Optimized Culture Conditions for the Detection of Selected Strains of Bacillus in Eye Creams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cosmetic Samples:
2.2. Preparation of Bacterial Strains
2.3. Sample Preparation and Inoculation
2.4. Enumeration of Bacillus
2.5. Testing for Protein Crystals in the Mislabeled Strain Using Two Staining Methods
2.6. Determination of Cry Genes in Purported B. Megaterium, Using PCR
2.7. Statistical Analysis
3. Results and Discussion
3.1. Challenge in Products without Neutralization
3.2. Challenge Organisms in Products B, C, D Neutralized with T80, TS and T20
3.3. Neutralizers
3.4. Comparing the Effects of Neutralizers by Products
3.5. Best Diluting Broth Used in Conjunction with the Neutralizer T80
3.6. Best Plating Media for Use with T80
3.7. Identification of Mislabeled Strain of Megaterium
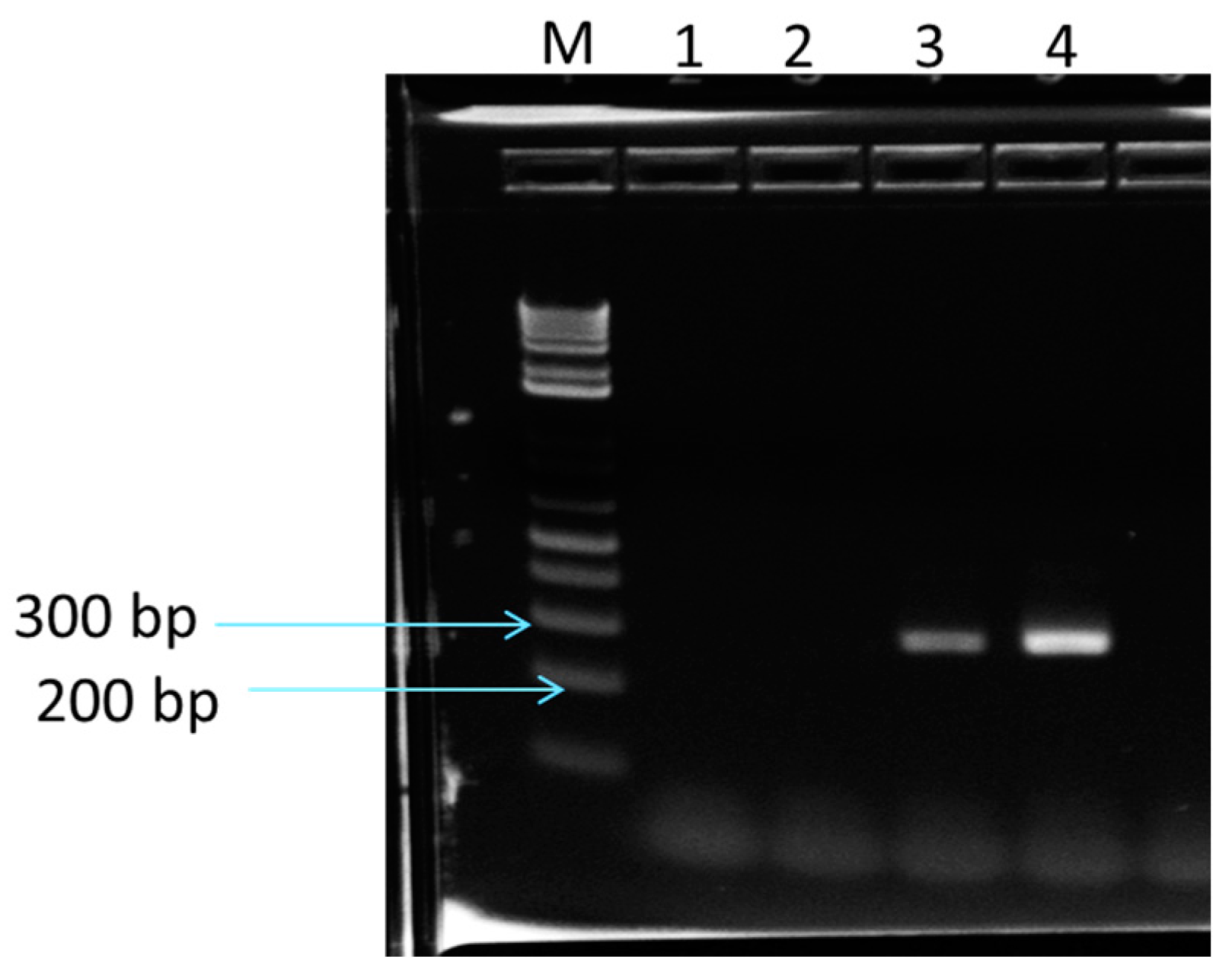
3.8. Determination of Cry Genes by PCR
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, S.; Street, D.; Delgado, S.I.; Klontz, K.C. Recalls of foods and cosmetics due to microbial contamination reported to the U.S. Food and drug Administration. J. Food Prot. 2000, 63, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Neza, E.; Centini, M. Microbiologically contaminated and over-preserved cosmetic products according Rapex 2008–2014. Cosmetics 2016, 3, 3. [Google Scholar] [CrossRef]
- Sutton, S.V.W. Antimicrobial preservative efficacy and microbial content testing. In Cosmetic Microbiology: A Practical Approach, 2nd ed.; Geis, P.A., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- EFSA Panel on biological hazards (BIOHAZ). Scientific opinion on the risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, 93. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony Count Technique at 30 °C; Beuth Verlang Gmbh: Köln, Germany, 2005. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Determination of Low Numbers of Presumptive Bacillus cereus—Most Probable Number Technique and Detection Method (ISO21871:2006); Beuth Verlang Gmbh: Köln, Germany, 2006. [Google Scholar]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Moyer, L.A.; Ramadan, R.T.; Novosad, B.D.; Callegan, M.C. Bacillus cereus—Induced permeability of the blood ocular barrier during experimental endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Donzis, P.B.; Mondino, B.J.; Weisman, B.A. Bacillus keratitis with contaminated contact lens case system. Am. J. Ophthaimol. 1988, 105, 194–197. [Google Scholar]
