Large Melanosome Complex Is Increased in Keratinocytes of Solar Lentigo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biopsies of SL Lesional and Perilesional Skin
2.2. Observations of Melanosomes in Keratinocytes Using Transmission Electron Microscopy (TEM)
2.3. Tyramide-Based TYR Assay
2.4. Immunohistochemistry
2.5. Semi-Quantitative mRNA Analyses
2.6. Enhanced Immunostaining of KIT in the Skin
2.7. Statistical Analysis
3. Results
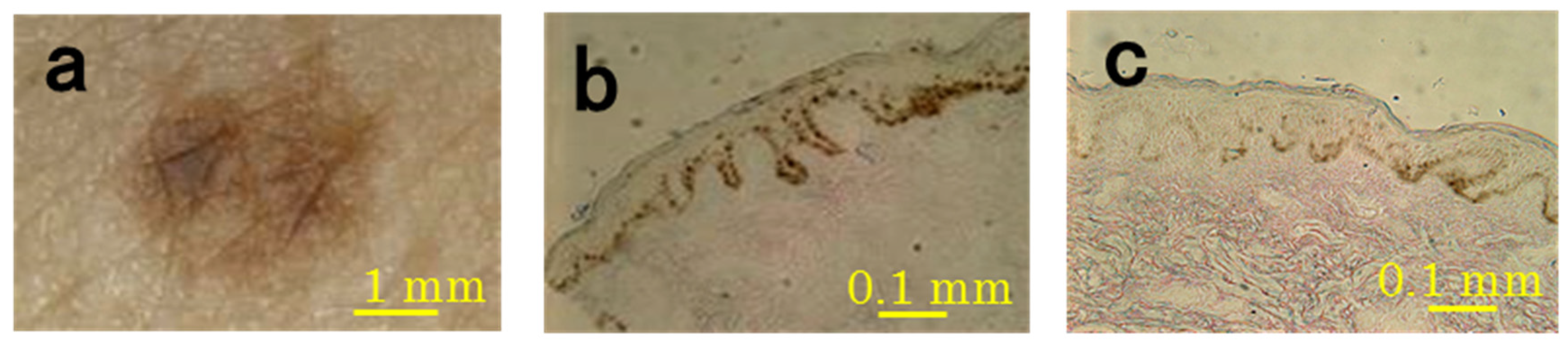
3.1. Microscopic and Histological Observations
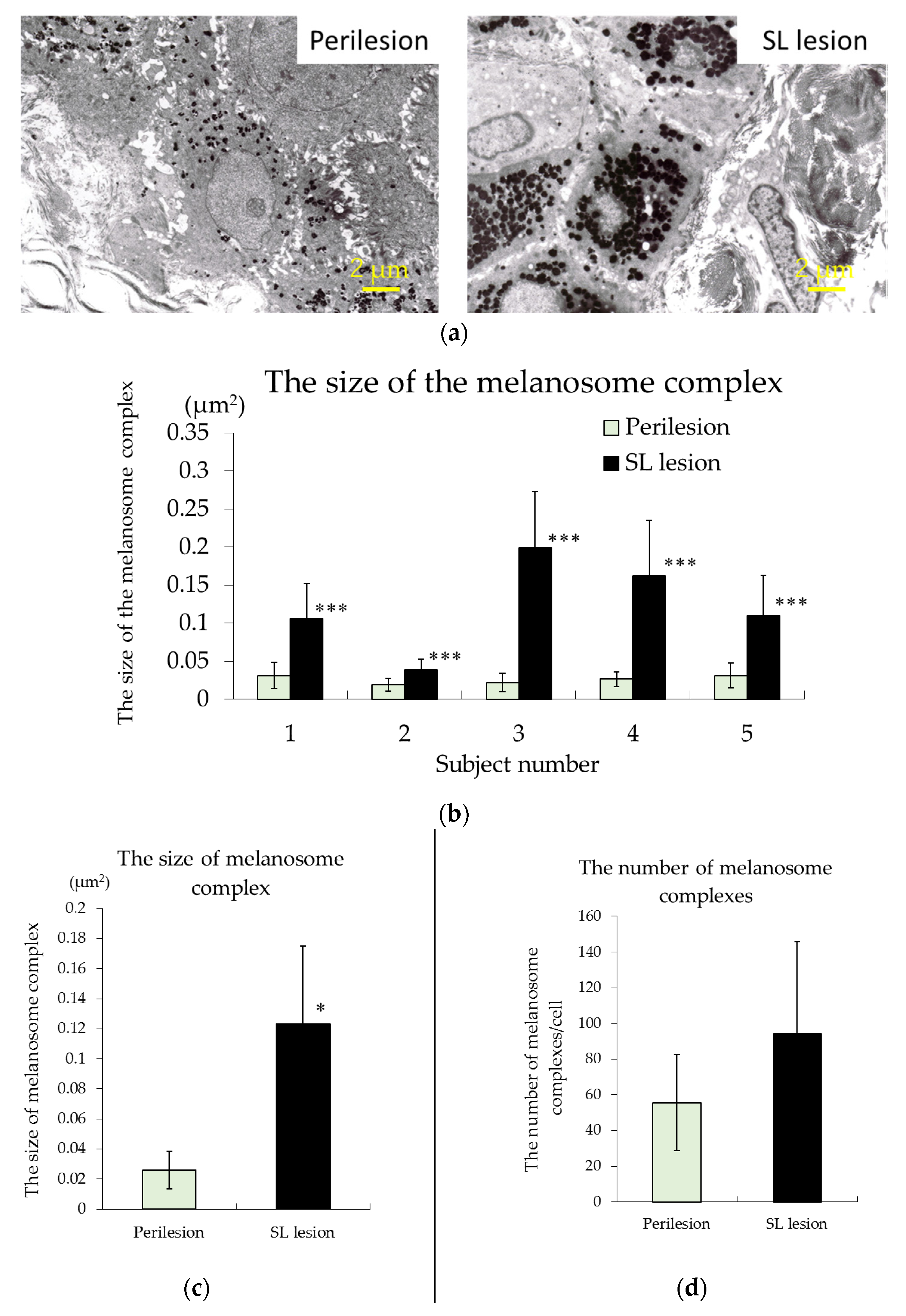
3.2. TEM Observations
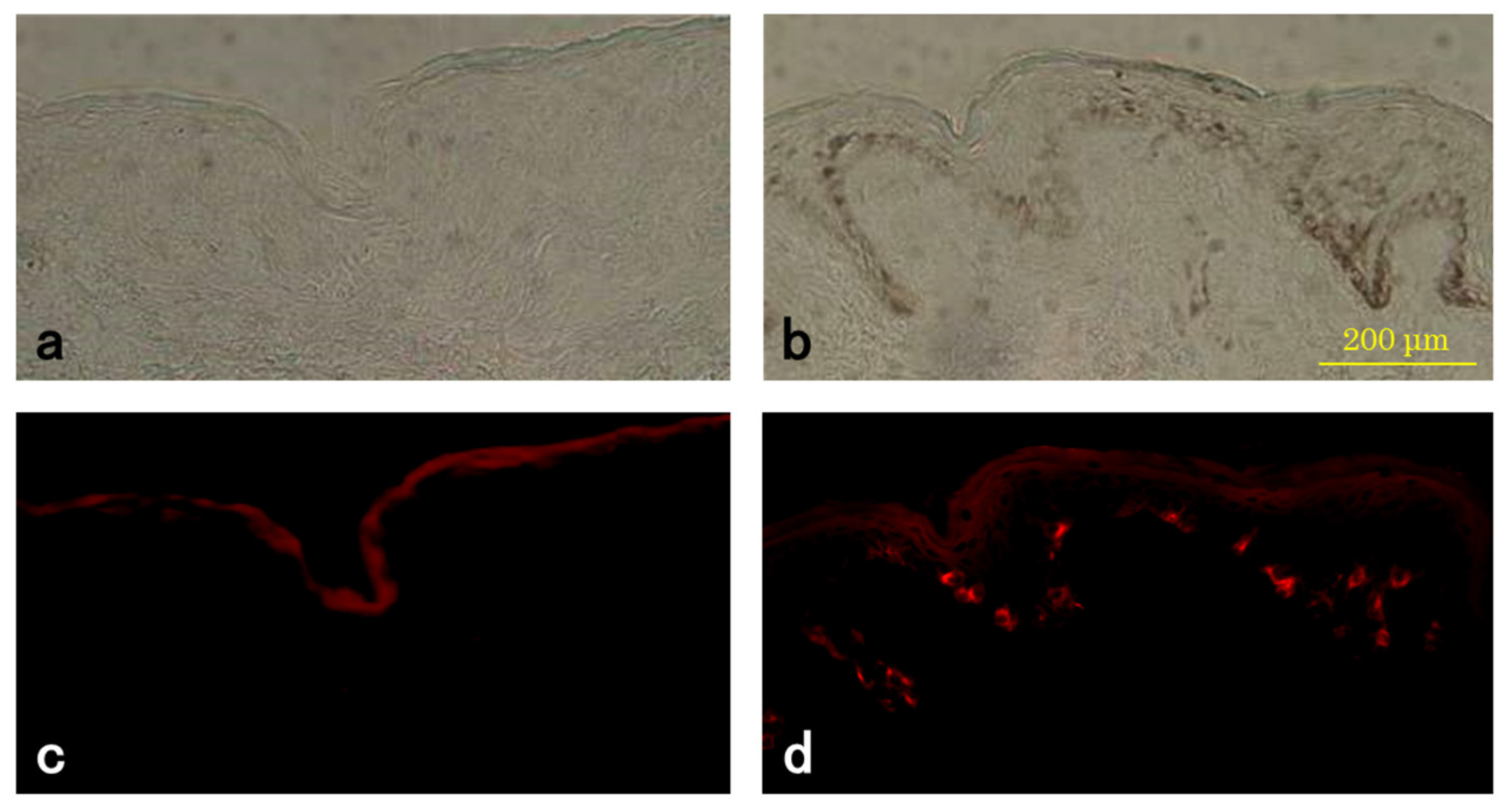
3.3. TYR Activity of Melanocytes
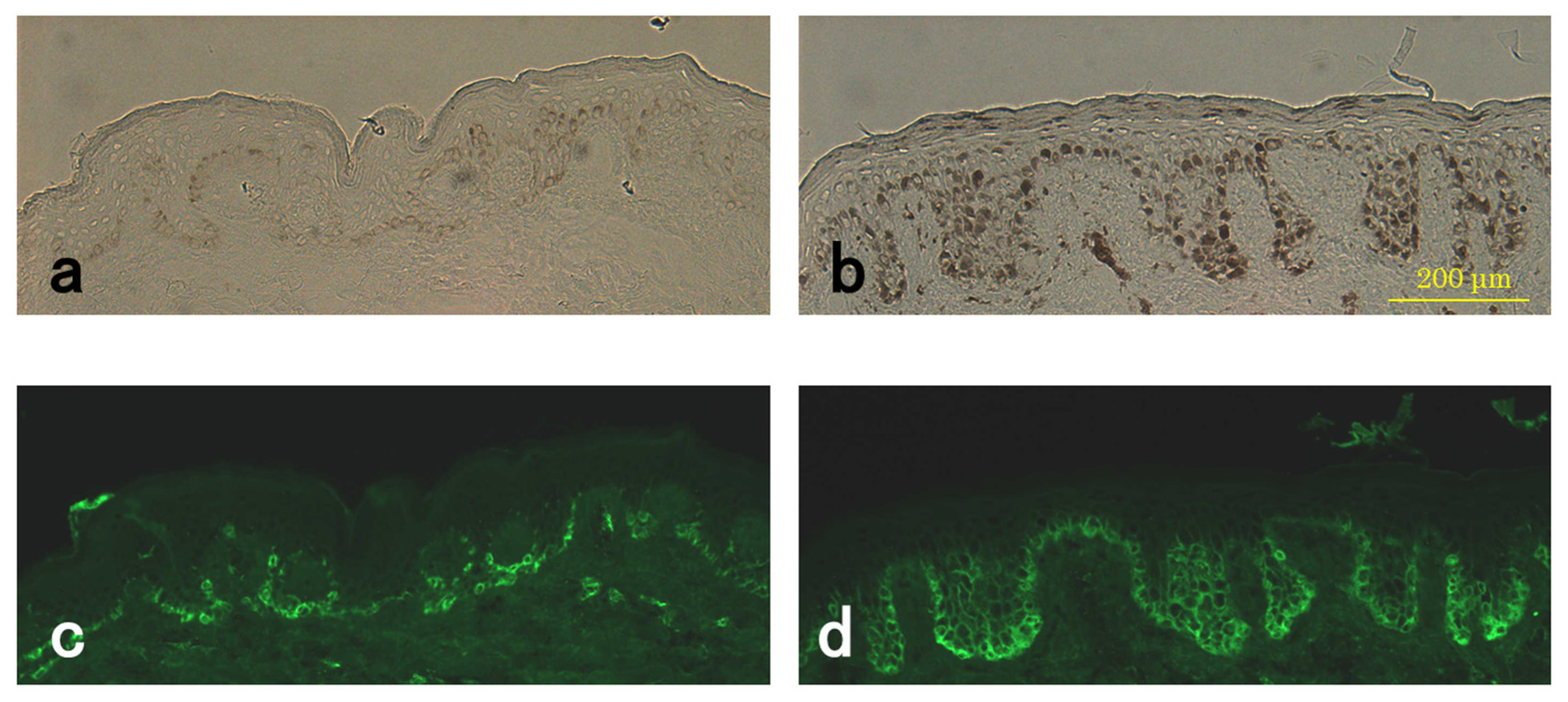
3.4. Level of Pmel17 in Melanocytes
3.5. mRNA Levels of TYR, MITF-m, KIT, BCL-2, and SCF
3.6. Enhanced Immunostaining of KIT in the Skin
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bastiaens, M.; Hoefnagel, J.; Westendorp, R.; Vermeer, B.J.; Bouwes Bavinck, J.N. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; ter Huurne, J.; Gruis, N.; Bergman, W.; Westendorp, R.; Vermeer, B.J.; Bavinck, J.N.B. The melanocortin-1-receptor gene is the major freckle gene. Hum. Mol. Genet. 2001, 10, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Miescher, G.; Haberlin, L.; Guggenheim, L. Uber fleckformige Alterspingmentierungen: Ihre Beziehungen zur melanotischen pracancerose und zur senilen Warze. Arch. Dermatol. Syph. 1936, 174, 105–125. [Google Scholar] [CrossRef]
- Andersen, W.K.; Labadie, R.R.; Bhawan, J. Histopathology of solar lentigines of the face: A semi-quantitative study. J. Am. Acad. Dermatol. 1997, 36, 444–447. [Google Scholar] [CrossRef]
- Hodgson, C. Senile lentigo. Arch. Dermatol. 1968, 87, 197–207. [Google Scholar] [CrossRef]
