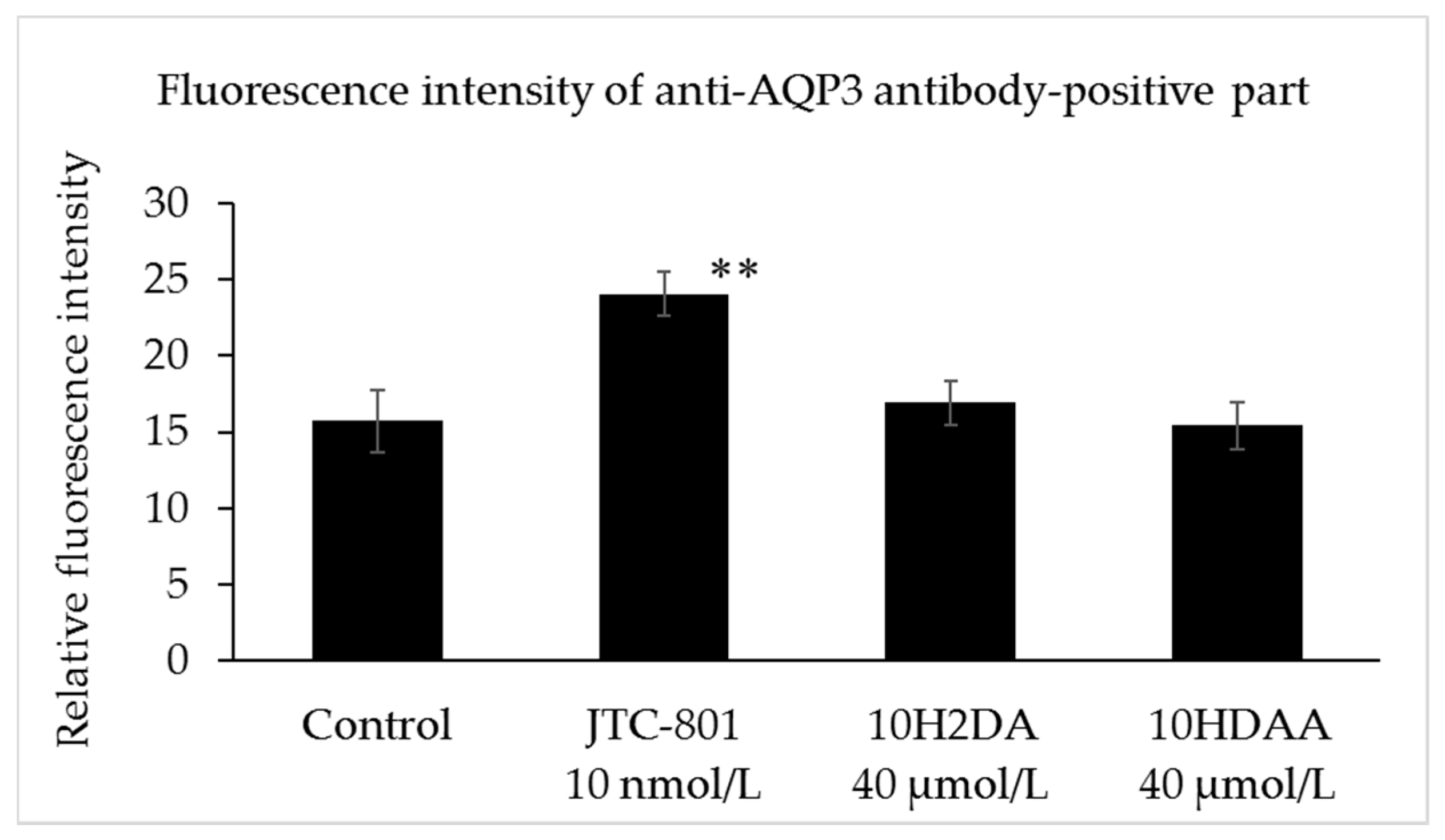
3.4. Immunohistochemical Staining of AQP3 in the Three-Dimensional Skin Model Treated with 10H2DA or 10HDAA
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Menon, G.K.; Norlén, L. Stratum corneum ceramides and their role in skin barrier function. In Skin Moisturization; Leyden, J.J., Rawlings, A.V., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 31–60. [Google Scholar]
- Tagami, H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch. Dermatol. Res. 2008, 300 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Kemperman, P.M.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Man, M.Q.; Hatano, Y.; Crumrine, D.; Gunathilake, R.; Sundberg, J.P.; Silva, K.A.; Mauro, T.M.; Hupe, M.; Cho, S.; et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritant and haptens. J. Allergy Clin. Immunol. 2009, 124, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.A.; Holbrook, K.A.; Steinert, P.M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature 1978, 276, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.; Taniguchi, A.; Yamamoto, M.; Nomura, J.; Ishihara, K.; Takahara, H.; Hibino, T.; Takeda, A. Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated FLG into amino acids. J. Biol. Chem. 2009, 284, 12829–12836. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.; Kemperman, P.; Devos, M.; Denecker, G.; Kezic, S.; Yau, N.; Gilbert, B.; Lippens, S.; De Groote, P.; Roelandt, R.; et al. Caspase-14 is required for FLG degradation to natural moisturizing factors in the skin. J. Investig. Dermatol. 2011, 131, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Irvine, A.D.; Terron-Kwiatkowski, A.; Sandilands, A.; Campbell, L.E.; Zhao, Y.; Liao, H.; Evans, A.T.; Goudie, D.R.; Lewis-Jones, S.; et al. Loss-of-function mutations in the gene encoding FLG cause ichthyosis vulgaris. Nat. Genet. 2006, 38, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Baurecht, H.; Herberich, E.; Wagenpfeil, S.; Brown, S.J.; Cordell, H.J.; Irvine, A.D.; Weidinger, S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. J. Allergy Clin. Immunol. 2009, 123, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Akiyama, M.; Sandilands, A.; Nemoto-Hasebe, I.; Sakai, K.; Nagasaki, A.; Ota, M.; Hata, H.; Evans, A.T.; Palmer, C.N.; et al. Specific FLG mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J. Investig. Dermatol. 2008, 128, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Sougrat, R.; Morand, M.; Gondran, C.; Barré, P.; Gobin, R.; Bonté, F.; Dumas, M.; Verbavatz, J.M. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Investig. Dermatol. 2002, 118, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Broberg, A.; Jernas, M.; Carlsson, L.; Rudemo, M.; Suurküla, M.; Svensson, P.A.; Benson, M. Increased expression of aquaporin 3 in atopic eczema. Allergy 2006, 61, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Nakahigashi, K.; Kabashima, K.; Ikoma, A.; Verkman, A.S.; Miyachi, Y.; Hara-Chikuma, M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J. Investig. Dermatol. 2011, 131, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Oribe, E.; Koshiishi, Y.; Tatefuji, T.; Hashimoto, K.; Akimoto, M.; Maeda, K. Effect of royal jelly extract on water holding ability and elasticity of forearm skin. Jpn. Aesthet. Dermatol. Symp. 2013, 6, 10–14. (In Japanese) [Google Scholar]
- Oribe, E. Effect of long term intake of enzyme-treated royal jelly in normal skin. Fine Chem. 2013, 42, 20–25. (In Japanese) [Google Scholar]
- Howe, S.R.; Dimick, P.S.; Benton, A.W. Composition of freshly harvested and commercial royal jelly. J. Apic. Res. 1985, 24, 52–61. [Google Scholar] [CrossRef]