- Van Setten, G.B.; Tervo, T.; Tarkkanen, A. Acute keratitis and contamination of contact lens care systems with B. cereus. Klin. Monbl. Augenheilkd. 1989, 195, 28–31. [Google Scholar] [PubMed]
- Pinna, A.; Sechi, L.A.; Zanetti, S.; Usai, D.; Delogu, G.; Cappuccinelli, P.; Carta, F. Bacillus cereus keratitis associated with contact lens wear. Ophthalmology 2001, 108, 1830–1834. [Google Scholar] [CrossRef]
- Gemdo Cosmetics, Inc. 4/16/15. Available online: https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm443701.htm (accessed on 12 December 2017).
- Import Alert 53-17. Available online: https://www.accessdata.fda.gov/cms_ia/importalert_136.html (accessed on 12 December 2017).
- Pitt, T.L.; McClure, J.; Parker, M.D.; Amézouita, A.; McClure, P.J. Bacillus cereus in personal care products: Risk to consumers. Int. J. Cosmet. Sci. 2015, 37, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.M. Surfactant Chemistry and Classification. In Surfactants in Cosmetics, 2nd ed.; Rieger, M., Rhein, L.D., Eds.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Orth, D.S. Inactivation of Preservatives by Surfactants. In Surfactants in Cosmetics, 2nd ed.; Rieger, M., Rhein, L.D., Eds.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- English, D.J. Factors in selecting and testing preservatives in product. In Cosmetic and Drug Microbiology; Orth, D.S., Kabara, J.J., Denyer, S.P., Tan, S.K., Eds.; Informa Healthcare: New York, NY, USA, 2006. [Google Scholar]
- Frentzel, H. Determination, Characterization and Survival of Bacillus cereus Group Members in Spices and Herbs. Ph.D. Thesis, Freien Universität Berlin, Berlin, Germany, June 2017. [Google Scholar]
- Cosmetic, Toiletry and Fragrance Association. Available online: http://cosmetictestlabs.com/ctfa_m-4_summary.html (accessed on 16 February 2015).
- BAM Appendix 2: Most Probable Number from Serial Dilutions. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm109656 (accessed on 13 December 2017).