- Montagna, W.; Hu, F.; Carlisle, K. A reinvestigation of solar lentigines. Arch. Dermatol. 1980, 116, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Kadono, S.; Manaka, I.; Kawashima, M.; Kobayashi, T.; Imokawa, G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J. Investig. Dermatol. 2001, 116, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Cario-Andre, M.; Lepreux, S.; Pain, C.; Nizard, C.; Noblesse, E.; Taieb, A. Perilesional vs. lesional skin changes in senile lentigo. J. Cutan. Pathol. 2004, 31, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Noblesse, E.; Nizard, C.; Cario-Andre, M.; Lepreux, S.; Pain, C.; Schnebert, S.; Taïeb, A.; Kurfurst, R. Skin ultrastructure in senile lentigo. Skin Pharmacol. Physiol. 2006, 19, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Mehregan, A.H. Lentigo senilis and its evolutions. J. Investig. Dermatol. 1975, 65, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Hölzle, E. Pigmented lesions as a sign of photodamage. Br. J. Dermatol. 1992, 127 (Suppl. 41), 48–50. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Rhodes, A.R.; Momtaz-T, K.; Fitzpatrick, T.B. Morphologic alterations of epidermal melanocytes and melanosomes in PUVA lentigines: A comparative ultrastructural investigation of lentigines induced by PUVA and sunlight. J. Investig. Dermatol. 1984, 82, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Berson, J.F.; Harper, D.C.; Tenza, D.; Raposo, G.; Marks, M.S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell 2001, 12, 3451–3464. [Google Scholar] [CrossRef] [PubMed]