- Blum, M.S.; Novak, A.F.; Taber, S., 3rd. 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science 1959, 130, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Fujii, A.; Kuboyama, N. Antitumor effects of royal jelly (RJ). Nihon Yakurigaku Zasshi 1987, 89, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sver, L.; Orsolić, N.; Tadić, Z.; Njari, B.; Valpotić, I.; Basić, I. A royal jelly as a new potential immunomodulator in rats and mice. Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 31–38. [Google Scholar] [CrossRef]
- Yang, X.Y.; Yang, D.S.; Zhang, W.; Wang, J.M.; Li, C.Y.; Ye, H.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; Wang, J.G. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Suzuki, K.M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid. Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nitta, Y.; Fukumitsu, H.; Soumiya, H.; Ikeno, K.; Nakamura, T.; Furukawa, S. Antidepressant-like activity of 10-hydroxy-trans-2-decenoic Acid, a unique unsaturated fatty acid of royal jelly, in stress-inducible depression-like mouse model. Evid. Based Complement. Altern. Med. 2012, 2012, 139140. [Google Scholar] [CrossRef] [PubMed]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid. Based Complement. Altern. Med. 2009, 6, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S.; et al. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Yoshimura, H. Effect of royal jelly on chills in Japanese young wamen. Jpn. Soc. Nutr. Food Sci. 2010, 63, 271–278. [Google Scholar] [CrossRef]
- Maeda, T.; Kuroda, H.; Motoyoshi, K. Effects of royal jelly and 10-hydroxy decenoic acid on the sebaceous glands of hamster ear. Nihon Hifuka Gakkai Zasshi 1988, 98, 469–475. (In Japanese) [Google Scholar] [PubMed]
- Taniguchi, Y.; Kohno, K.; Inoue, S.; Koya-Miyata, S.; Okamoto, I.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2003, 3, 1313–1324. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In vitro anti-Inflammatory effects of three fatty acids from royal jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [PubMed]
- Duplan, H.; Questel, E.; Hernandez-Pigeon, H.; Galliano, M.F.; Caruana, A.; Ceruti, I.; Ambonati, M.; Mejean, C.; Damour, O.; Castex-Rizzi, N.; et al. Effects of Hydroxydecine® (10-hydroxy-2-decenoic acid) on skin barrier structure and function in vitro and clinical efficacy in the treatment of UV-induced xerosis. Eur. J. Dermatol. 2011, 21, 906–915. [Google Scholar] [PubMed]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Doi, H.; Egawa, G.; Maekawa, A.; Fujita, T.; Nakamizo, S.; Nakashima, C.; Nakajima, S.; Watanabe, T.; Miyachi, Y.; et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J. Allergy Clin. Immunol. 2014, 133, 139–146. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Zeng, H.; Maeda, K. 10-Hydroxy-2-Decenoic Acid in Royal Jelly Extract Induced Both Filaggrin and Amino Acid in a Cultured Human Three-Dimensional Epidermis Model. Cosmetics 2017, 4, 48. https://doi.org/10.3390/cosmetics4040048
Gu L, Zeng H, Maeda K. 10-Hydroxy-2-Decenoic Acid in Royal Jelly Extract Induced Both Filaggrin and Amino Acid in a Cultured Human Three-Dimensional Epidermis Model. Cosmetics. 2017; 4(4):48. https://doi.org/10.3390/cosmetics4040048
Chicago/Turabian StyleGu, Lihao, Haifeng Zeng, and Kazuhisa Maeda. 2017. "10-Hydroxy-2-Decenoic Acid in Royal Jelly Extract Induced Both Filaggrin and Amino Acid in a Cultured Human Three-Dimensional Epidermis Model" Cosmetics 4, no. 4: 48. https://doi.org/10.3390/cosmetics4040048