- FDA. Bacteriological Analytical Manuel (BAM). Bacillus cereus. Available online: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070875.htm (accessed on 13 December 2017).
- Ammons, D.; Rampersad, J.; Khan, A. Usefulness of staining parasporal bodies when screening for Bacillus thuringiensis. J. Invertebr. Pathol. 2002, 79, 203–204. [Google Scholar] [PubMed]
- Voundi, S.O.; Nyegue, M.; Lazar, I.; Raducanu, D.; Foe Ndoye, F.; Stamate, M.; Etoa, F.-X. Effect of essential oils on germination and growth of some pathogenic and spoilage spore-forming bacteria. Foodborne Pathog. Dis. 2015, 12, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Nazarro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Hayley, A.L.; Palombo, E.A. Activity of essential oils against Bacillus subtilis spores. J. Microbiol. Biotechnol. 2009, 19, 1590–1595. [Google Scholar]
- Geis, P.A. Common cosmetic preservatives. In Cosmetic Microbiology: A Practical Approach, 2nd ed.; Geis, P.A., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ibarra, F.; Johnson, C. Natural Preservation from Concepts in Nature—Microorganisms and Cosmetics; Allured Books: Carol Stream, IL, USA, 2009. [Google Scholar]
- Sox, T.E. Mechanisms of action of cosmetic preservatives. In Cosmetic Microbiology: A Practical Handbook; Brannan, D.K., Ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Cummings, P.; Goldstein, M. Preservatives boosters: Up against the wall. In Cosmetic Microbiology: A Practical Approach, 2nd ed.; Geis, P.A., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Marx, H.; Sabalitschka, T.; Boehm, E. Behavior of antimicrobial materials in nonionic systems. Am. Perfum. Cosmet. 1968, 83, 39–47. [Google Scholar]
- Yossa, N.; Arce, G.; Huang, M.-C.J.; Yin, L.; Brown, E.; Hammack, T. Factors of detection of Bacillus cereus strains in eye cream. Int. J. Cosmet. Sci. 2017, 39, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wedderburn, D.L. Preservation of toilet preparations containing nonionics. J. Soc. Cosmet. Chem. 1957, 210–228. [Google Scholar]
- Sharaf, E.F.; El-Sayed, W.S.; Abosaif, R.M. Lecithicinase-producing bacteria in commercial and home-made foods: Evalustion of toxic propertis and identification of potent producers. J. Taibah Univ. Sci. 2014, 8, 207–215. [Google Scholar] [CrossRef]