- Tsuura, Y.; Hiraki, H.; Watanabe, K.; Igarashi, S.; Shimamura, K.; Fukuda, T.; Suzuki, T.; Seito, T. Preferential localization of c-kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminoma/dysgerminoma in human: Immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Virchows Arch. 1994, 424, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Simak, R.; Capodieci, P.; Cohen, D.W.; Fair, W.R.; Scher, H.; Melamed, J.; Drobnjak, M.; Heston, W.D.; Stix, U.; Steiner, G.; et al. Expression of c-kit and kit-ligand in benign and malignant prostatic tissues. Histol. Histopathol. 2000, 15, 365–374. [Google Scholar] [PubMed]
- Han, R.; Baden, H.P.; Brissette, J.L.; Weiner, L. Redefining the skin’s pigmentary system with a novel tyrosinase assay. Pigment Cell Res. 2002, 15, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Hoashi, T.; Muller, J.; Vieira, W.D.; Rouzaud, F.; Kikuchi, K.; Tamaki, K.; Hearing, V.J. The repeat domain of the melanosomal matrix protein PMEL17/GP100 is required for the formation of organellar fibers. J. Biol. Chem. 2006, 281, 21198–21208. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Takeda, K.; Nobukuni, Y.; Urabe, K.; Long, J.E.; Meyers, K.A.; Aaronson, S.A.; Miki, T. Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat. Genet. 1996, 14, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Udono, T.; Yasumoto, K.; Takeda, K.; Amae, S.; Watanabe, K.; Saito, H.; Fuse, N.; Tachibana, M.; Takahashi, K.; Tamai, M.; et al. Structural organization of the human microphthalmia-associated transcription factor gene containing four alternative promoters. Biochim. Biophys. Acta 2000, 1491, 205–219. [Google Scholar] [CrossRef]
- Du, J.; Miller, A.J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Fisher, D.E. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol. 2003, 163, 333–343. [Google Scholar] [CrossRef]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar] [PubMed]
- Grichnik, J.M.; Ali, W.N.; Burch, J.A.; Byers, J.D.; Garcia, C.A.; Clark, R.E.; Shea, C.R. KIT expression reveals a population of precursor melanocytes in human skin. J. Investig. Dermatol. 1996, 106, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Otaki, N.; Seiji, M. Degradation of melanosomes by lysosomes. J. Investig. Dermatol. 1971, 57, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Schwemberger, S.; Babcock, G.; Hakozaki, T.; Boissy, R.E. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Investig. Dermatol. 2011, 131, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Harashima, A.; Kato, K.; Gu, L.; Motomura, Y.; Otsuka, R.; Maeda, K. Degradation of tyrosinase by melanosomal pH change and a new mechanism of whitening with propylparaben. Cosmetics 2017, 4, 43. [Google Scholar] [CrossRef]
- Thong, H.Y.; Jee, S.H.; Sun, C.C.; Boissy, R.E. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br. J. Dermatol. 2003, 149, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Chabot, B.; Stephenson, D.A.; Chapman, V.M.; Besmer, P.; Bernstein, A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 1988, 335, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ashman, L.K.; Du, Z.; Schwartz, L.B. Internalization of Kit together with stem cell factor on human fetal liver-derived mast cells: New protein and RNA synthesis are required for reappearance of Kit. J. Immunol. 1996, 156, 3443–3449. [Google Scholar] [PubMed]
- Grichnik, J.M.; Burch, J.A.; Burchette, J.; Shea, C.R. The SCF/KIT pathway plays a critical role in the control normal human melanocytes homeostasis. J. Investig. Dermatol. 