- Kushner, D.J. The effect of alcohols on the synthesis of lecithinase by Bacillus cereus. Nature 1957, 179, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Kushner, D.J. The effect of alcohols on the synthesis of lipase, lecithinase and other enzymes by Bacillus cereus. Biochem. J. 1960, 75, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Tallent, S.M.; Kotewicz, K.M.; Strain, E.A.; Bennett, R.W. Efficient isolation and identification of Bacillus cereus group. J. AOAC Int. 2012, 95, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.I.; Fritz-James, P. Structures and morphogenesis of the bacterial pore coat. Bacteriol. Rev. 1976, 40, 360–402. [Google Scholar] [PubMed]
- Ohba, M.; Aizawa, K. Distribution of Bacillus thuringiensis in soils of Japan. J. Invertebr. Pathol. 1986, 47, 277–282. [Google Scholar] [CrossRef]
- Federici, B.A.; Luethy, P.; Ibarra, J.E. Parasporal Body of Bacillus thuringiensis Subs. Israelensis: Structure, Protein Composition and Toxicity. In Bacterial Control of Mosquitos and Blackflies: Biochemistry, Genetics and Applications of Bacillus thuringiensis and Bacillus sphaericus; de Barjac, H., Sutherland, D.J., Eds.; Rutgers University Press: New Brunswick, NJ, USA, 1990. [Google Scholar]
- Herrnstand, C.H.; Soares, G.G.; Wilcox, E.R.; Edwards, D.I. A new strain of Bacillus thuringiensis with activity against coleopteran insects. Nat. Biotechnol. 1986, 4, 305–308. [Google Scholar] [CrossRef]
- Lopez-meza, J.E.; Ibarra, J.E. Characterization of a novel strain of Bacillus thuringiensis. Appl. Environ. Microbiol. 1996, 62, 1306–1310. [Google Scholar] [PubMed]
- Arrieta, G.; Espinoza, H.A. Characterization of Bacillus thuringiensis strain collection isolated from diverse Costa Rican natural ecosystems. Rev. Biol. Trop. 2006, 54, 13–27. [Google Scholar] [CrossRef] [PubMed]


| Product B | Product C (Organic) | Product D |
|---|---|---|
| Bisabolol | Aloe barbadensis | Camellia sinensis leaf |
| Diazolidinyl Urea | Aspalathus lineans | Citric acid |
| Methylparaben | Calendula officinalis | Potassium sorbate |
| Propylparaben | Citric acid | Sodium benzoate |
| Lavendula angustifolia | ||
| Oenotheris biennis | ||
| Olea europea | ||
| Punica granatum | ||
| Rhodiola Roots | ||
| Simmondsia chinensis |
| Bacillus spp. | Strain ID |
|---|---|
| B. cereus | F 4227 A |
| B. cereus | F 60006 |
| B. cereus | ATCC 14579 |
| B. mycoides | ATCC 6264 |
| B. thuringiensis | ATCC 35866 |
| B. megaterium | ATCC 6458 |
| B. subtilis | ATCC 15563 |
| Strains | B | C* | D |
|---|---|---|---|
| cereus ATCC 14579 | 1.99 ± 0.83 | NG | 0.13 ± 0.23 |
| cereus F 60006 | 2.56 ± 0.80 | 0.03 ± 0.05 | 0.87 ± 1.50 |
| cereus F4227A | 2.68 ±0.43 | 0.05 ± 0.05 | 1.44 ± 1.72 |
| megaterium ATCC 6458 | 2.39 ± 0.89 | NG | 3.98 ± 1.07 |