1998, 111, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Tobin, D.J.; Botchkareva, N.; Maurer, M.; Paus, R. Migration of melanoblasts into the developing murine hair follicle is accompanied by transient c-Kit expression. J. Histochem. Cytochem. 2002, 50, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Maurer, M.; Botchkarev, V.A.; Jensen, K.D.; Welker, P.; Scot, G.A.; Paus, R. Kit is expressed by epithelial cells in vivo. J. Investig. Dermatol. 2003, 121, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.; Da Silva, C.A.; Frossard, N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur. J. Pharmacol. 2006, 533, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Rönnstrand, L. Signal transduction via the stem cell factor receptor/c-Kit. Cell. Mol. Life Sci. 2004, 61, 2535–2548. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, T.; Hashimoto, K.; Kitayama, H.; Ikeda, H.; Sugahara, H.; Matsumura, I.; Kaisho, T.; Terada, N.; Kitamura, Y.; Kanakura, Y. Activating mutation in the catalytic domain of c-kit elicits hematopoietic transformation by receptor self-association not at the ligand-induced dimerization site. Blood 1999, 93, 1319–1329. [Google Scholar] [PubMed]
- Nishida, T.; Hirota, S.; Taniguchi, M.; Hashimoto, K.; Isozaki, K.; Nakamura, H.; Kanakura, Y.; Tanaka, T.; Takabayashi, A.; Matsuda, H.; et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat. Genet. 1998, 19, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, K.; Terris, B.; Belghiti, J.; Schiffmann, S.; Hirota, S.; Vanderwinden, J.M. Germline-activating mutation in the kinase domain of KIT gene in familial gastrointestinal stromal tumors. Am. J. Pathol. 2000, 157, 1581–1585. [Google Scholar] [CrossRef]
- Beghini, A.; Tibiletti, M.G.; Roversi, G.; Chiaravalli, A.M.; Serio, G.; Capella, C.; Larizza, L. Germline mutation in the juxtamembrane domain of the kit gene in a family with gastrointestinal stromal tumors and urticaria pigmentosa. Cancer 2001, 92, 657–662. [Google Scholar] [CrossRef]
- Hattori, H.; Kawashima, M.; Ichikawa, Y.; Imokawa, G. The epidermal stem cell factor is over-expressed in lentigo senilis: Implication for the mechanism of hyperpigmentation. J. Investig. Dermatol. 2004, 122, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, T.; Lu, S.Z.; Yoshida, H.; Nishikawa, S.; Nishikawa, S.; Mizoguchi, M.; Hayashi, S.; Tyrrell, L.; Williams, D.A.; Wang, X.; et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J. Exp. Med. 1998, 187, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Grichnik, J.M.; Crawford, J.; Jimenez, F.; Kurtzberg, J.; Buchanan, M.; Blackwell, S.; Clark, R.E.; Hitchcock, M.G. Human recombinant stem-cell factor induces melanocytic hyperplasia in susceptible patients. J. Am. Acad. Dermatol. 1995, 33, 577–583. [Google Scholar] [CrossRef]

| mRNA | Relative Expression of mRNA | |
|---|---|---|
| Perilesion | SL Lesion (Mean ± Standard Deviation) | |
| TYR | 1.0 | 4.9 ± 2.1 |
| MITF-m | 1.0 | 2.5 ± 1.4 |
| KIT | 1.0 | 3.6 ± 0.9 |
| BCL-2 | 1.0 | 1.0 ± 0.5 |
| SCF | 1.0 | 1.7 ± 1.2 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, K. Large Melanosome Complex Is Increased in Keratinocytes of Solar Lentigo. Cosmetics 2017, 4, 49. https://doi.org/10.3390/cosmetics4040049
Maeda K. Large Melanosome Complex Is Increased in Keratinocytes of Solar Lentigo. Cosmetics. 2017; 4(4):49. https://doi.org/10.3390/cosmetics4040049
Chicago/Turabian StyleMaeda, Kazuhisa. 2017. "Large Melanosome Complex Is Increased in Keratinocytes of Solar Lentigo" Cosmetics 4, no. 4: 49. https://doi.org/10.3390/cosmetics4040049