| mycoides ATCC 6462 | 3.32 ± 1.09 | NG | 1.69 ± 1.56 |
| subtilis ATCC 15563 | 2.69 ± 2.35 | 0.03 ± 0.05 | 1.18 ± 2.04 |
| thuringiensis ATCC 35866 | 1.75 ± 1.14 | NG | 1.59 ±1.50 |
| Type 3 Tests of Fixed Effects | ||
|---|---|---|
| Effect | F Value | Pr > F |
| Product | 99.83 | <0.0001 |
| Neutralizer | 183.03 | <0.0001 |
| Bacteria | 0.03 | 0.9998 |
| Broth | 10.73 | 0.0011 |
| Media | 19.42 | <0.0001 |
| Neutralizers | Strains | Broth | B | C | D | |||
|---|---|---|---|---|---|---|---|---|
| BACARA | MYP | BACARA | MYP | BACARA | MYP | |||
| T80 | 4227 | MLB | 2.0 ± 1.9 | 3.5 ± 0.4 | 3.4 ± 0.6 | 4.1 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2 |
| TAT | 2.1 ± 1.5 | 3.5 ± 0.3 | 3.6 ± 0.6 | 4.1 ± 0.5 | 5.0 ± 0.3 | 4.9 ± 0.3 | ||
| 60006 | MLB | 2.1 ± 1.9 | 3.6 ± 0.6 | 3.9 ± 0.7 | 4.4 ± 0.6 | 5.2 ± 0.0 | 5.2 ± 0.0 | |
| TAT | 2.7 ± 1.7 | 3.3 ± 0.2 | 4.1 ± 0.8 | 4.5 ± 0.7 | 5.2 ± 0.0 | 5.2 ± 0.0 | ||
| 14579 | MLB | 3.9 ± 0.1 | 3.9 ± 0.2 | 3.5 ± 0.6 | 4.3 ± 0.4 | 5.3 ± 0.1 | 5.3 ± 0.0 | |
| TAT | 4.3 ± 0.4 | 4.1 ± 0.5 | 4.1 ± 0.7 | 4.4 ± 0.6 | 5.2 ± 0.2 | 5.3 ± 0.0 | ||
| 6458 | MLB | 2.5 ± 2.1 | 3.9 ± 0.2 | 2.8 ± 2.1 | 3.0 ± 2.3 | 5.2 ± 0.1 | 5.1 ± 0.0 | |
| TAT | 3.2 ± 1.7 | 4.0 ± 0.1 | 2.9 ± 1.7 | 3.9 ± 0.8 | 5.2 ± 0.1 | 5.0 ± 0.2 | ||
| 6264 | MLB | 3.4 ± 0.5 | 3.7 ± 0.5 | 2.7 ± 2.4 | 3.0 ± 2.6 | 5.0 ± 0.4 | 5.0 ± 0.5 | |
| TAT | 3.4 ± 1.5 | 3.8 ± 0.8 | 3.8 ± 0.4 | 4.0 ± 0.9 | 5.1 ± 0.4 | 5.1 ± 0.4 | ||
| 15563 | MLB | NG | 3.8 ± 0.5 | NG | 3.8 ± 1.5 | NG | 4.5± 0.4 | |
| TAT | NG | 4.2 ± 0.4 | NG | 4.2 ± 0.8 | NG | 4.5 ± 0.3 | ||
| 35866 | MLB | 2.2 ± 1.5 | 3.6 ± 0.2 | 3.8 ± 0.5 | 4.5 ± 0.3 | 5.1 ± 0.1 | 5.0 ± 0.1 | |
| TAT | 3.6 ± 0.2 | 4.0 ± 0.8 | 4.3 ± 0.4 | 4.6 ± 0.5 | 5.1 ± 0.1 | 5.0 ± 0.1 | ||
| T20 | 4227 | MLB | 2.0 ± 0.7 | 1.6 ± 1.4 | 0.5 ± 0.4 | 0.9 ± 1.0 | 2.4 ± 0.8 | 3.2 ± 0.6 |
| TAT | 1.9 ± 1.0 | 2.2 ± 1.3 | 0.4 ± 0.5 | 0.4 ± 0.5 | 2.4 ± 0.4 | 2.7 ± 0.7 | ||
| 60006 | MLB | 1.4 ± 2.4 | 0.9 ± 1.6 | NG | NG | 3.0 ± 1.2 | 2.6 ± 1.0 | |
| TAT | 0.6 ± 1.0 | 0.6 ± 1.0 | 0.2 ± 0.2 | 0.7 ± 1.1 | 1.8 ± 1.1 | 2.2 ± 1.5 | ||
| 14579 | MLB | 1.3 ± 1.3 | 1.3 ± 1.3 | NG | NG | 2.5 ± 0.7 | 2.9 ± 0.8 | |
| TAT | 1.2 ± 1.6 | 1.2 ± 1.6 | NG | NG | 2.9 ± 0.6 | 3.0 ± 0.6 | ||
| 6458 | MLB | 0.4 ± 0.6 | 0.4 ± 0.6 | NG | 0.3 ± 0.5 | 3.4 ± 0.3 | 3.6 ± 0.2 | |
| TAT | 0.6 ± 0.5 | 0.6 ± 0.6 | 0.2 ± 0.2 | 0.2 ± 0.2 | 3.2±1.3 | 3.4 ± 1.2 | ||
| 6264 | MLB | 2.3 ± 1.8 | 2.3 ± 1.8 | NG | NG | 2.5 ± 0.6 | 2.5 ± 0.1 | |
| TAT | 1.6 ± 1.6 | 1.6 ± 1.6 | NG | NG | 3.6 ± 1.2 | 3.3 ± 1.6 | ||
| 15563 | MLB | NG | 2.6 ± 2.0 | NG | 0.3 ± 0.5 | NA | 2.7±0.8 | |
| TAT | NG | 1.1 ± 1.3 | NG | 0.4 ± 0.6 | NA | 2.8 ± 2.4 | ||
| 35866 | MLB | 0.9 ± 0.7 | 0.8 ± 0.7 | NG | NG | 2.7±0.9 | 2.9 ± 1.1 | |
| TAT | 0.5 ± 0.5 | 0.7 ± 0.8 | NG | NG | 2.7±0.8 | 3.2 ± 1.0 | ||
| TS | 4227 | MLB | 1.8 ± 1.8 | 3.5 ± 0.6 | 2.3 ± 1.4 | 2.7 ± 1.7 | 4.9 ± 0.2 | 4.8 ± 0.2 |
| TAT | 2.0 ± 1.8 | 3.3 ± 0.6 | 2.3 ± 1.5 | 3.1 ± 1.0 | 4.8 ± 0.3 | 4.8 ± 0.2 | ||
| 60006 | MLB | 3.0 ± 0.4 | 3.7 ± 0.1 | 1.7 ± 2.1 | 2.4 ± 1.7 | 5.2 ± 0.2 | 5.1 ± 0.1 | |
| TAT | 2.8 ± 1.8 | 3.6 ± 0.1 | 2.3 ± 1.7 | 2.5 ± 2.0 | 5.2 ± 0.0 | 5.1 ± 0.1 | ||
| 14579 | MLB | 3.5 ± 0.1 | 3.8 ± 0.2 | 0.8 ± 1.3 | 1.1 ± 1.8 | 5.2 ± 0.1 | 5.2 ± 0.1 | |
| TAT | 3.5 ± 1.5 | 4.3 ± 0.7 | 0.2 ± 1.4 | 1.3 ± 1.6 | 5.2 ± 0.2 | 5.3 ± 0.1 | ||
| 6458 | MLB | 3.0 ± 1.8 | 3.9 ± 0.3 | 2.0 ± 1.3 | 2.7 ± 1.8 | 5.1± 0.1 | 5.0 ± 0.1 | |
| TAT | 3.1 ± 1.8 | 4.0 ± 0.2 | 2.4 ± 1.0 | 2.7 ± 1.3 | 5.1 ± 0.1 | 5.1 ± 0.1 | ||
| 6264 | MLB | 4.0 ± 0.7 | 4.0 ± 0.8 | 2.2 ± 1.5 | 2.8 ± 1.8 | 5.0 ± 0.3 | 4.9 ± 0.3 | |
| TAT | 3.2 ± 1.5 | 4.0 ± 0.6 | 2.2 ± 0.7 | 3.1 ± 1.4 | 5.0 ± 0.3 | 5.0 ± 0.3 | ||
| 15563 | MLB | NA | 3.5 ± 0.8 | NA | 2.5 ± 2.3 | NA | 4.5 ± 1.0 | |
| TAT | NA | 4.3 ± 0.7 | NA | 2.6 ± 2.4 | NA | 4.4±0.6 | ||
| 35866 | MLB | 2.7 ± 2.3 | 3.9 ± 0.6 | 1.9 ± 1.8 | 2.5 ± 2.2 | 5.1 ± 0.0 | 5.0 ± 0.1 | |
| TAT | 2.8 ± 2.5 | 3.9 ± 0.6 | 1.8 ± 1.3 | 2.8 ± 2.1 | 5.0 ± 0.1 | 5.0 ± 0.1 | ||
| Type 3 Tests of Fixed Effects | ||
|---|---|---|
| Effect | F Value | Pr > F |
| Product | 24.84 | <0.0001 |
| Bacteria | 0.67 | 0.6704 |
| Broth | 16.14 | <0.0001 |
| Media | 14.67 | <0.0001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yossa, N.; Arce, G.; Smiley, J.; Huang, M.-C.J.; Yin, L.; Bell, R.; Tallent, S.; Brown, E.; Hammack, T. Optimized Culture Conditions for the Detection of Selected Strains of Bacillus in Eye Creams. Cosmetics 2017, 4, 56. https://doi.org/10.3390/cosmetics4040056
Yossa N, Arce G, Smiley J, Huang M-CJ, Yin L, Bell R, Tallent S, Brown E, Hammack T. Optimized Culture Conditions for the Detection of Selected Strains of Bacillus in Eye Creams. Cosmetics. 2017; 4(4):56. https://doi.org/10.3390/cosmetics4040056
Chicago/Turabian StyleYossa, Nadine, Gabriela Arce, James Smiley, Mei-Chiung Jo Huang, Lanlan Yin, Rebecca Bell, Sandra Tallent, Eric Brown, and Thomas Hammack. 2017. "Optimized Culture Conditions for the Detection of Selected Strains of Bacillus in Eye Creams" Cosmetics 4, no. 4: 56. https://doi.org/10.3390/cosmetics4040056




